

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50088653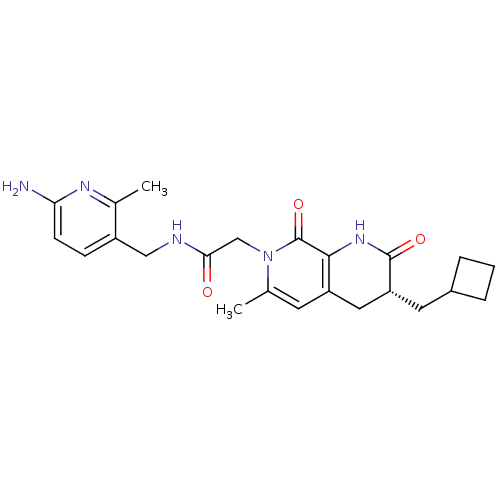 (CHEMBL274152 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit thrombin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50088660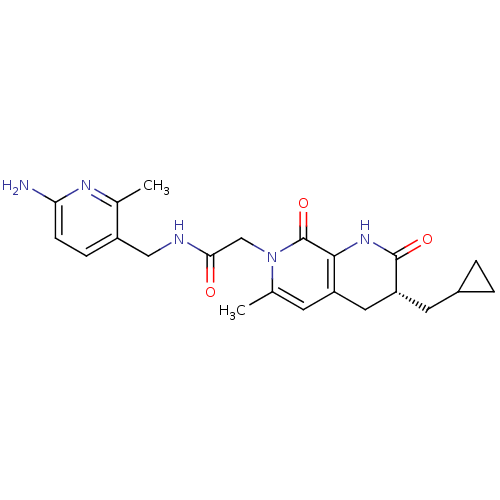 (CHEMBL10785 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit thrombin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50088657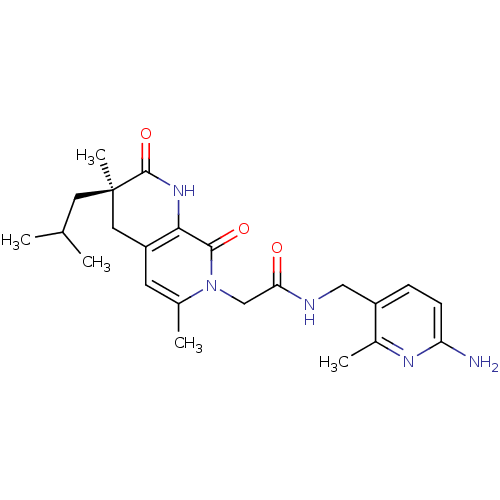 (CHEMBL11181 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit thrombin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50088659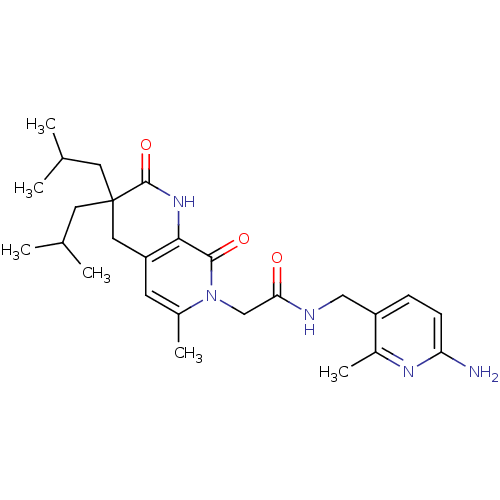 (CHEMBL10346 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit thrombin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50088658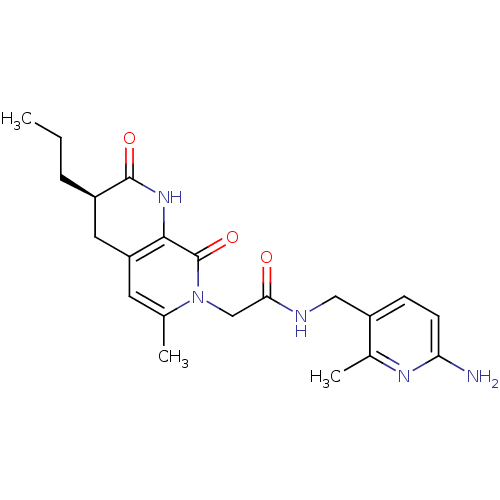 (CHEMBL10800 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit thrombin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50088655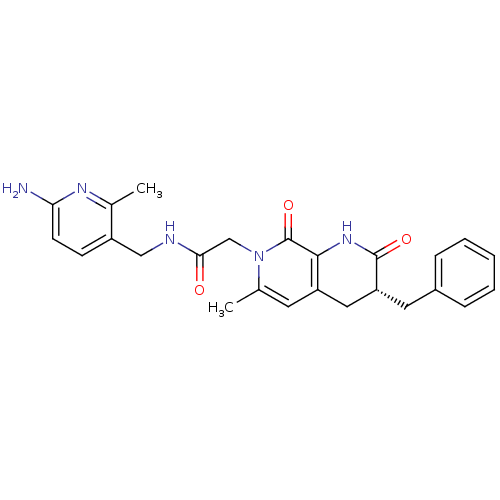 (CHEMBL10545 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit thrombin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50088652 (CHEMBL275520 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit thrombin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50088656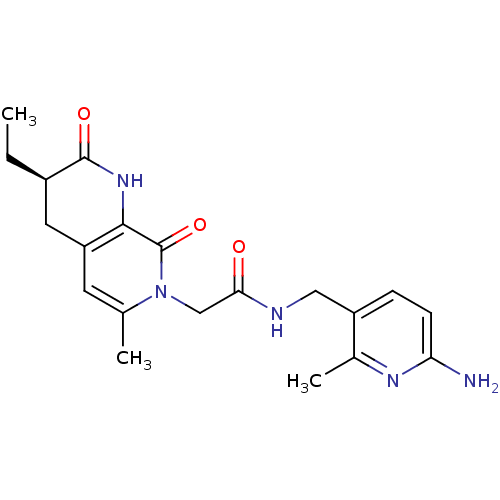 (CHEMBL10657 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit thrombin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50088661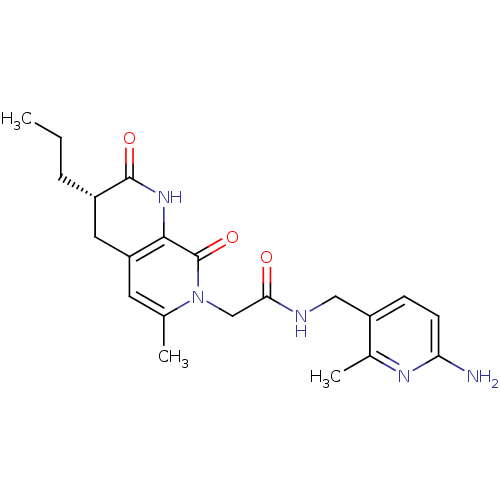 (CHEMBL275110 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit thrombin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50088654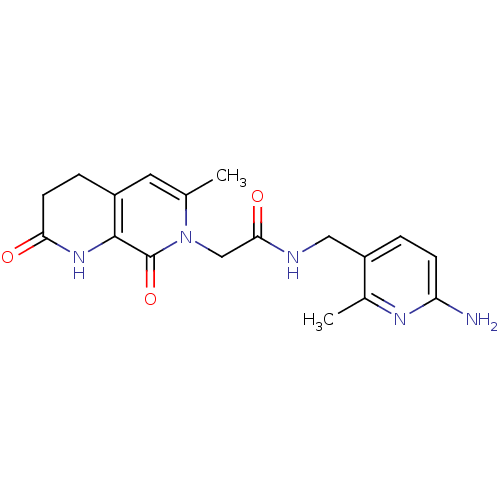 (CHEMBL273861 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit thrombin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50088653 (CHEMBL274152 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit trypsin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50088658 (CHEMBL10800 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit trypsin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50088652 (CHEMBL275520 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit trypsin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50088660 (CHEMBL10785 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit trypsin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50088657 (CHEMBL11181 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit trypsin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50088659 (CHEMBL10346 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit trypsin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50088656 (CHEMBL10657 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit trypsin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50088655 (CHEMBL10545 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit trypsin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50088661 (CHEMBL275110 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit trypsin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50088654 (CHEMBL273861 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for its ability to inhibit trypsin. | Bioorg Med Chem Lett 10: 1069-72 (2000) BindingDB Entry DOI: 10.7270/Q2DZ07J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16694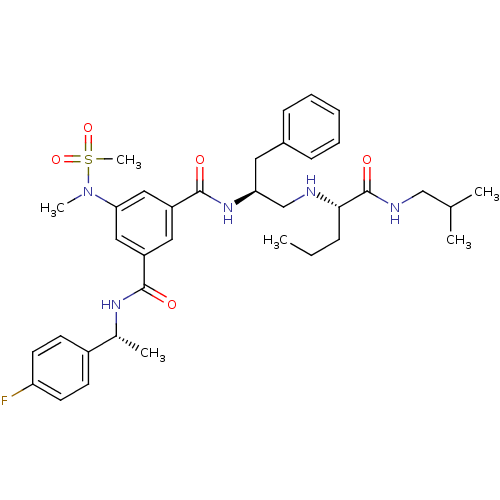 (1-N-[(1R)-1-(4-fluorophenyl)ethyl]-5-(N-methylmeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 16: 3635-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.076 BindingDB Entry DOI: 