

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (MOUSE) | BDBM50007002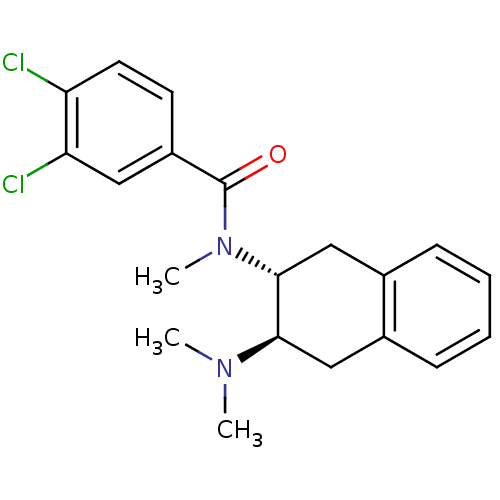 (3,4-Dichloro-N-(3-dimethylamino-1,2,3,4-tetrahydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50229403 (CHEMBL2311130) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Compound was evaluated for time-dependent inactivation of Ribonucleotide diphosphate reductase (RDPR) in E. coli | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50006996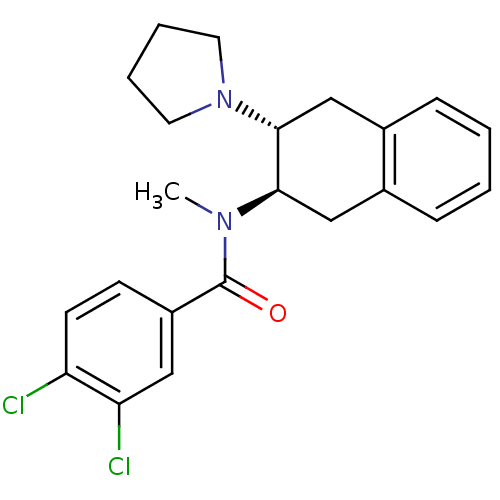 (3,4-Dichloro-N-methyl-N-(3-pyrrolidin-1-yl-1,2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 374 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50007004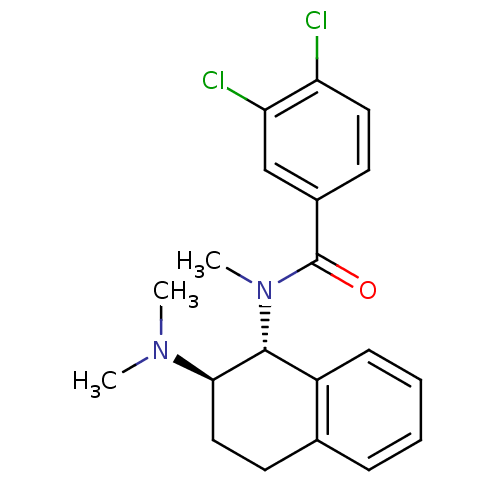 (3,4-Dichloro-N-(2-dimethylamino-1,2,3,4-tetrahydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 505 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50006998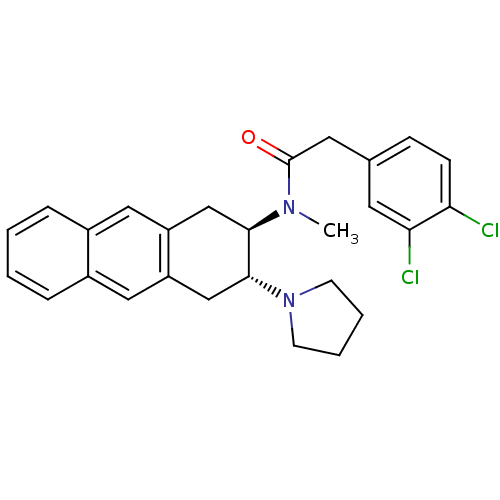 (2-(3,4-Dichloro-phenyl)-N-methyl-N-(3-pyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50006994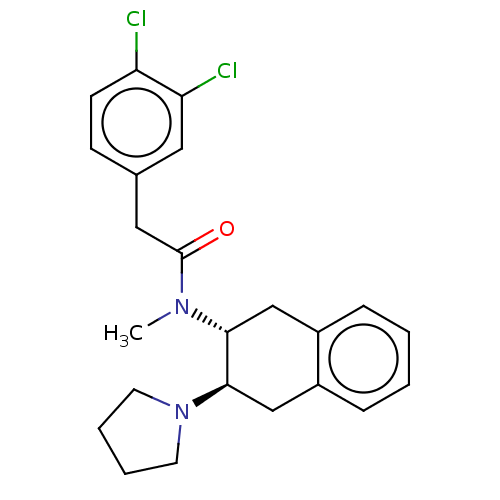 (2-(3,4-Dichloro-phenyl)-N-methyl-N-(3-pyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50006999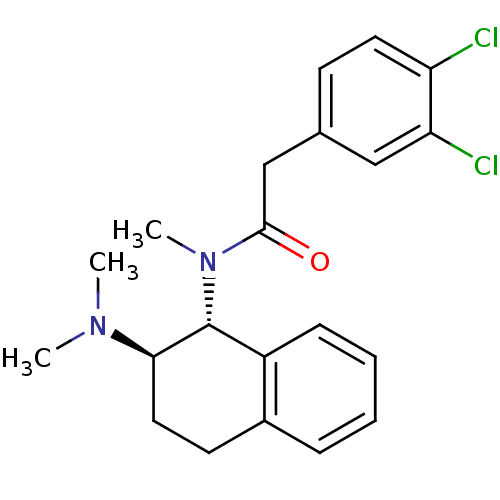 (2-(3,4-Dichloro-phenyl)-N-(2-dimethylamino-1,2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50007000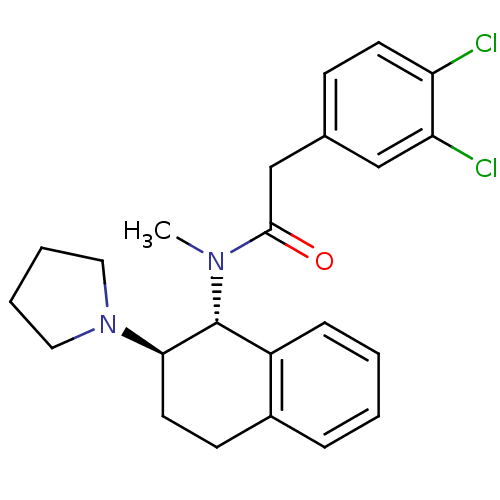 (2-(3,4-Dichloro-phenyl)-N-methyl-N-(2-pyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor