

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50121272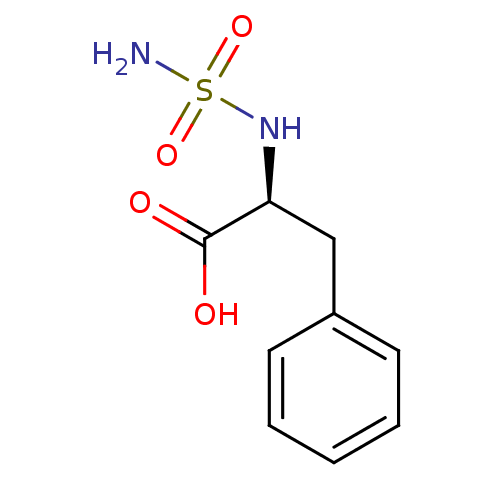 ((2S)-2-[(aminosulfonyl)amino]-3-phenylpropanoic ac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB PubMed | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Carboxypeptidase A | J Med Chem 45: 5295-302 (2002) BindingDB Entry DOI: 10.7270/Q2BR8RJM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50253594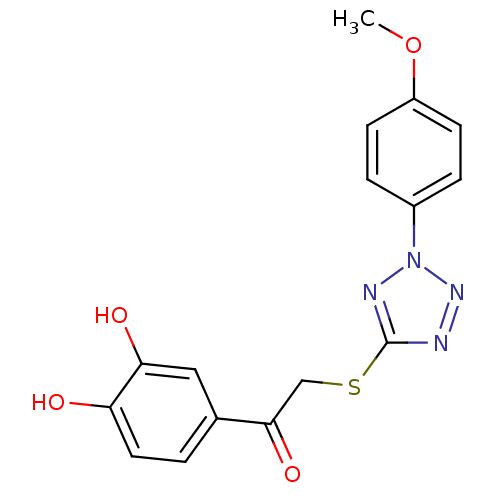 (1-(3,4-dihydroxyphenyl)-2-[2-(4-methoxyphenyl)-2H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Binding affinity to Cdc25A (336-523) (unknown origin) expressed in Escherichia coli by steady-state kinetic assay | J Med Chem 51: 5533-41 (2008) Article DOI: 10.1021/jm701157g BindingDB Entry DOI: 10.7270/Q20V8CMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50253593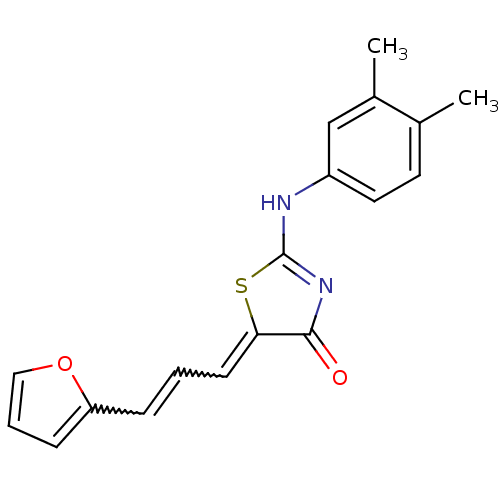 (2-(3,4-Dimethylphenylamino)-5-(3-furan-2-ylallylid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Binding affinity to Cdc25A (336-523) (unknown origin) expressed in Escherichia coli by steady-state kinetic assay | J Med Chem 51: 5533-41 (2008) Article DOI: 10.1021/jm701157g BindingDB Entry DOI: 10.7270/Q20V8CMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50237948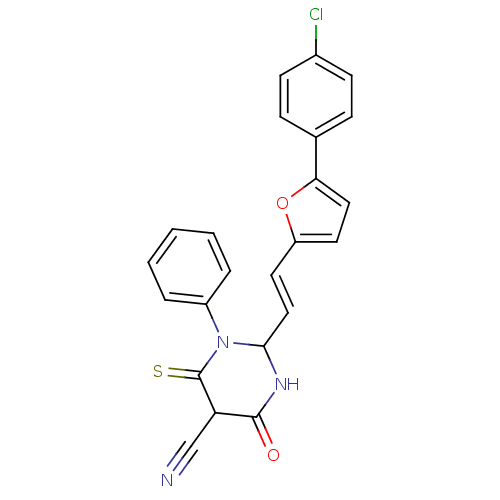 ((E)-2-(2-(5-(4-chlorophenyl)furan-2-yl)vinyl)-6-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Binding affinity to Cdc25A (336-523) (unknown origin) expressed in Escherichia coli by steady-state kinetic assay | J Med Chem 51: 5533-41 (2008) Article DOI: 10.1021/jm701157g BindingDB Entry DOI: 10.7270/Q20V8CMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50121270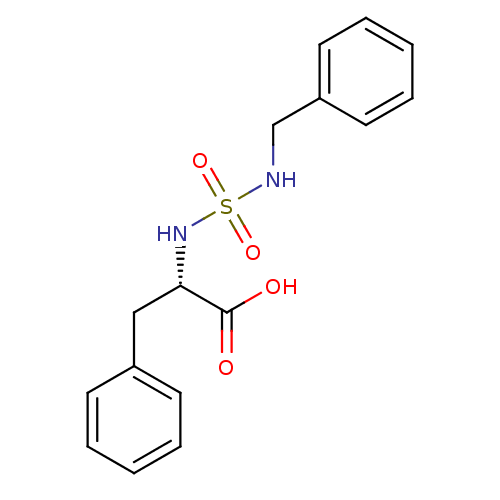 ((2S)-2-({[(phenylmethyl)amino]sulfonyl}amino)-3-ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Carboxypeptidase A | J Med Chem 45: 5295-302 (2002) BindingDB Entry DOI: 10.7270/Q2BR8RJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50253622 (2-[4-(4-chlorophenyl)-5-p-tolyl-4H-[1,2,4]triazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Binding affinity to Cdc25A (336-523) (unknown origin) expressed in Escherichia coli by steady-state kinetic assay | J Med Chem 51: 5533-41 (2008) Article DOI: 10.1021/jm701157g BindingDB Entry DOI: 10.7270/Q20V8CMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50121276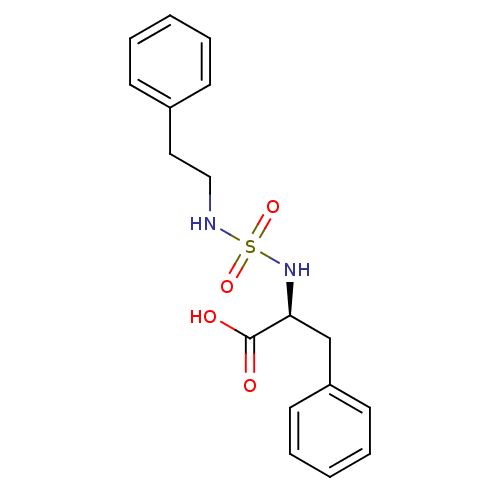 ((2S)-2-({[(2-phenylethyl)amino]sulfonyl}amino)-3-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Carboxypeptidase A | J Med Chem 45: 