

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50100263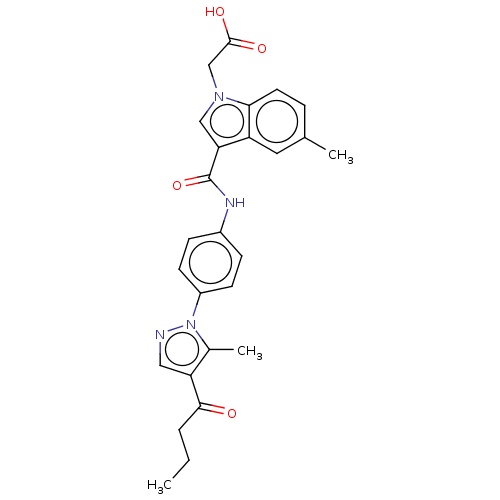 (CHEMBL3326907) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Displacement of [33P]2MeS-ADP from P2Y12 receptor (unknown origin) transfected in CHO cells after 30 mins by scintillation counting analysis | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50100269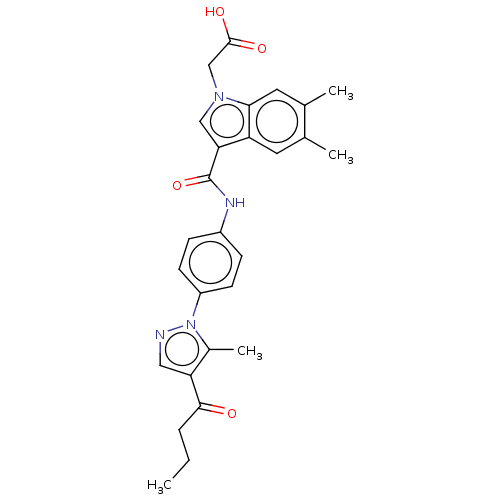 (CHEMBL3325627) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Displacement of [33P]2MeS-ADP from P2Y12 receptor (unknown origin) transfected in CHO cells after 30 mins by scintillation counting analysis | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153099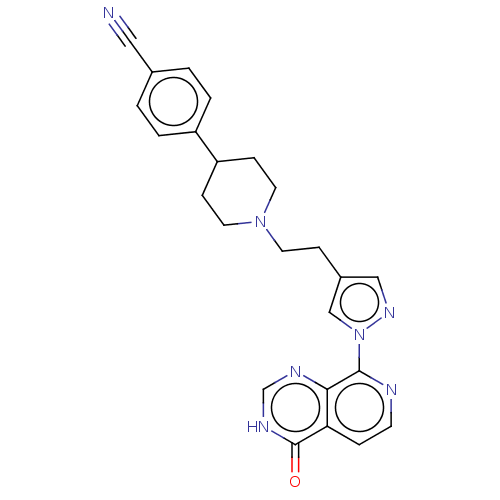 (CHEMBL3775814) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50100195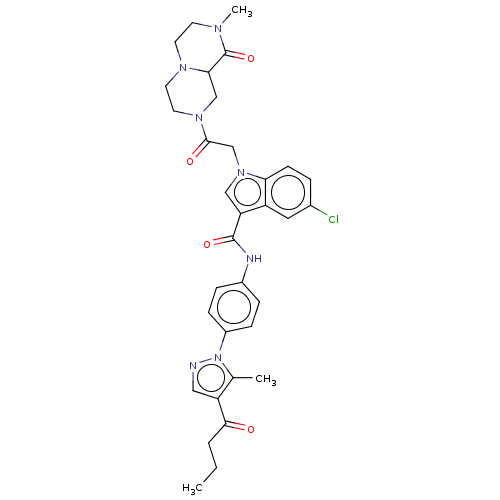 (CHEMBL3325805) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor in Sprague-Dawley rat platelet rich plasma assessed as inhibition of ADP-induced aggregation | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50100262 (CHEMBL3326906) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Displacement of [33P]2MeS-ADP from P2Y12 receptor (unknown origin) transfected in CHO cells after 30 mins by scintillation counting analysis | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50100195 (CHEMBL3325805) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor in Sprague-Dawley rat platelet rich plasma assessed as inhibition of ADP-induced aggregation | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50100245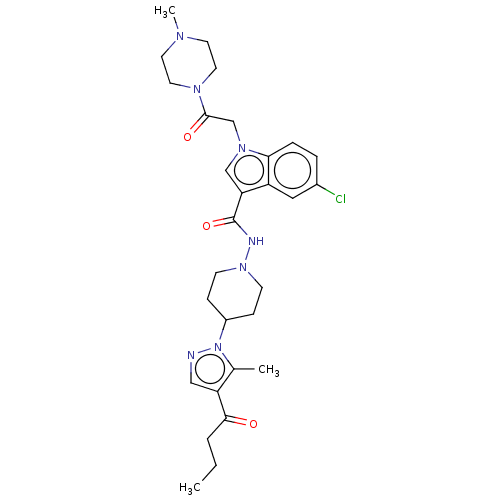 (CHEMBL3325894) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Displacement of [33P]2MeS-ADP from P2Y12 receptor (unknown origin) transfected in CHO cells after 30 mins by scintillation counting analysis | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peregrin (Homo sapiens (Human)) | BDBM50249810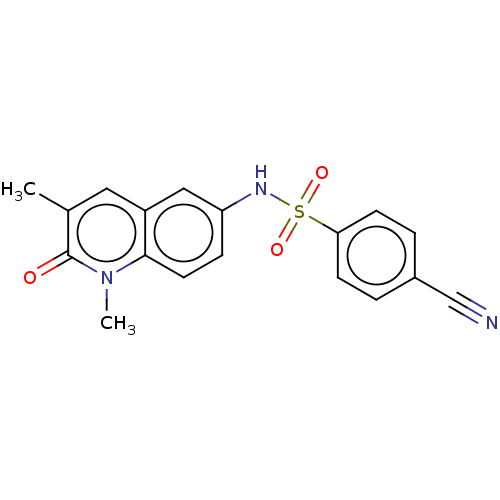 (CHEMBL4102050) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of human BRPF1 expressed in Escherichia coli BL21 after 1 hr by BROMOscan assay | J Med Chem 60: 668-680 (2017) Article DOI: 10.1021/acs.jmedchem.6b01583 BindingDB Entry DOI: 10.7270/Q29K4DQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50100195 (CHEMBL3325805) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor in Sprague-Dawley rat platelet rich plasma assessed as inhibition of ADP-induced aggregation | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peregrin (Homo sapiens (Human)) | BDBM50249785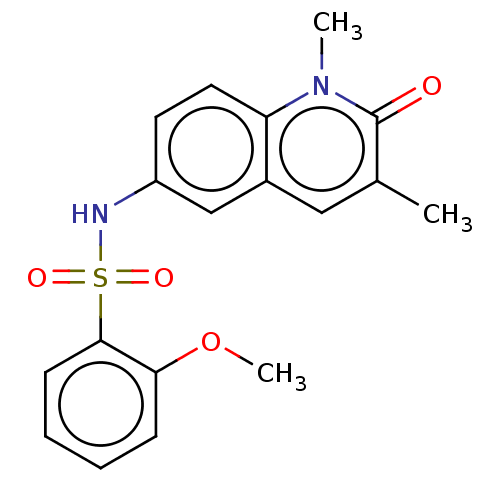 (CHEMBL4069814) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of human BRPF1 expressed