

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Antagonist activity on agonist stimulated [35S]GTP-gamma-S, binding in guinea pig caudate membranes (10 uM SNC-80 as the agonist ligand for delta-rec... | Bioorg Med Chem Lett 13: 529-32 (2003) BindingDB Entry DOI: 10.7270/Q27S7P9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Apparent antagonist activity determined by measuring the ability to inhibit stimulation of [35S]GTP-gamma-S, binding to opioid receptor delta 1 in gu... | J Med Chem 47: 1400-12 (2004) Article DOI: 10.1021/jm030311v BindingDB Entry DOI: 10.7270/Q2639QGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Antagonist activity on agonist stimulated [35S]GTP-gamma-S, binding in guinea pig caudate membranes (10 uM U69,593 as the agonist ligand for kappa-re... | Bioorg Med Chem Lett 13: 529-32 (2003) BindingDB Entry DOI: 10.7270/Q27S7P9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50545673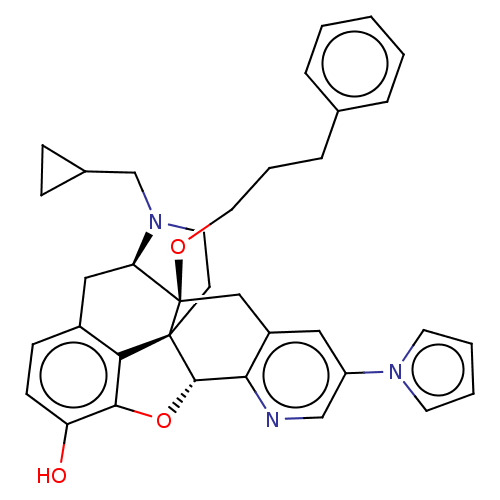 (CHEMBL4634079) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.169 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human delta opioid receptor expressed in CHO cell membranes incubated for 1 hr by competition radioligand bin... | J Med Chem 63: 7663-7694 (2020) Article DOI: 10.1021/acs.jmedchem.0c00503 BindingDB Entry DOI: 10.7270/Q2B56P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50080467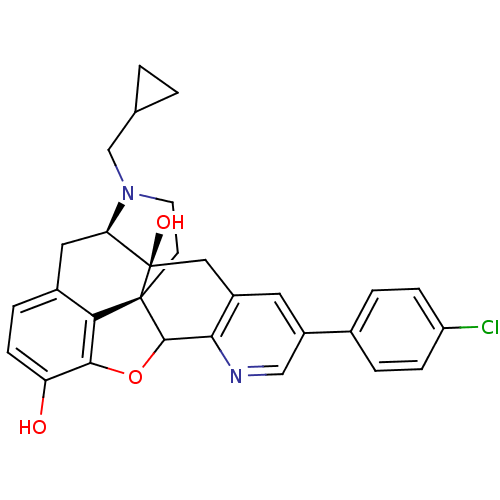 (5'-(Chlorophenyl)-17-(cyclopropylmethyl) -6,7-dide...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Apparent antagonist activity determined by measuring the ability to inhibit stimulation of [35S]GTP-gamma-S, binding to opioid receptor delta 1 in gu... | J Med Chem 47: 1400-12 (2004) Article DOI: 10.1021/jm030311v BindingDB Entry DOI: 10.7270/Q2639QGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50057761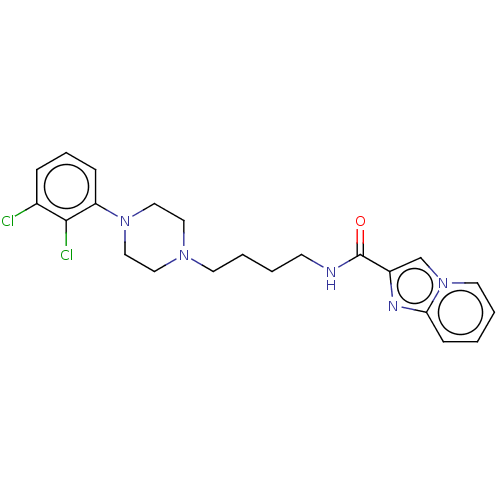 (CHEMBL3322994) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]IABN from human D3R expressed in HEK293 cell membranes by gamma counting method | J Med Chem 57: 7042-60 (2014) Article DOI: 10.1021/jm500801r BindingDB Entry DOI: 10.7270/Q27D2WSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50123279 (19-cyclopropylmethyl-6-(1H-1-pyrrolyl)-11-oxa-8,19...