5295-302 (2002) BindingDB Entry DOI: 10.7270/Q2BR8RJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50121275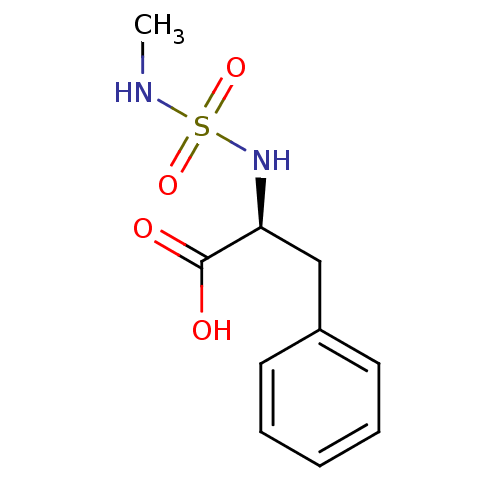 ((2S)-2-{[(methylamino)sulfonyl]amino}-3-phenylprop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Carboxypeptidase A | J Med Chem 45: 5295-302 (2002) BindingDB Entry DOI: 10.7270/Q2BR8RJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50121269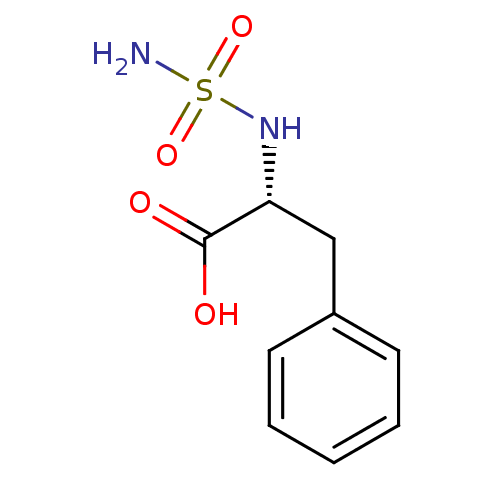 ((2R)-2-[(aminosulfonyl)amino]-3-phenylpropanoic ac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Carboxypeptidase A | J Med Chem 45: 5295-302 (2002) BindingDB Entry DOI: 10.7270/Q2BR8RJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50121273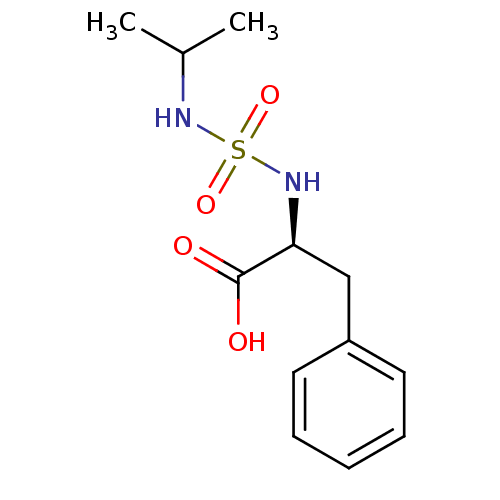 ((2S)-2-{[(isopropylamino)sulfonyl]amino}-3-phenylp...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Carboxypeptidase A | J Med Chem 45: 5295-302 (2002) BindingDB Entry DOI: 10.7270/Q2BR8RJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50121274 ((2R)-2-benzyl-3-{[(methyl-lambda~5~-azanyl)sulfony...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Carboxypeptidase A | J Med Chem 45: 5295-302 (2002) BindingDB Entry DOI: 10.7270/Q2BR8RJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50121277 (2-benzyl-3-{[(methyl-lambda~5~-azanyl)sulfonyl]ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Carboxypeptidase A | J Med Chem 45: 5295-302 (2002) BindingDB Entry DOI: 10.7270/Q2BR8RJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50121271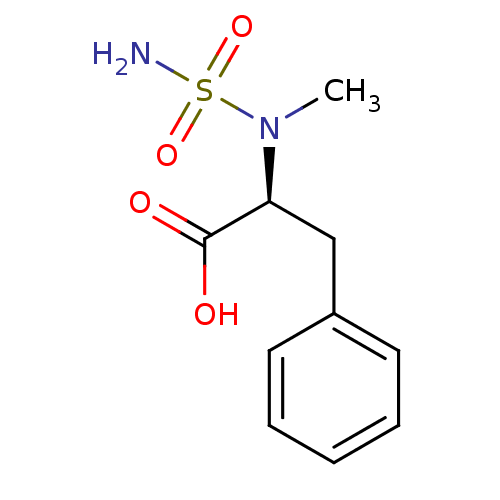 ((2S)-2-[(aminosulfonyl)(methyl)amino]-3-phenylprop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Carboxypeptidase A | J Med Chem 45: 5295-302 (2002) BindingDB Entry DOI: 10.7270/Q2BR8RJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine-phosphatase (Homo sapiens (Human)) | BDBM50391713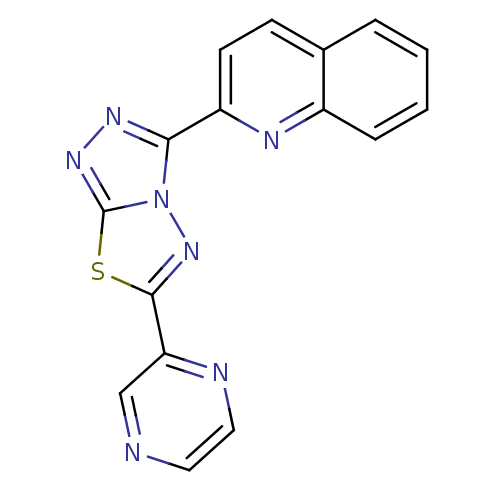 (CHEMBL2146956) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Inhibition of His-tagged PTPsigma catalytic domain amino acid 1369 to 1948 using DiFMUP as substrate after 20 mins by spectrofluorometric analysis | Bioorg Med Chem Lett 22: 6333-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.081 BindingDB Entry DOI: 10.7270/Q2HT2QDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine-phosphatase (Homo sapiens (Human)) | BDBM50237957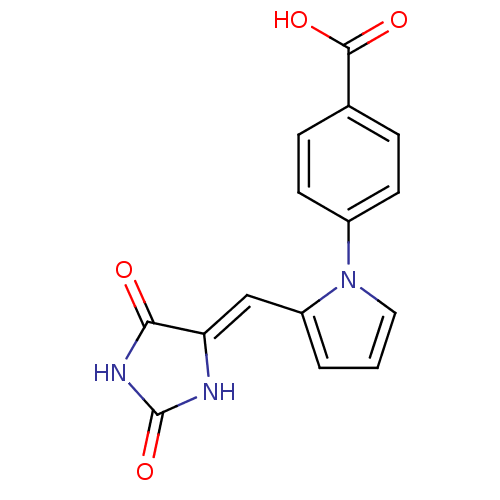 (4-(2-((2,5-dioxoimidazolidin-4-ylidene)methyl)-1H-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Inhibition of His-tagged PTPsigma catalytic