in Escherichia coli BL21 after 1 hr by BROMOscan assay | J Med Chem 60: 668-680 (2017) Article DOI: 10.1021/acs.jmedchem.6b01583 BindingDB Entry DOI: 10.7270/Q29K4DQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153101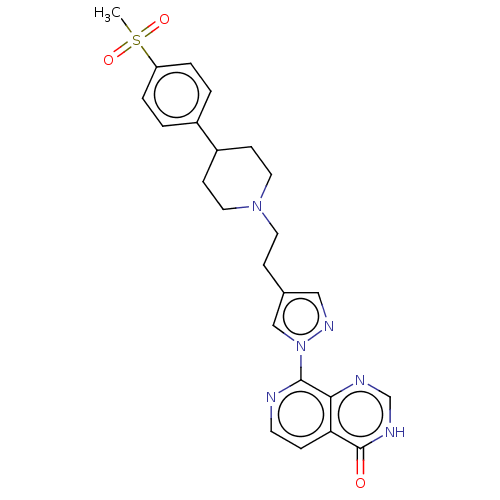 (CHEMBL3774665) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153161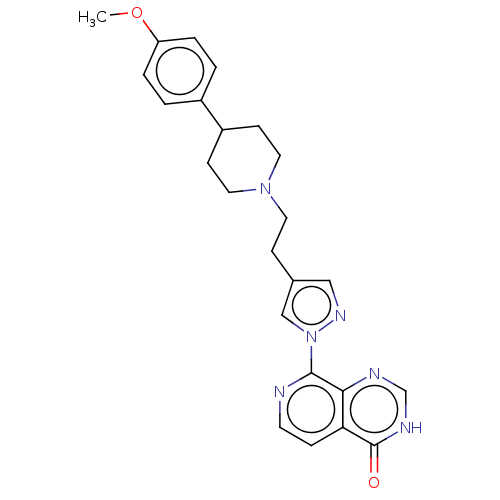 (CHEMBL3775545) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50151919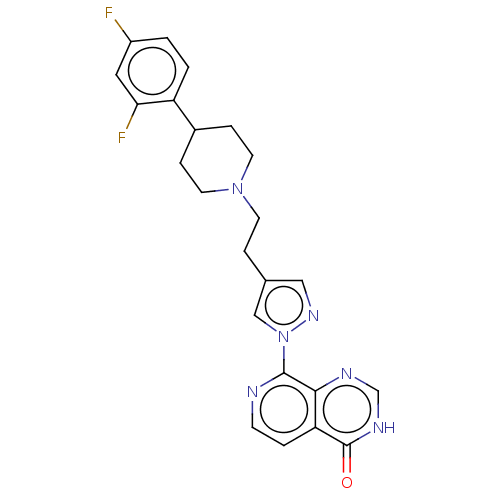 (CHEMBL3775121) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM6B (unknown origin) using biotin-H3K27me3 (21 to 44 residues) as substrate preincubated for 15 mins followed by substrate addition m... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50100194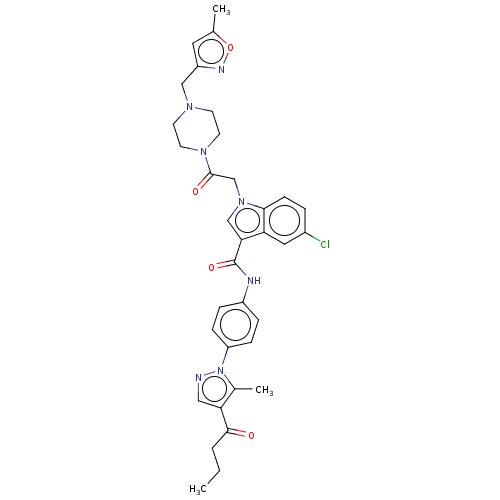 (CHEMBL3325804) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor in Sprague-Dawley rat platelet rich plasma assessed as inhibition of ADP-induced aggregation | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50100197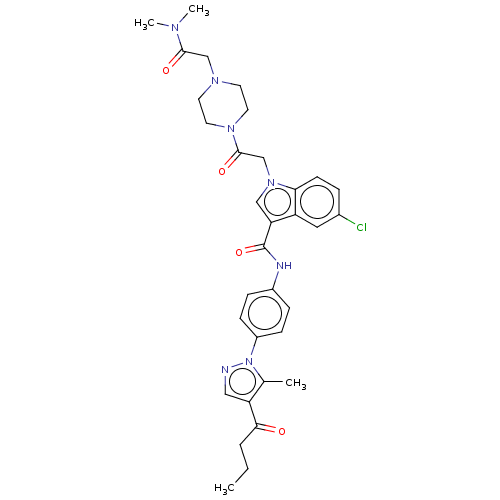 (CHEMBL3325803) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor in Sprague-Dawley rat platelet rich plasma assessed as inhibition of ADP-induced aggregation | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50100196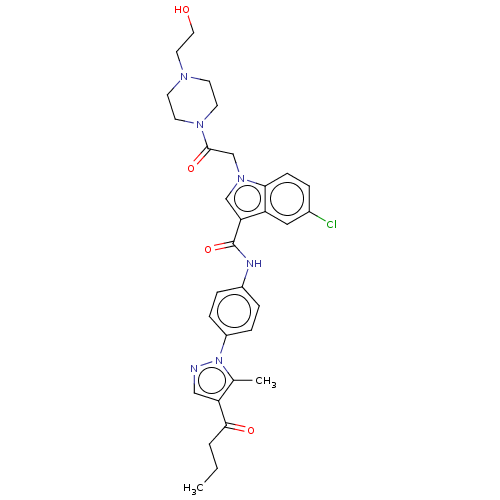 (CHEMBL3325802) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor in Sprague-Dawley rat platelet rich plasma assessed as inhibition of ADP-induced aggregation | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153181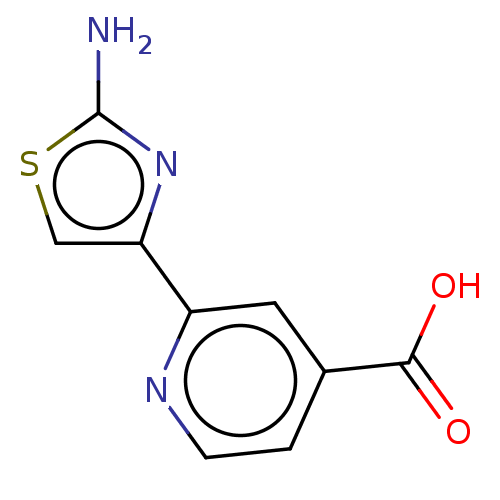 (CHEMBL3774692) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50055846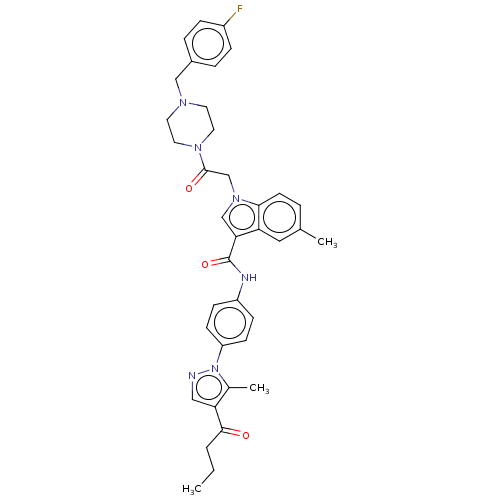 (CHEMBL3325788) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Displacement of [33P]2MeS-ADP from P2Y12 receptor (unknown origin) transfected in CHO cells after 30 mins by scintillation counting analysis | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5C (Homo sapiens (Human)) | BDBM50153181 (CHEMBL3774692) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5C (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50152029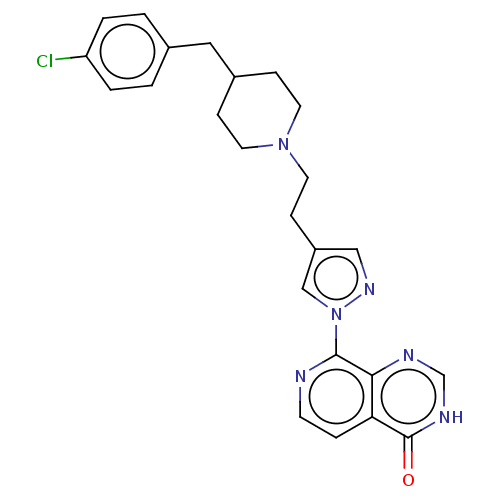 (CHEMBL3775899) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153092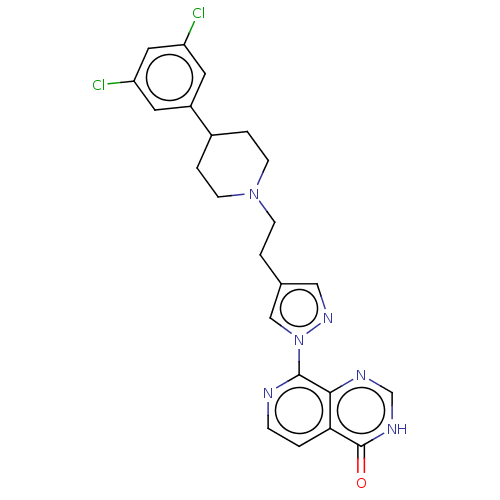 (CHEMBL3775894) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153094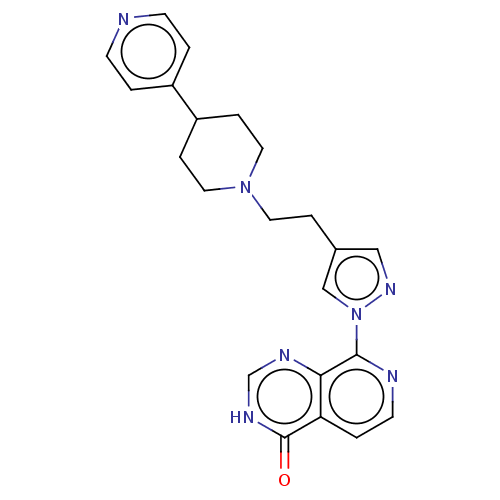 (CHEMBL3775277) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peregrin (Homo sapiens (Human)) | BDBM50267002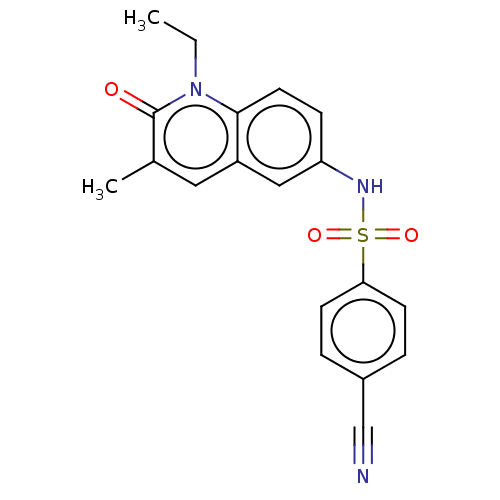 (CHEMBL4097011) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of human BRPF1 expressed in