mu 1 using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50006997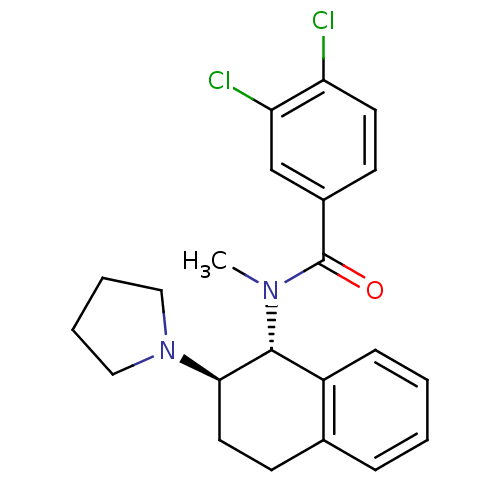 (3,4-Dichloro-N-methyl-N-(2-pyrrolidin-1-yl-1,2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50007001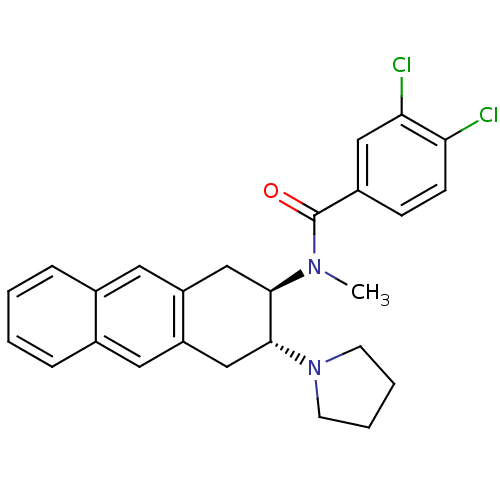 (3,4-Dichloro-N-methyl-N-(3-pyrrolidin-1-yl-1,2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor mu using [3H]-etorphine as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50006994 (2-(3,4-Dichloro-phenyl)-N-methyl-N-(3-pyrrolidin-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa using [3H]U-69,593 as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor kappa 1 using [3H]U-69,593 as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50007000 (2-(3,4-Dichloro-phenyl)-N-methyl-N-(2-pyrrolidin-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa using [3H]U-69,593 as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50006999 (2-(3,4-Dichloro-phenyl)-N-(2-dimethylamino-1,2,3,4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa using [3H]U-69,593 as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50367718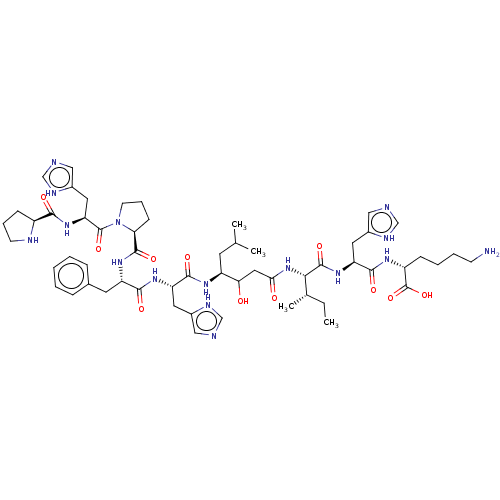 (CHEMBL3037868) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against renin from lyophilized human plasma | J Med Chem 31: 1377-82 (1988) BindingDB Entry DOI: 10.7270/Q2T43TN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50006996 (3,4-Dichloro-N-methyl-N-(3-pyrrolidin-1-yl-1,2,3,4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa using [3H]U-69,593 as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50367714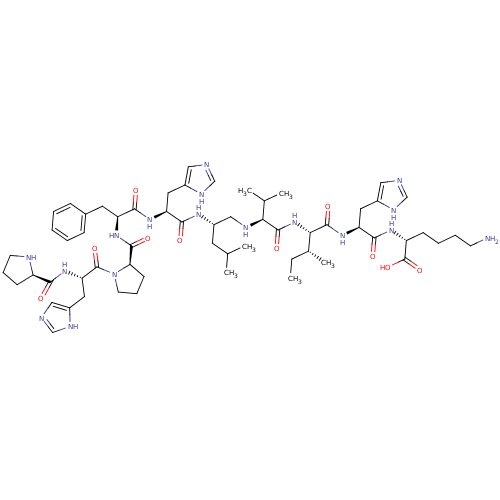 (CHEMBL1790111) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against renin from lyophilized human plasma | J Med Chem 31: 1377-82 (1988) BindingDB Entry DOI: 10.7270/Q2T43TN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50007002 (3,4-Dichloro-N-(3-dimethylamino-1,2,3,4-tetrahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa using [3H]U-69,593 as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50007001 (3,4-Dichloro-N-methyl-N-(3-pyrrolidin-1-yl-1,2,3,4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa using [3H]U-69,593 as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50006998 (2-(3,4-Dichloro-phenyl)-N-methyl-N-(3-pyrrolidin-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa using [3H]U-69,593 as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50006997 (3,4-Dichloro-N-methyl-N-(2-pyrrolidin-1-yl-1,2,3,4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 368 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa using [3H]U-69,593 as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50007004 (3,4-Dichloro-N-(2-dimethylamino-1,2,3,4-tetrahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa using [3H]U-69,593 as a radioligand | J Med Chem 34: 1891-6 (1991) BindingDB Entry DOI: 