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Antagonist activity on agonist stimulated [35S]GTP-gamma-S, binding in guinea pig caudate membranes (10 uM SNC-80 as the agonist ligand for delta-rec... | Bioorg Med Chem Lett 13: 529-32 (2003) BindingDB Entry DOI: 10.7270/Q27S7P9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity to delta opioid receptor was measured using [3H]DADLE | Bioorg Med Chem Lett 13: 529-32 (2003) BindingDB Entry DOI: 10.7270/Q27S7P9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398759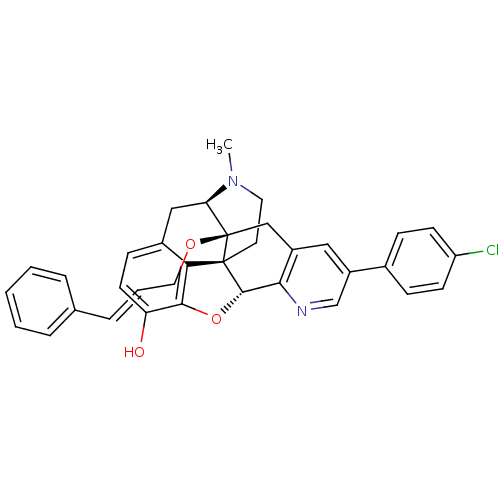 (CHEMBL2179655) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor delta 1 was determined by inhibition of binding of [3H]DADLE (1.3-2.0 nM) to rat brain membranes | J Med Chem 42: 3527-38 (1999) Article DOI: 10.1021/jm990039i BindingDB Entry DOI: 10.7270/Q2QR4XSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50545677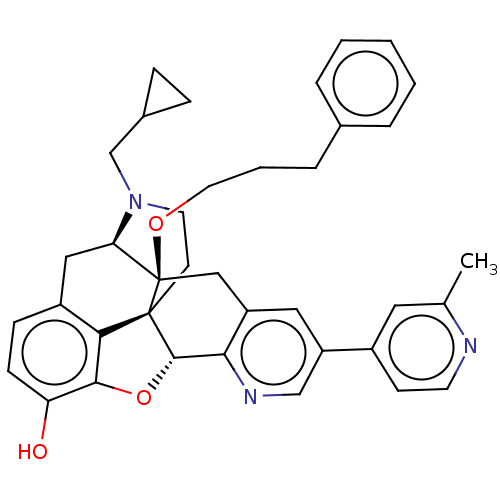 (CHEMBL4645508) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by competition radioligand bin... | J Med Chem 63: 7663-7694 (2020) Article DOI: 10.1021/acs.jmedchem.0c00503 BindingDB Entry DOI: 10.7270/Q2B56P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398758 (CHEMBL2179656) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123279 (19-cyclopropylmethyl-6-(1H-1-pyrrolyl)-11-oxa-8,19...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity to delta opioid receptor was measured using [3H]DADLE | Bioorg Med Chem Lett 13: 529-32 (2003) BindingDB Entry DOI: 10.7270/Q27S7P9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398760 (CHEMBL2179652) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123274 (6-amino(imino)methylamino-19-cyclopropylmethyl-11-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity for kappa opioid receptor was measured using [3H]U-69593 | Bioorg Med Chem Lett 13: 529-32 (2003) BindingDB Entry DOI: 