domain amino acid 1369 to 1948 using DiFMUP as substrate after 20 mins by spectrofluorometric analysis | Bioorg Med Chem Lett 22: 6333-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.081 BindingDB Entry DOI: 10.7270/Q2HT2QDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase S (Homo sapiens (Human)) | BDBM50456401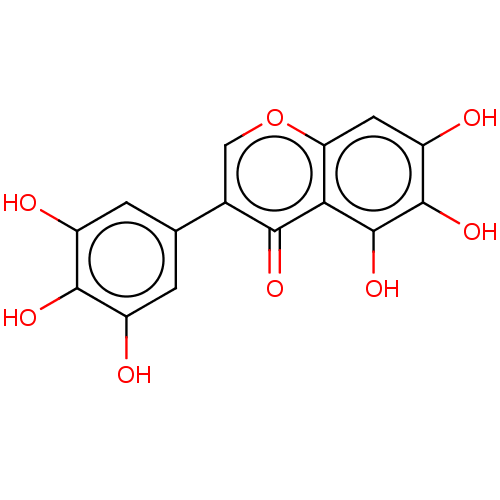 (IRIGENOL) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP-sigma (residues 1367 to 1948) using para-nitrophenylphosphate as substrate for 60 mins by fluorescence analysis | Bioorg Med Chem Lett 26: 87-93 (2016) Article DOI: 10.1016/j.bmcl.2015.11.026 BindingDB Entry DOI: 10.7270/Q2M048FV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine-phosphatase (Homo sapiens (Human)) | BDBM50391714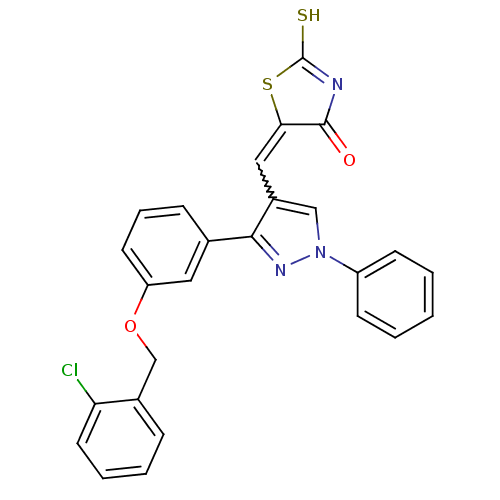 (CHEMBL2146957) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Inhibition of His-tagged PTPsigma catalytic domain amino acid 1369 to 1948 using DiFMUP as substrate after 20 mins by spectrofluorometric analysis | Bioorg Med Chem Lett 22: 6333-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.081 BindingDB Entry DOI: 10.7270/Q2HT2QDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase S (Homo sapiens (Human)) | BDBM23411 (5,6,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP-sigma (residues 1367 to 1948) using para-nitrophenylphosphate as substrate for 60 mins by fluorescence analysis | Bioorg Med Chem Lett 26: 87-93 (2016) Article DOI: 10.1016/j.bmcl.2015.11.026 BindingDB Entry DOI: 10.7270/Q2M048FV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine-phosphatase (Homo sapiens (Human)) | BDBM50391715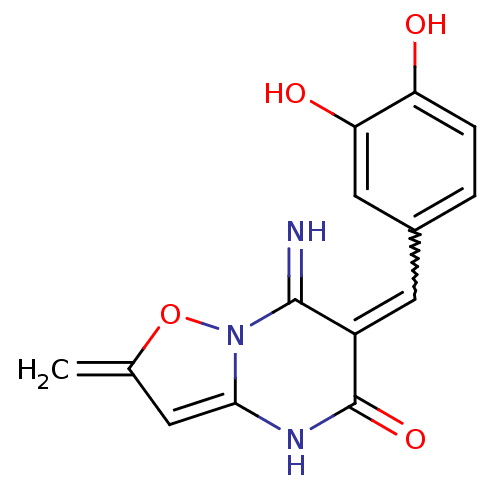 (CHEMBL2146960) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Inhibition of His-tagged PTPsigma catalytic domain amino acid 1369 to 1948 using DiFMUP as substrate after 20 mins by spectrofluorometric analysis | Bioorg Med Chem Lett 22: 6333-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.081 BindingDB Entry DOI: 10.7270/Q2HT2QDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase S (Homo sapiens (Human)) | BDBM50499947 (CHEMBL3739950) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP-sigma (residues 1367 to 1948) using para-nitrophenylphosphate as substrate for 60 mins by fluorescence analysis | Bioorg Med Chem Lett 26: 87-93 (2016) Article DOI: 10.1016/j.bmcl.2015.11.026 BindingDB Entry DOI: 10.7270/Q2M048FV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50253622 (2-[4-(4-chlorophenyl)-5-p-tolyl-4H-[1,2,4]triazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Inhibition of Cdc25A (336-523) (unknown origin) expressed in Escherichia coli | J Med Chem 51: 5533-41 (2008) Article DOI: 10.1021/jm701157g BindingDB Entry DOI: 10.7270/Q20V8CMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine-phosphatase (Homo sapiens (Human)) | BDBM50391711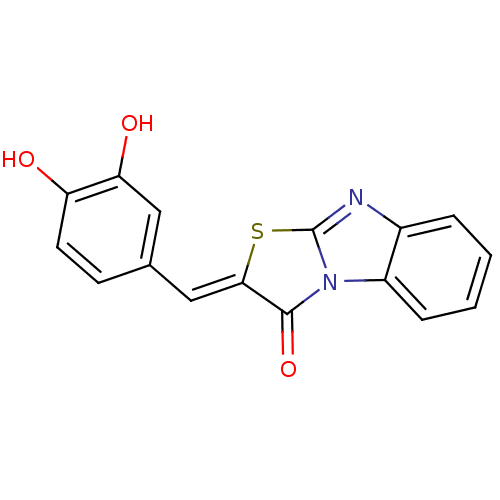 (CHEMBL2146958) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Inhibition of His-tagged PTPsigma catalytic domain amino acid 1369 to 1948 using DiFMUP as substrate after 20 mins by spectrofluorometric analysis | Bioorg Med Chem Lett 22: 6333-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.081 BindingDB Entry DOI: 10.7270/Q2HT2QDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase S (Homo sapiens (Human)) | BDBM50499946 (CHEMBL3740878) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of recombinant human PTP-sigma (residues 1367 to 1948) using para-nitrophenylphosphate as substrate for 60 mins by fluorescence analysis | Bioorg Med Chem Lett 26: 87-93 (2016) Article DOI: 10.1016/j.bmcl.2015.11.026 BindingDB Entry DOI: 10.7270/Q2M048FV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein tyrosine phosphatase type IVA 3 (Homo sapiens (Human)) | BDBM50184297 (5-(5-(benzo[b]thiophen-3-yl)-2-(benzyloxy)benzylid...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PRL-3 | Bioorg Med Chem Lett 16: 2996-9 (2006) Article DOI: 10.1016/j.bmcl.2006.02.060 BindingDB Entry DOI: 10.7270/Q2RB746W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein tyrosine phosphatase type IVA 3 (Homo sapiens (Human)) | BDBM50184288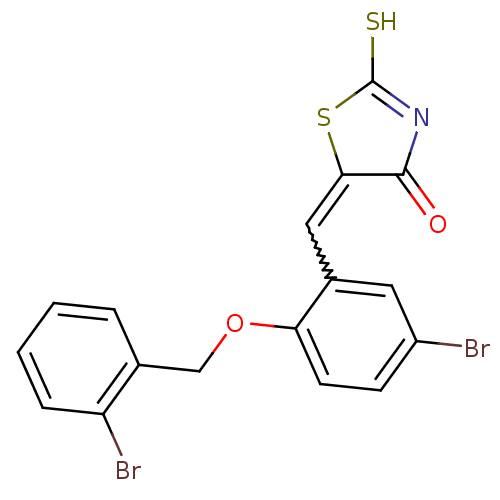 (5-(2-(2-bromobenzyloxy)-5-bromobenzylidene)-2-thio...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PRL-3 | Bioorg Med Chem Lett 16: 2996-9 (2006) Article DOI: 10.1016/j.bmcl.2006.02.060 BindingDB Entry DOI: 10.7270/Q2RB746W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50296442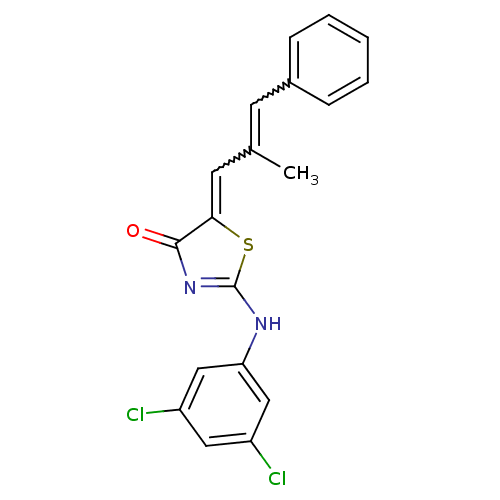 (5-(2-methyl-3-phenylallylidene)-2-(phenylamino)thi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Inhibition of N-terminal 6xHis-tagged (378-566) catalytic domain of Cdc25B expressed in Escherichia coli after 20 mins by by fluorescent plate reader... | Bioorg Med Chem Lett 19: 4330-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.084 BindingDB Entry DOI: 10.7270/Q2NZ87P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine-phosphatase (Homo sapiens (Human)) | BDBM50387974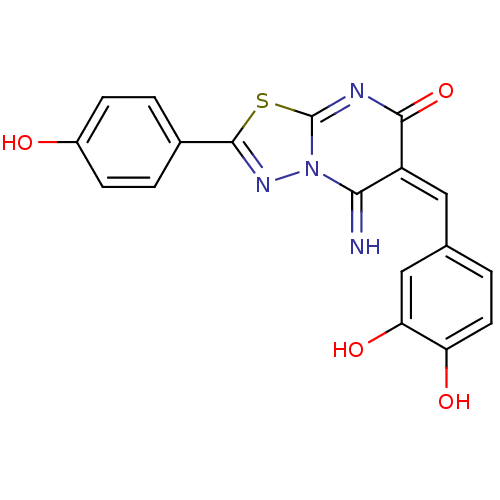 (CHEMBL2057830) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Inhibition of His-tagged PTPsigma catalytic domain amino acid 1369 to 1948 using DiFMUP as substrate after 20 mins by spectrofluorometric analysis | Bioorg Med Chem Lett 22: 6333-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.081 BindingDB Entry DOI: 10.7270/Q2HT2QDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein tyrosine phosphatase type IVA 3 (Homo sapiens (Human)) | BDBM50184287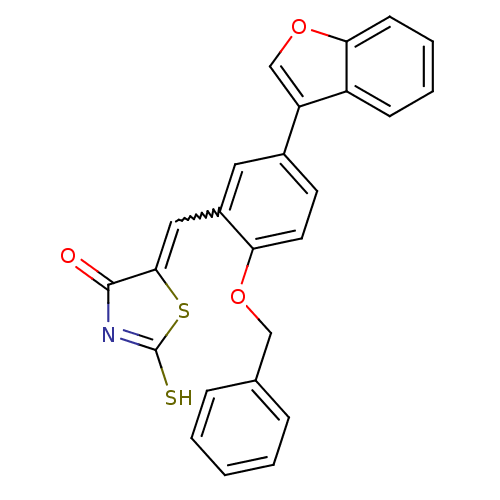 (5-(5-(benzofuran-3-yl)-2-(benzyloxy)benzylidene)-2...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PRL-3 | Bioorg Med Chem Lett 16: 2996-9 (2006) Article DOI: 10.1016/j.bmcl.2006.02.060 BindingDB Entry DOI: 10.7270/Q2RB746W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine-phosphatase (Homo sapiens (Human)) | BDBM50391712 (CHEMBL2146959) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Inhibition of His-tagged PTPsigma catalytic domain amino acid 1369 to 1948 using DiFMUP as substrate after 20 mins by spectrofluorometric analysis | Bioorg Med Chem Lett 22: 6333-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.081 BindingDB Entry DOI: 10.7270/Q2HT2QDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50296467 (2-(5-(4-chlorophenyl)-4-phenyl-4H-1,2,4-triazol-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Inhibition of N-terminal 6xHis-tagged (378-566) catalytic domain of Cdc25B expressed in Escherichia coli after 20 mins by by fluorescent plate reader... | Bioorg Med Chem Lett 19: 4330-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.084 BindingDB Entry DOI: 10.7270/Q2NZ87P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein tyrosine phosphatase type IVA 3 (Homo sapiens (Human)) | BDBM50184285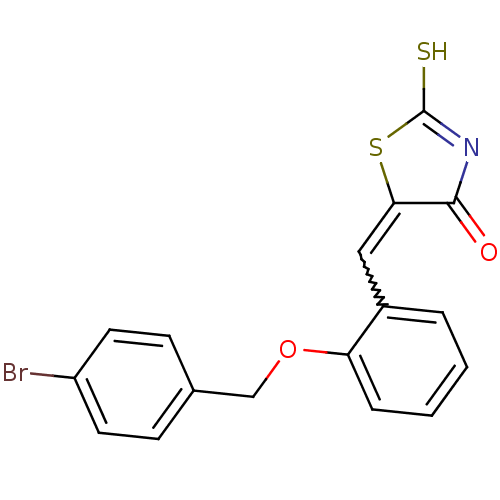 (5-(2-(4-bromobenzyloxy)benzylidene)-2-thioxothiazo...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PRL-3 | Bioorg Med Chem Lett 16: 2996-9 (2006) Article DOI: 10.1016/j.bmcl.2006.02.060 BindingDB Entry DOI: 10.7270/Q2RB746W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50179012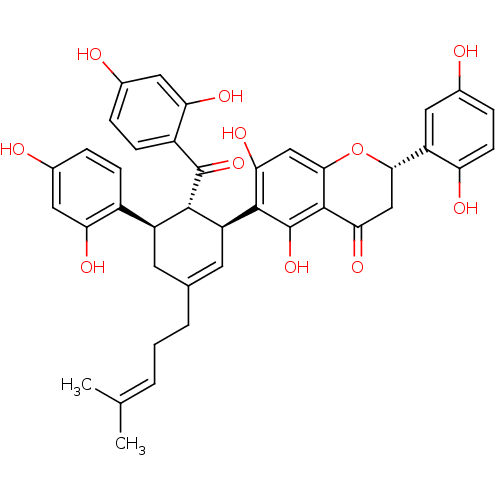 (CHEMBL382338 | Sanggenon G) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology (KRIBB) Curated by ChEMBL | Assay Description Inhibition of PTP1B | Bioorg Med Chem Lett 16: 1426-9 (2006) Article DOI: 10.1016/j.bmcl.2005.11.071 BindingDB Entry DOI: 10.7270/Q2JQ10KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein tyrosine phosphatase type IVA 3 (Homo sapiens (Human)) | BDBM50184296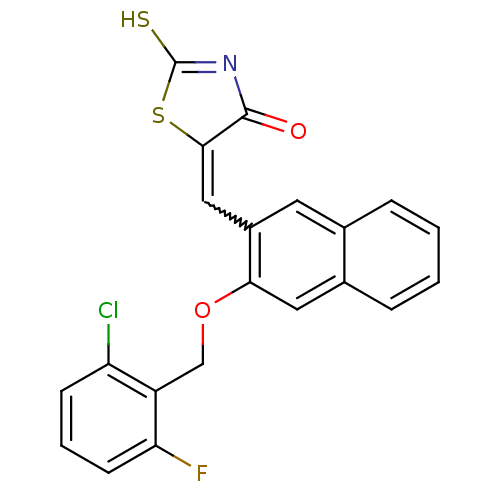 (5-((3-(2-chloro-6-fluorobenzyloxy)naphthalen-2-yl)...