10.7270/Q27P8WN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16693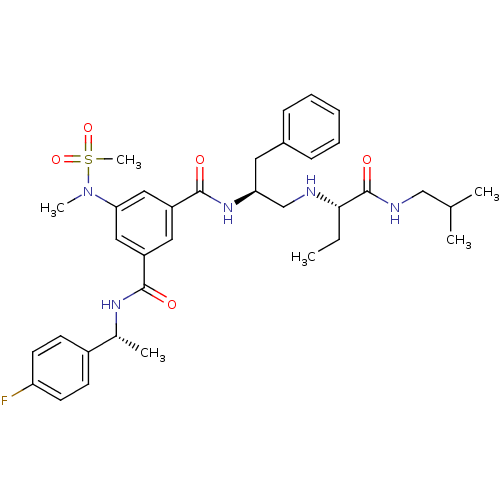 (1-N-[(1R)-1-(4-fluorophenyl)ethyl]-5-(N-methylmeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 16: 3635-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.076 BindingDB Entry DOI: 10.7270/Q27P8WN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16692 (1-N-[(1R)-1-(4-fluorophenyl)ethyl]-5-(N-methylmeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 16: 3635-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.076 BindingDB Entry DOI: 10.7270/Q27P8WN0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16696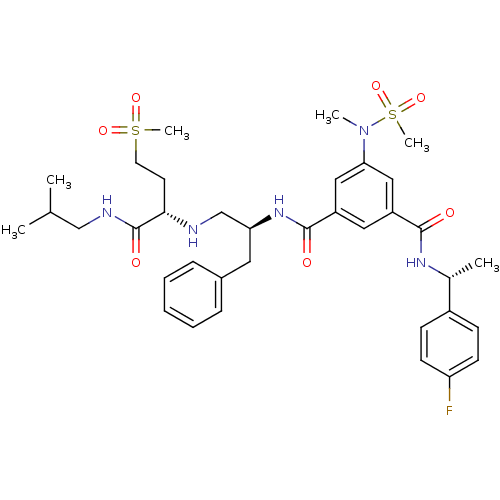 (1-N-[(1R)-1-(4-fluorophenyl)ethyl]-3-N-[(2S)-1-{[(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 16: 3635-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.076 BindingDB Entry DOI: 10.7270/Q27P8WN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16695 (1-N-[(1R)-1-(4-fluorophenyl)ethyl]-3-N-[(2S)-1-{[(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 16: 3635-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.076 BindingDB Entry DOI: 10.7270/Q27P8WN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16701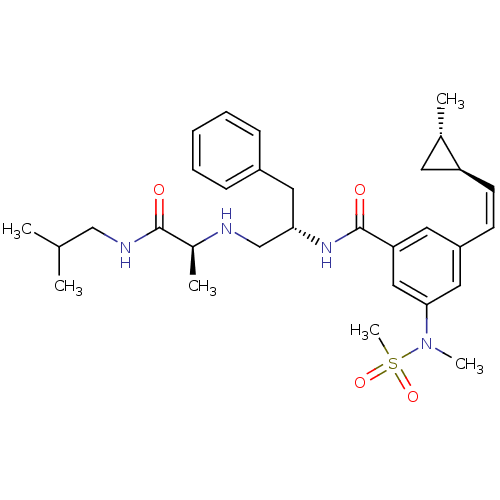 ((2S)-2-{[(2S)-2-({3-[(Z)-2-[(1S,2S)-2-methylcyclop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 16: 3635-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.076 BindingDB Entry DOI: 10.7270/Q27P8WN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16698 (1-N-[(2S)-1-{[(1S)-1-(cyclopropylcarbamoyl)ethyl]a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 16: 3635-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.076 BindingDB Entry DOI: 10.7270/Q27P8WN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16697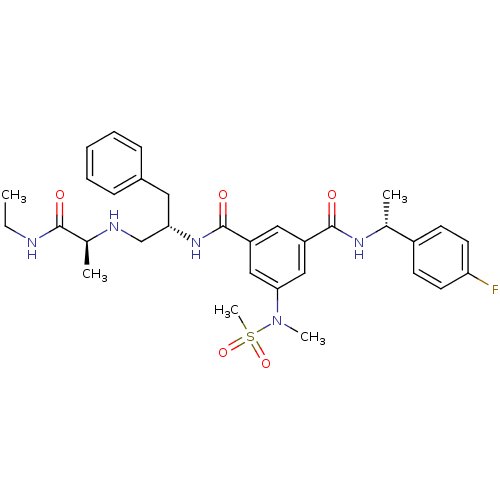 (1-N-[(2S)-1-{[(1S)-1-(ethylcarbamoyl)ethyl]amino}-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 16: 3635-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.076 BindingDB Entry DOI: 10.7270/Q27P8WN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16691 (1-N-[(1R)-1-(4-fluorophenyl)ethyl]-5-(N-methylmeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 117 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 16: 3635-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.076 BindingDB Entry DOI: 10.7270/Q27P8WN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16699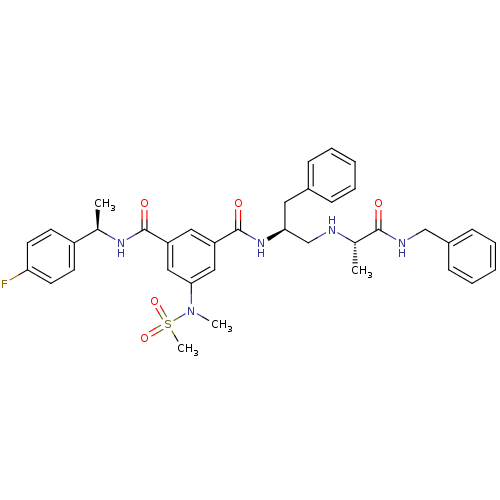 (1-N-[(2S)-1-{[(1S)-1-(benzylcarbamoyl)ethyl]amino}...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 139 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 16: 3635-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.076 BindingDB Entry DOI: 10.7270/Q27P8WN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16689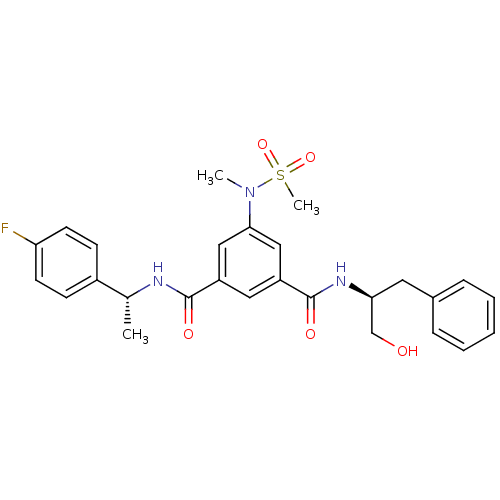 (1,3,5-trisubstituted aromatic scaffold, 2a | 1-N-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 16: 3635-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.076 BindingDB Entry DOI: 10.7270/Q27P8WN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16046 (3-{[(5-aminopentyl)carbamoyl]methoxy}-5-{[(1R)-1-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | J Med Chem 47: 6117-9 (2004) Article DOI: 10.1021/jm049388p BindingDB Entry DOI: 10.7270/Q21C1V47 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16044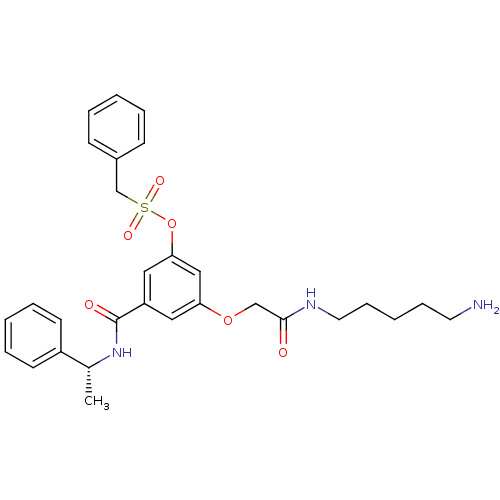 (3-{[(5-aminopentyl)carbamoyl]methoxy}-5-{[(1R)-1-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | J Med Chem 47: 6117-9 (2004) Article DOI: 10.1021/jm049388p BindingDB Entry DOI: 