10.7270/Q27S7P9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123273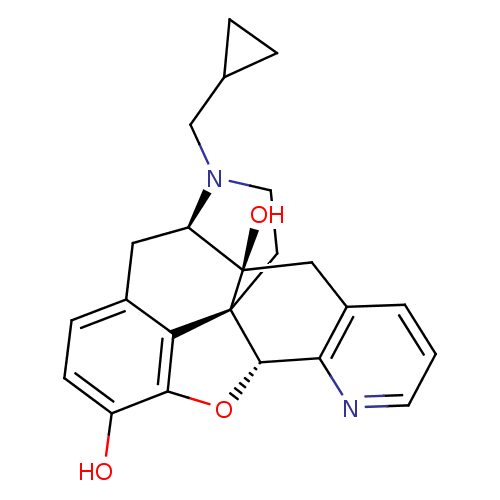 (19-cyclopropylmethyl-(2S,10R)-11-oxa-8,19-diazahex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity to delta opioid receptor was measured using [3H]DADLE | Bioorg Med Chem Lett 13: 529-32 (2003) BindingDB Entry DOI: 10.7270/Q27S7P9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123273 (19-cyclopropylmethyl-(2S,10R)-11-oxa-8,19-diazahex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor delta 1 was determined by inhibition of binding of [3H]DADLE (1.3-2.0 nM) to rat brain membranes | J Med Chem 42: 3527-38 (1999) Article DOI: 10.1021/jm990039i BindingDB Entry DOI: 10.7270/Q2QR4XSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123273 (19-cyclopropylmethyl-(2S,10R)-11-oxa-8,19-diazahex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity at opioid receptor delta 1 by displacement of [3H]DADLE in rat brain membrane | J Med Chem 47: 1400-12 (2004) Article DOI: 10.1021/jm030311v BindingDB Entry DOI: 10.7270/Q2639QGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001196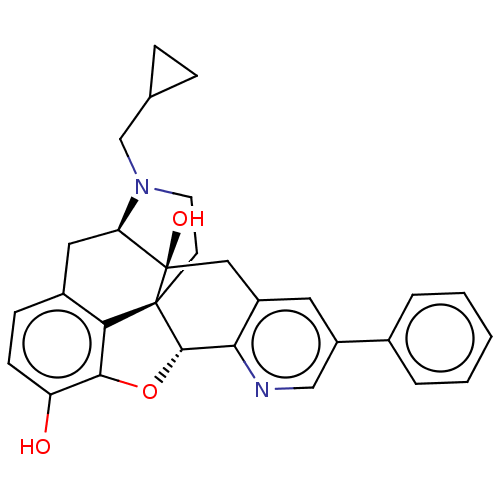 (CHEMBL610522) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor delta 1 was determined by inhibition of binding of [3H]DADLE (1.3-2.0 nM) to rat brain membranes | J Med Chem 42: 3527-38 (1999) Article DOI: 10.1021/jm990039i BindingDB Entry DOI: 10.7270/Q2QR4XSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50080459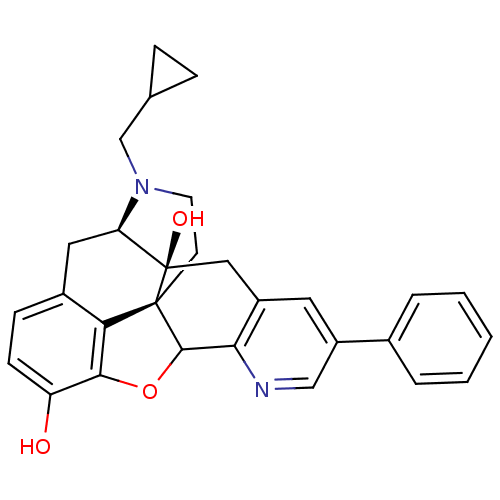 (17-(Cyclopropyl-methyl) -6, 7-didehydro-3,14-dihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity at opioid receptor delta 1 by displacement of [3H]DADLE in rat brain membrane | J Med Chem 47: 1400-12 (2004) Article DOI: 10.1021/jm030311v BindingDB Entry DOI: 10.7270/Q2639QGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398750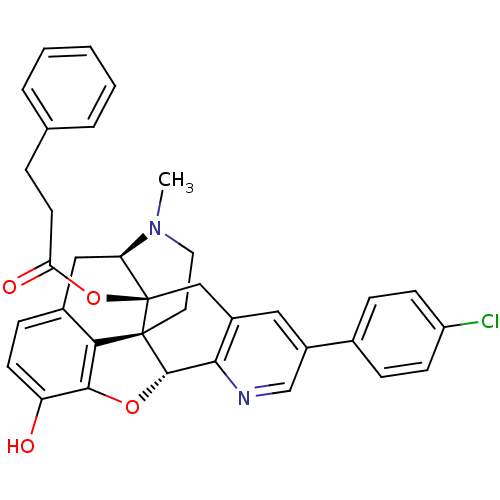 (CHEMBL2179662) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398750 (CHEMBL2179662) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398751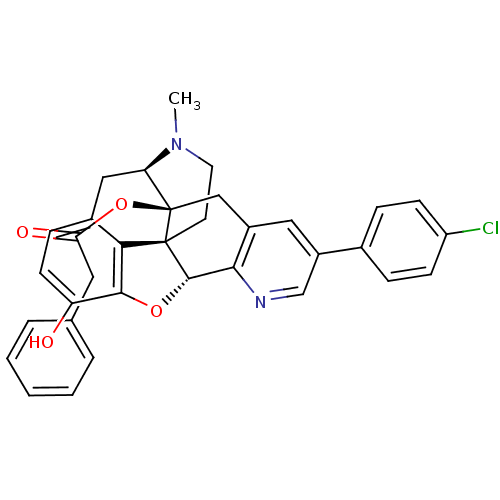 (CHEMBL2179661) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50123274 (6-amino(imino)methylamino-19-cyclopropylmethyl-11-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Antagonist activity on agonist stimulated [35S]GTP-gamma-S, binding in guinea pig caudate membranes (10 uM U69,593 as the agonist ligand for kappa-re... | Bioorg Med Chem Lett 13: 529-32 (2003) BindingDB Entry DOI: 10.7270/Q27S7P9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398753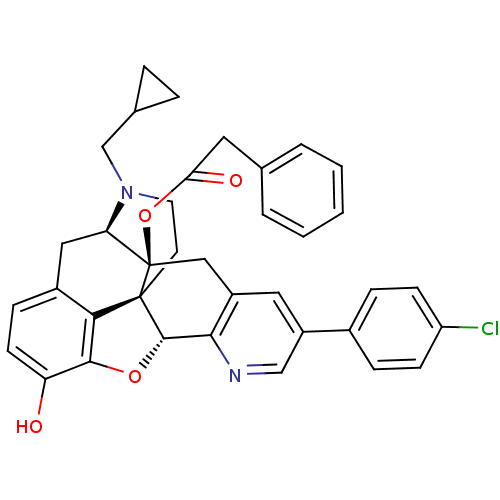 (CHEMBL2179658) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398758 (CHEMBL2179656) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398759 (CHEMBL2179655) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50545677 (CHEMBL4645508) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human mu opioid receptor expressed in CHO cell membranes incubated for 1 hr by competition radioligand bindin... | J Med Chem 63: 7663-7694 (2020) Article DOI: 10.1021/acs.jmedchem.0c00503 BindingDB Entry DOI: 10.7270/Q2B56P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50140900 (6-(2,4-dichlorophenyl)-19-methyl-(2R,10R)-11-oxa-8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity at opioid receptor delta 1 by displacement of [3H]DADLE in rat brain membrane | J Med Chem 47: 1400-12 (2004) Article DOI: 10.1021/jm030311v BindingDB Entry DOI: 10.7270/Q2639QGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50140900 (6-(2,4-dichlorophenyl)-19-methyl-(2R,10R)-11-oxa-8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Apparent antagonist activity determined by measuring the ability to inhibit stimulation of [35S]GTP-gamma-S, binding to opioid receptor delta 1 in gu... | J Med Chem 47: 1400-12 (2004) Article DOI: 10.1021/jm030311v BindingDB Entry DOI: 10.7270/Q2639QGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398760 (CHEMBL2179652) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123271 (19-cyclopropylmethyl-6-bromo-11-oxa-8,19-diazahexa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity to delta opioid receptor was measured using [3H]DADLE | Bioorg Med Chem Lett 13: 529-32 (2003) BindingDB Entry DOI: 10.7270/Q27S7P9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123275 (19-cyclopropylmethyl-6-benzylamino-11-oxa-8,19-dia...