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PRL-3 | Bioorg Med Chem Lett 16: 2996-9 (2006) Article DOI: 10.1016/j.bmcl.2006.02.060 BindingDB Entry DOI: 10.7270/Q2RB746W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein tyrosine phosphatase type IVA 3 (Homo sapiens (Human)) | BDBM50184298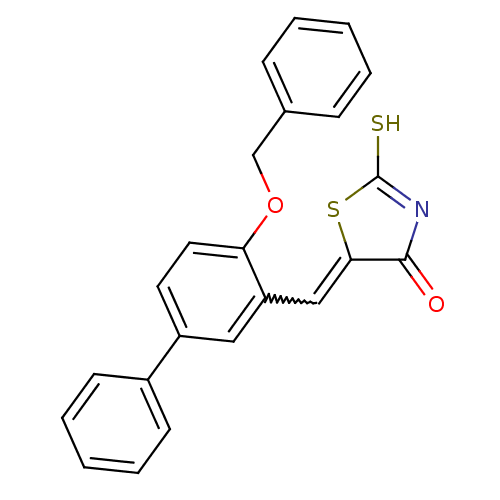 (5-(4-Benzyloxy-biphenyl-3-ylmethylene)-2-thioxo-th...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PRL-3 | Bioorg Med Chem Lett 16: 2996-9 (2006) Article DOI: 10.1016/j.bmcl.2006.02.060 BindingDB Entry DOI: 10.7270/Q2RB746W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50296456 (1-(3,4-dihydroxyphenyl)-2-(5-(4-methoxyphenyl)-4-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Inhibition of N-terminal 6xHis-tagged (378-566) catalytic domain of Cdc25B expressed in Escherichia coli after 20 mins by by fluorescent plate reader... | Bioorg Med Chem Lett 19: 4330-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.084 BindingDB Entry DOI: 10.7270/Q2NZ87P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50253593 (2-(3,4-Dimethylphenylamino)-5-(3-furan-2-ylallylid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Inhibition of N-terminal 6xHis-tagged (378-566) catalytic domain of Cdc25B expressed in Escherichia coli after 20 mins by by fluorescent plate reader... | Bioorg Med Chem Lett 19: 4330-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.084 BindingDB Entry DOI: 10.7270/Q2NZ87P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein tyrosine phosphatase type IVA 3 (Homo sapiens (Human)) | BDBM50184293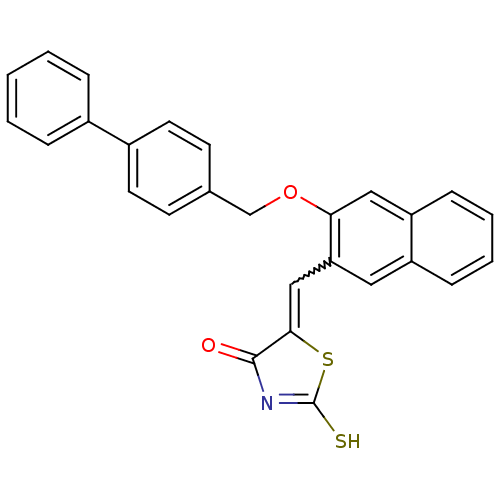 (5-[1-[3-(biphenyl-4-ylmethoxy)-naphthalen-2-yl]-me...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PRL-3 | Bioorg Med Chem Lett 16: 2996-9 (2006) Article DOI: 10.1016/j.bmcl.2006.02.060 BindingDB Entry DOI: 10.7270/Q2RB746W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50253622 (2-[4-(4-chlorophenyl)-5-p-tolyl-4H-[1,2,4]triazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Inhibition of Cdc25B (378-566) (unknown origin) expressed in Escherichia coli | J Med Chem 51: 5533-41 (2008) Article DOI: 10.1021/jm701157g BindingDB Entry DOI: 10.7270/Q20V8CMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein tyrosine phosphatase type IVA 3 (Homo sapiens (Human)) | BDBM50184291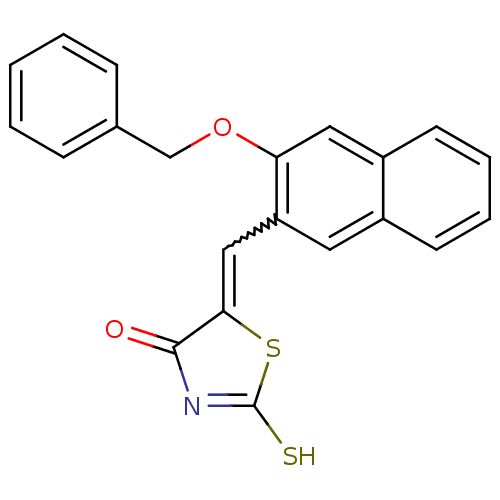 (5-((3-(benzyloxy)naphthalen-2-yl)methylene)-2-thio...