Escherichia coli BL21 after 1 hr by BROMOscan assay | J Med Chem 60: 668-680 (2017) Article DOI: 10.1021/acs.jmedchem.6b01583 BindingDB Entry DOI: 10.7270/Q29K4DQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50100193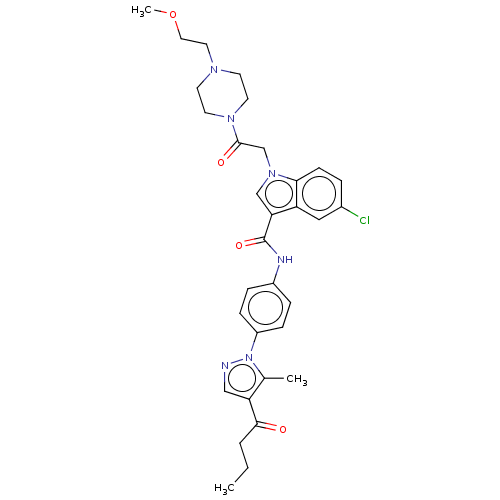 (CHEMBL3325801) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor in Sprague-Dawley rat platelet rich plasma assessed as inhibition of ADP-induced aggregation | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153076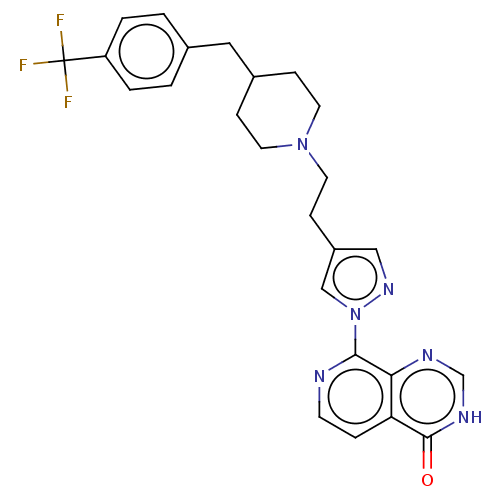 (CHEMBL3775516) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153075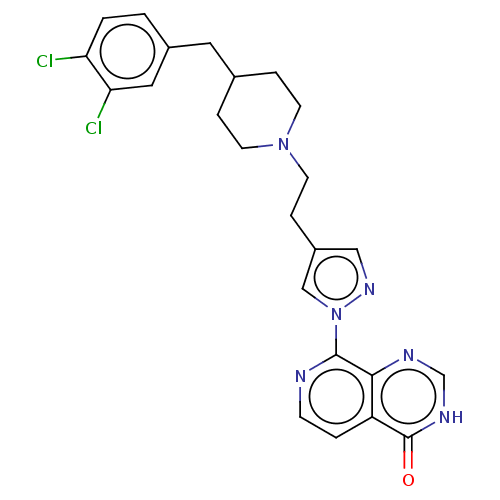 (CHEMBL3774940) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50055843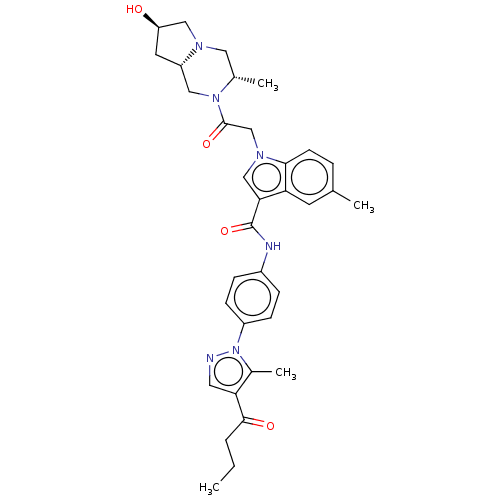 (CHEMBL3325785) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Displacement of [33P]2MeS-ADP from P2Y12 receptor (unknown origin) transfected in CHO cells after 30 mins by scintillation counting analysis | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153073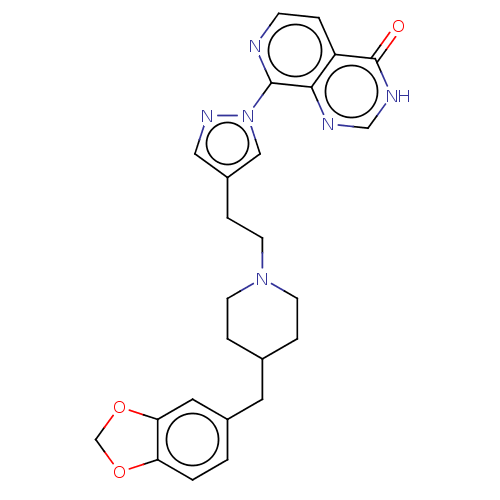 (CHEMBL3775451) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153077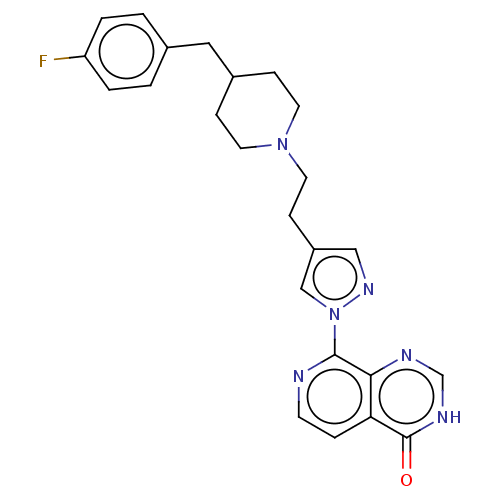 (CHEMBL3775040) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50100314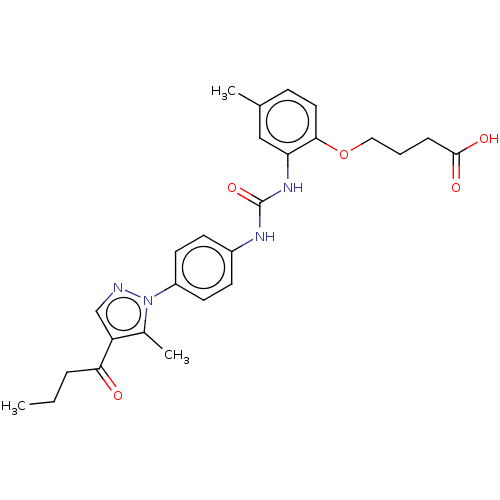 (CHEMBL3326903) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Displacement of [33P]2MeS-ADP from P2Y12 receptor (unknown origin) transfected in CHO cells after 30 mins by scintillation counting analysis | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50100247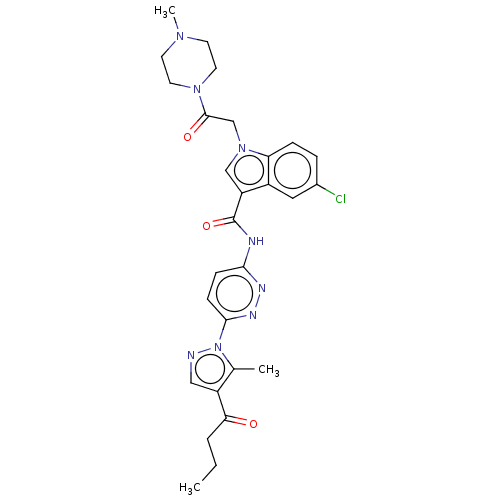 (CHEMBL3325891) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Displacement of [33P]2MeS-ADP from P2Y12 receptor (unknown origin) transfected in CHO cells after 30 mins by scintillation counting analysis | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4B (Homo sapiens (Human)) | BDBM50153092 (CHEMBL3775894) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged KDM4B (1 to 500 residues) expressed in baculovirus infected sf9 cells using biotin-H3K9me3 as s... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153069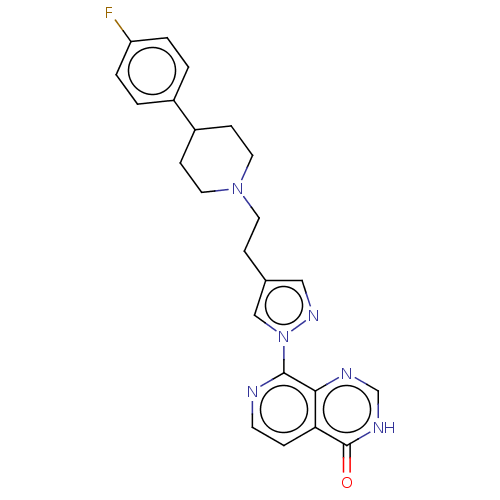 (CHEMBL3775953) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50151917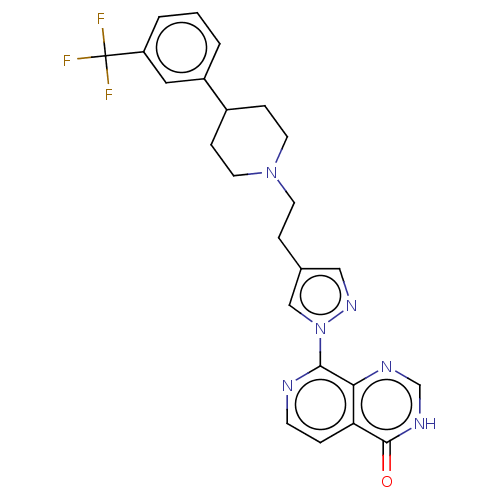 (CHEMBL3775548) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM6B (unknown origin) using biotin-H3K27me3 (21 to 44 residues) as substrate preincubated for 15 mins followed by substrate addition m... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50055840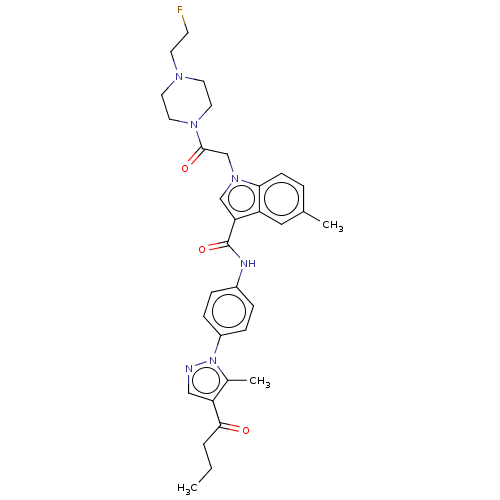 (CHEMBL3325782) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Displacement of [33P]2MeS-ADP from P2Y12 receptor (unknown origin) transfected in CHO cells after 30 mins by scintillation counting analysis | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM223320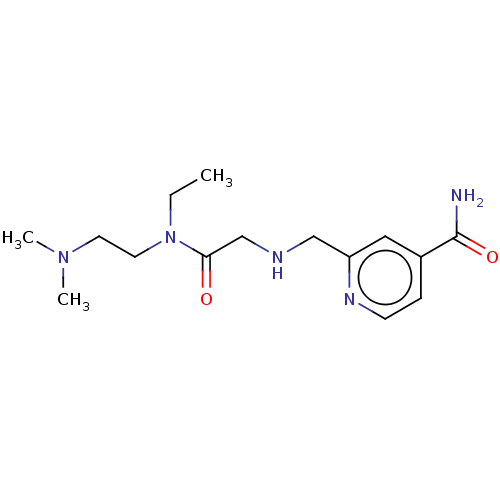 (KDOAM-25) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50055841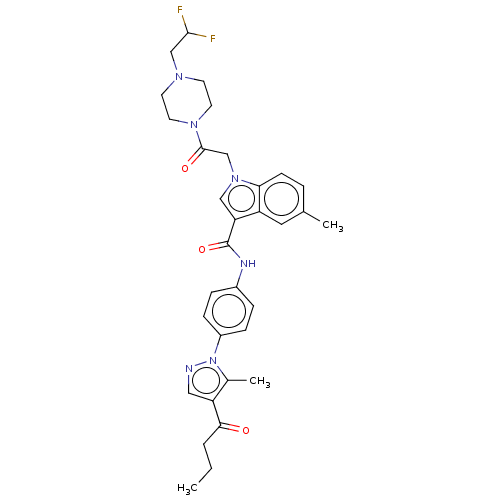 (CHEMBL3325783) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Displacement of [33P]2MeS-ADP from P2Y12 receptor (unknown origin) transfected in CHO cells after 30 mins by scintillation counting analysis | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50055816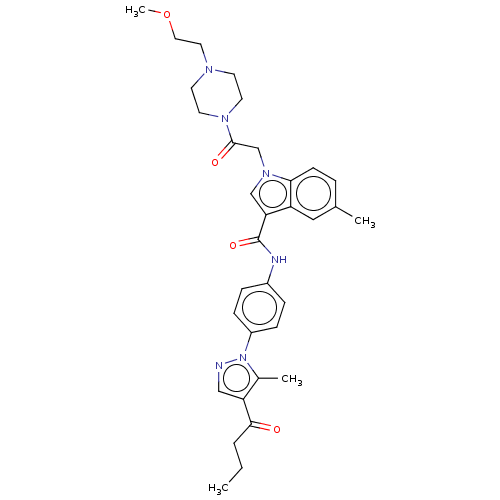 (CHEMBL3325660) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Displacement of [33P]2MeS-ADP from P2Y12 receptor (unknown origin) transfected in CHO cells after 30 mins by scintillation counting analysis | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM223320 (KDOAM-25) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 12 (Rattus norvegicus) | BDBM50100245 (CHEMBL3325894) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor in Sprague-Dawley rat platelet rich plasma assessed as inhibition of ADP-induced aggregation | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5D (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5C (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford | Assay Description We previously described 4-carboxy-2-triazolopyridines as selective KDM2 inhibitors (England et al., 2014). In efforts to find alternative scaffolds b... | Cell Chem Biol 24: 371-380 (2017) Article DOI: 10.1016/j.chembiol.2017.02.006 BindingDB Entry DOI: 10.7270/Q23N2288 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5C (Homo sapiens (Human)) | BDBM50153077 (CHEMBL3775040) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5C (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153078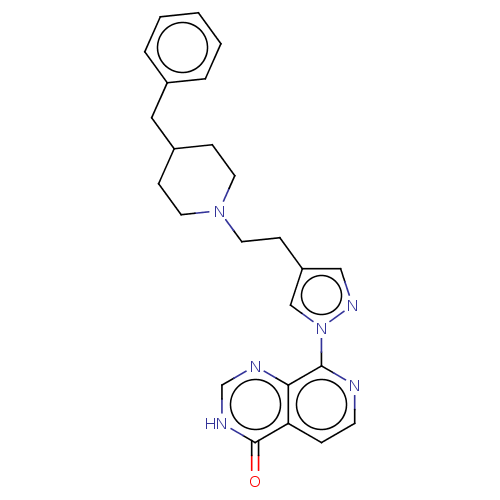 (CHEMBL3774392) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5C (Homo sapiens (Human)) | BDBM50153073 (CHEMBL3775451) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5C (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50055847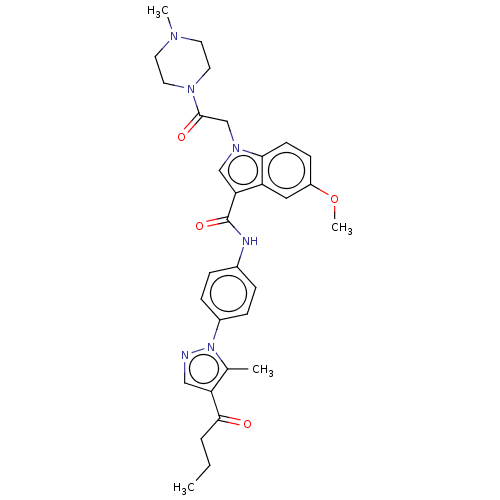 (CHEMBL3325789) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Displacement of [33P]2MeS-ADP from P2Y12 receptor (unknown origin) transfected in CHO cells after 30 mins by scintillation counting analysis | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5C (Homo sapiens (Human)) | BDBM50153076 (CHEMBL3775516) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5C (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 757 total ) | Next | Last >> |