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity to delta opioid receptor was measured using [3H]DADLE | Bioorg Med Chem Lett 13: 529-32 (2003) BindingDB Entry DOI: 10.7270/Q27S7P9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398753 (CHEMBL2179658) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398751 (CHEMBL2179661) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123273 (19-cyclopropylmethyl-(2S,10R)-11-oxa-8,19-diazahex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity to mu opioid receptor was measured using [3H]DAMGO | Bioorg Med Chem Lett 13: 529-32 (2003) BindingDB Entry DOI: 10.7270/Q27S7P9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398758 (CHEMBL2179656) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123273 (19-cyclopropylmethyl-(2S,10R)-11-oxa-8,19-diazahex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor mu 1 was determined by inhibition of binding of [3H]DAMGO (1.4-3 nM) to rat brain membranes | J Med Chem 42: 3527-38 (1999) Article DOI: 10.1021/jm990039i BindingDB Entry DOI: 10.7270/Q2QR4XSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123273 (19-cyclopropylmethyl-(2S,10R)-11-oxa-8,19-diazahex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity at Opioid receptor mu 1 by displacement of [3H]DAMGO in rat brain membrane | J Med Chem 47: 1400-12 (2004) Article DOI: 10.1021/jm030311v BindingDB Entry DOI: 10.7270/Q2639QGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity at opioid receptor delta 1 by displacement of [3H]DADLE in rat brain membrane | J Med Chem 47: 1400-12 (2004) Article DOI: 10.1021/jm030311v BindingDB Entry DOI: 10.7270/Q2639QGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398759 (CHEMBL2179655) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50140913 (6-(4-chlorophenyl)-19-cyclopropylmethyl-(2R,10R)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Apparent antagonist activity