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PRL-3 | Bioorg Med Chem Lett 16: 2996-9 (2006) Article DOI: 10.1016/j.bmcl.2006.02.060 BindingDB Entry DOI: 10.7270/Q2RB746W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50296462 (1-(biphenyl-4-yl)-2-(5-(furan-2-yl)-4-methyl-4H-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Inhibition of N-terminal 6xHis-tagged (378-566) catalytic domain of Cdc25B expressed in Escherichia coli after 20 mins by by fluorescent plate reader... | Bioorg Med Chem Lett 19: 4330-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.084 BindingDB Entry DOI: 10.7270/Q2NZ87P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50296443 (2-(2-chlorophenylamino)-5-(3-(furan-2-yl)allyliden...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Inhibition of N-terminal 6xHis-tagged (378-566) catalytic domain of Cdc25B expressed in Escherichia coli after 20 mins by by fluorescent plate reader... | Bioorg Med Chem Lett 19: 4330-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.084 BindingDB Entry DOI: 10.7270/Q2NZ87P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50296444 (2-(2,3-dichlorophenylamino)-5-(3-(furan-2-yl)allyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Inhibition of N-terminal 6xHis-tagged (378-566) catalytic domain of Cdc25B expressed in Escherichia coli after 20 mins by by fluorescent plate reader... | Bioorg Med Chem Lett 19: 4330-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.084 BindingDB Entry DOI: 10.7270/Q2NZ87P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase 4 (Homo sapiens (Human)) | BDBM50391713 (CHEMBL2146956) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Inhibition of MKP2 | Bioorg Med Chem Lett 22: 6333-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.081 BindingDB Entry DOI: 10.7270/Q2HT2QDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50296466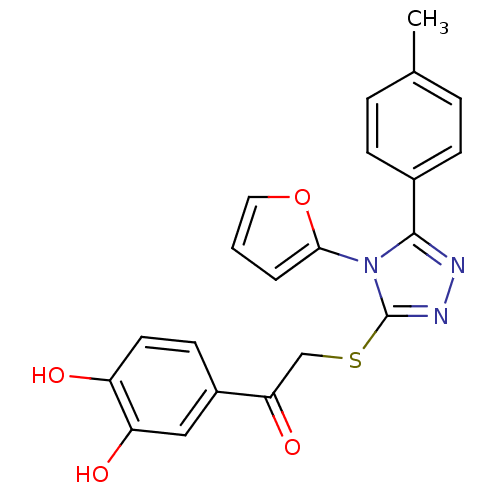 (1-(3,4-dihydroxyphenyl)-2-(4-(furan-2-yl)-5-p-toly...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Inhibition of N-terminal 6xHis-tagged (378-566) catalytic domain of Cdc25B expressed in Escherichia coli after 20 mins by by fluorescent plate reader... | Bioorg Med Chem Lett 19: 4330-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.084 BindingDB Entry DOI: 10.7270/Q2NZ87P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50296447 (2-(3,4-dimethylphenylamino)-5-(2-methyl-3-phenylal...