10.7270/Q2WW7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50367716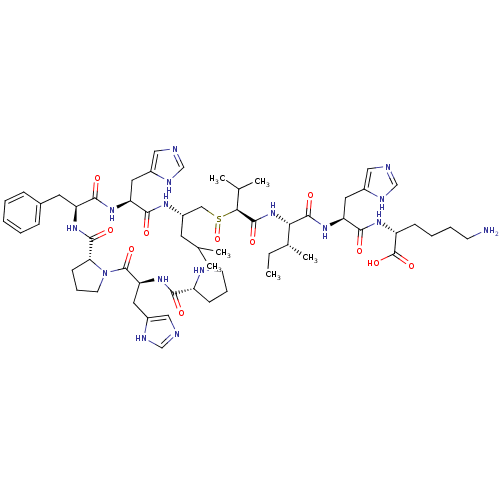 (CHEMBL1790120) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against renin from lyophilized human plasma | J Med Chem 31: 1377-82 (1988) BindingDB Entry DOI: 10.7270/Q2T43TN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50367717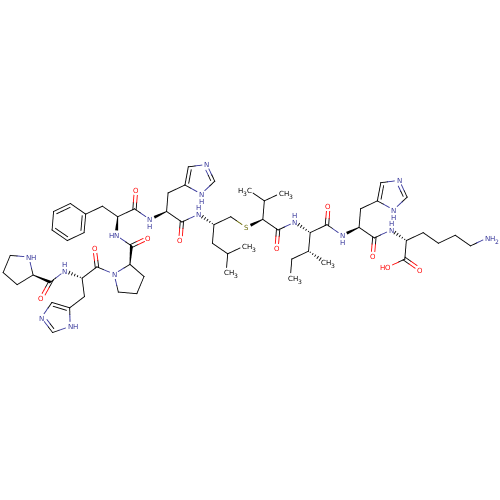 (CHEMBL1790115) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against renin from lyophilized human plasma | J Med Chem 31: 1377-82 (1988) BindingDB Entry DOI: 10.7270/Q2T43TN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50367713 (CHEMBL1790122) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against renin from lyophilized human plasma | J Med Chem 31: 1377-82 (1988) BindingDB Entry DOI: 10.7270/Q2T43TN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022938 (CHEMBL438719 | H-Pro-His-Pro-Phe-His-Phe-Phe-Val-T...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against renin from lyophilized human plasma | J Med Chem 31: 1377-82 (1988) BindingDB Entry DOI: 10.7270/Q2T43TN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50367715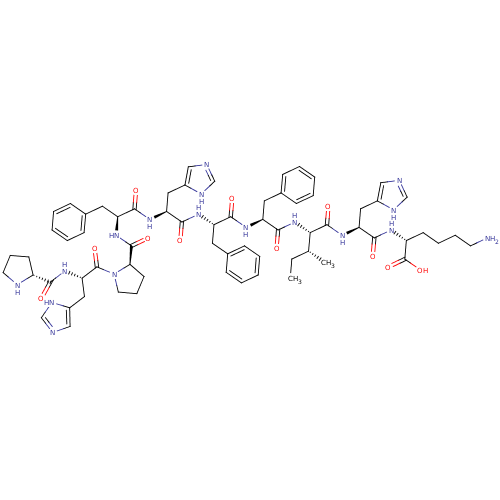 (CHEMBL1790121) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against renin from lyophilized human plasma | J Med Chem 31: 1377-82 (1988) BindingDB Entry DOI: 10.7270/Q2T43TN1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||