determined by measuring the ability to inhibit stimulation of [35S]GTP-gamma-S, binding to opioid receptor delta 1 in gu... | J Med Chem 47: 1400-12 (2004) Article DOI: 10.1021/jm030311v BindingDB Entry DOI: 10.7270/Q2639QGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398755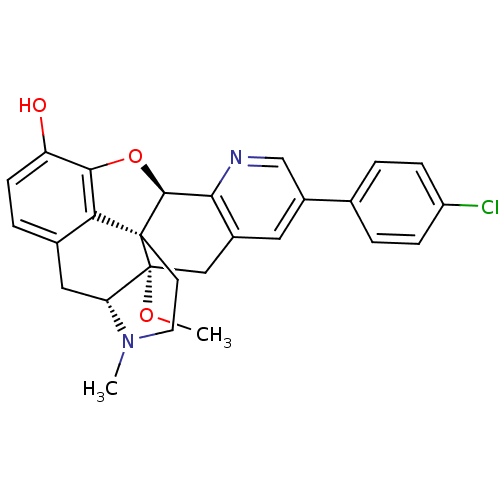 (CHEMBL2179653) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Antagonist activity on agonist stimulated [35S]GTP-gamma-S, binding in guinea pig caudate membranes (10 uM DAMGO as the agonist ligand for mu-recepto... | Bioorg Med Chem Lett 13: 529-32 (2003) BindingDB Entry DOI: 10.7270/Q27S7P9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50398760 (CHEMBL2179652) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123277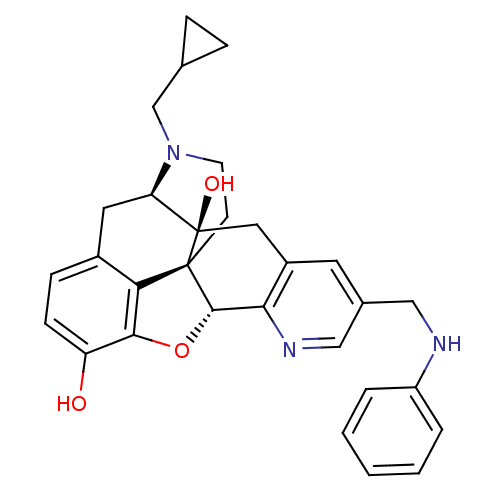 (19-cyclopropylmethyl-6-phenyleaminomethyl-11-oxa-8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity to delta opioid receptor was measured using [3H]DADLE | Bioorg Med Chem Lett 13: 529-32 (2003) BindingDB Entry DOI: 10.7270/Q27S7P9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001190 (CHEMBL2112576) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor delta 1 was determined by inhibition of binding of [3H]DADLE (1.3-2.0 nM) to rat brain membranes | J Med Chem 42: 3527-38 (1999) Article DOI: 10.1021/jm990039i BindingDB Entry DOI: 10.7270/Q2QR4XSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398754 (CHEMBL2179654) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398756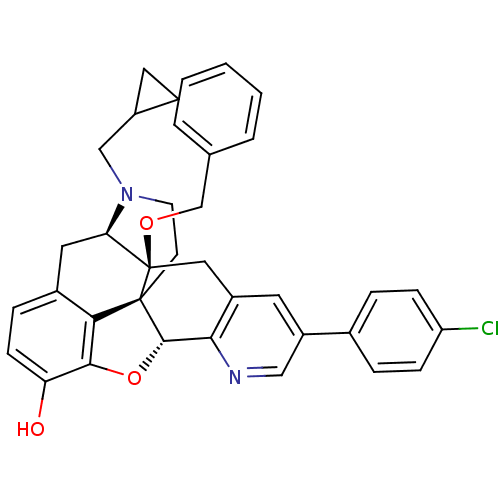 (CHEMBL2179650) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in CHO cells by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50140905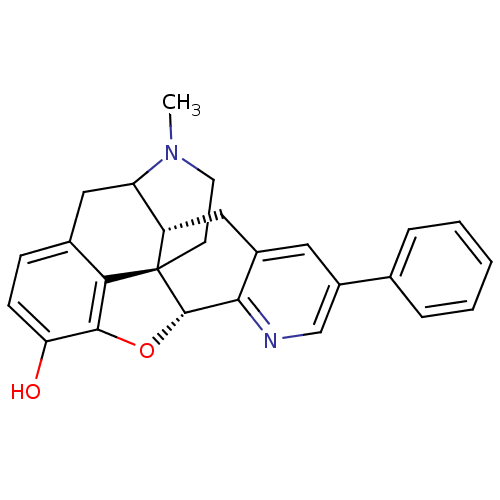 (19-methyl-6-phenyl-(2R,10R)-11-oxa-8,19-diazahexac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity at opioid receptor delta 1 by displacement of [3H]DADLE in rat brain membrane | J Med Chem 47: 1400-12 (2004) Article DOI: 10.1021/jm030311v BindingDB Entry DOI: 10.7270/Q2639QGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 895 total ) | Next | Last >> |