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Inhibition of N-terminal 6xHis-tagged (378-566) catalytic domain of Cdc25B expressed in Escherichia coli after 20 mins by by fluorescent plate reader... | Bioorg Med Chem Lett 19: 4330-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.084 BindingDB Entry DOI: 10.7270/Q2NZ87P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50296450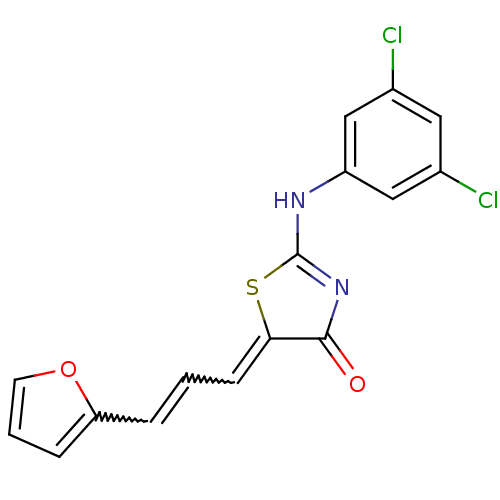 (2-(3,5-dichlorophenylamino)-5-(3-(furan-2-yl)allyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Inhibition of N-terminal 6xHis-tagged (378-566) catalytic domain of Cdc25B expressed in Escherichia coli after 20 mins by by fluorescent plate reader... | Bioorg Med Chem Lett 19: 4330-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.084 BindingDB Entry DOI: 10.7270/Q2NZ87P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50296453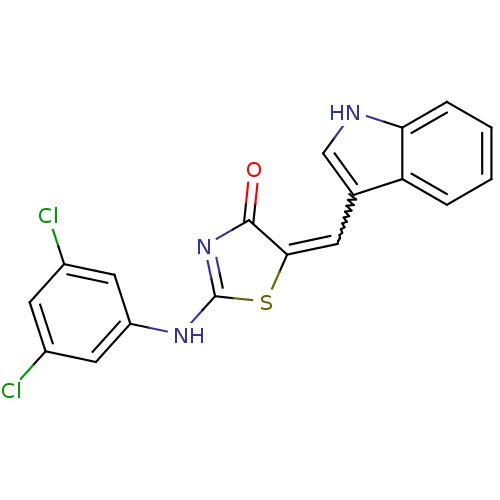 (5-((1H-indol-3-yl)methylene)-2-(3,5-dichlorophenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Inhibition of N-terminal 6xHis-tagged (378-566) catalytic domain of Cdc25B expressed in Escherichia coli after 20 mins by by fluorescent plate reader... | Bioorg Med Chem Lett 19: 4330-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.084 BindingDB Entry DOI: 10.7270/Q2NZ87P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein tyrosine phosphatase type IVA 3 (Homo sapiens (Human)) | BDBM50184290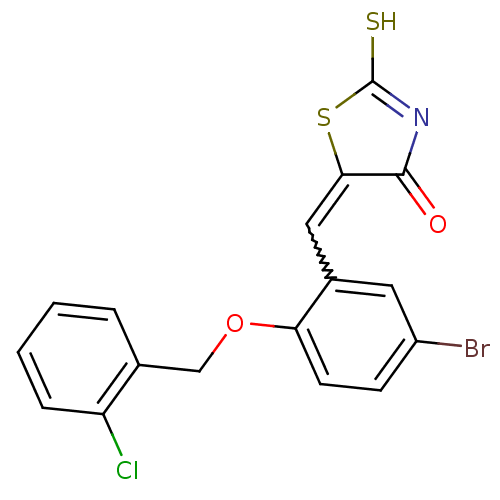 (5-(2-(2-chlorobenzyloxy)-5-bromobenzylidene)-2-thi...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PRL-3 | Bioorg Med Chem Lett 16: 2996-9 (2006) Article DOI: 10.1016/j.bmcl.2006.02.060 BindingDB Entry DOI: 10.7270/Q2RB746W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50296465 (1-(3,4-dihydroxyphenyl)-2-(4-phenyl-5-p-tolyl-4H-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sejong University Curated by ChEMBL | Assay Description Inhibition of N-terminal 6xHis-tagged (378-566) catalytic domain of Cdc25B expressed in Escherichia coli after 20 mins by by fluorescent plate reader... | Bioorg Med Chem Lett 19: 4330-4 (2009) Article DOI: 10.1016/j.bmcl.2009.05.084 BindingDB Entry DOI: 10.7270/Q2NZ87P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50179008 (CHEMBL204813 | sanggenon C) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology (KRIBB) Curated by ChEMBL | Assay Description Inhibition of PTP1B | Bioorg Med Chem Lett 16: 1426-9 (2006) Article DOI: 10.1016/j.bmcl.2005.11.071 BindingDB Entry DOI: 10.7270/Q2JQ10KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 187 total ) | Next | Last >> |