10.7270/Q21C1V47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16690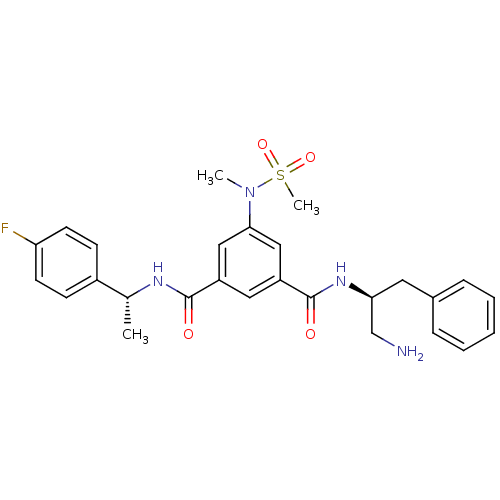 (1,3,5-trisubstituted aromatic scaffold, 2b | 1-N-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 16: 3635-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.076 BindingDB Entry DOI: 10.7270/Q27P8WN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16700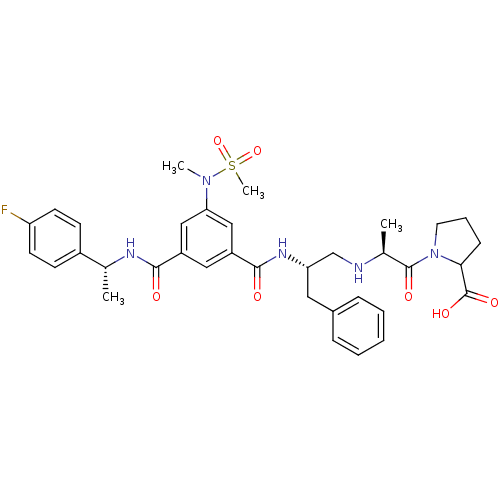 (1-[(2S)-2-{[(2S)-2-[(3-{[(1R)-1-(4-fluorophenyl)et...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.72E+4 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | Bioorg Med Chem Lett 16: 3635-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.076 BindingDB Entry DOI: 10.7270/Q27P8WN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16043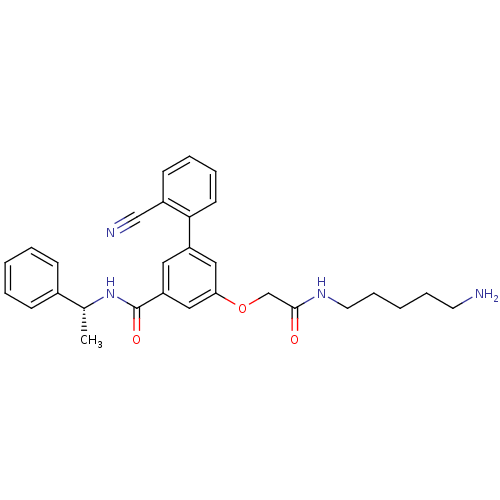 (3-{[(5-aminopentyl)carbamoyl]methoxy}-5-(2-cyanoph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | J Med Chem 47: 6117-9 (2004) Article DOI: 10.1021/jm049388p BindingDB Entry DOI: 10.7270/Q21C1V47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16042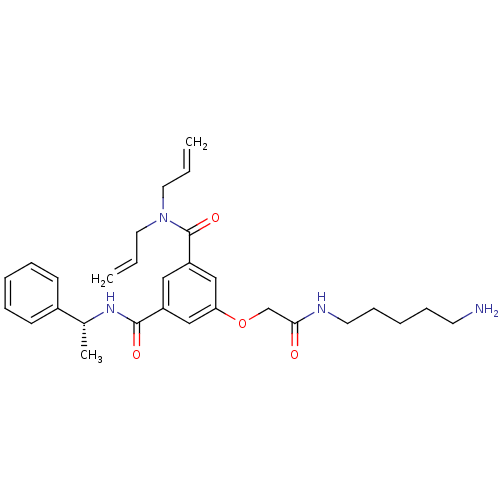 (5-{[(5-aminopentyl)carbamoyl]methoxy}-3-N-[(1R)-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | J Med Chem 47: 6117-9 (2004) Article DOI: 10.1021/jm049388p BindingDB Entry DOI: 10.7270/Q21C1V47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16045 (3-{[(5-aminopentyl)carbamoyl]methoxy}-5-{[(1S)-1-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
Merck Research Laboratories | Assay Description The assay was performed using a 96-well format on a HPLC equipped with four 96-well plate holders. Test compounds were preincubated with enzymes for ... | J Med Chem 47: 6117-9 (2004) Article DOI: 10.1021/jm049388p BindingDB Entry DOI: 10.7270/Q21C1V47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||