 Found 161 hits with Last Name = 'scheerens' and Initial = 'h'
Found 161 hits with Last Name = 'scheerens' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM111939

(US8618107, 105)Show SMILES Cn1cc(cc(Nc2ccncn2)c1=O)-c1cccc(N2CCc3c4CC(C)(C)Cc4sc3C2=O)c1CO Show InChI InChI=1S/C29H29N5O3S/c1-29(2)12-20-19-8-10-34(28(37)26(19)38-24(20)13-29)23-6-4-5-18(21(23)15-35)17-11-22(27(36)33(3)14-17)32-25-7-9-30-16-31-25/h4-7,9,11,14,16,35H,8,10,12-13,15H2,1-3H3,(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length His-tagged BTK expressed in baculovirus expression system by Z-LYTE assay |
ACS Med Chem Lett 8: 608-613 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00103
BindingDB Entry DOI: 10.7270/Q24M96ZH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187800

((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccccc3)cc2)n1 Show InChI InChI=1S/C26H32N4O5/c1-2-33-26(32)28-22(10-6-7-16-27)24(31)25-29-23(35-30-25)18-20-11-13-21(14-12-20)34-17-15-19-8-4-3-5-9-19/h3-5,8-9,11-14,22H,2,6-7,10,15-18,27H2,1H3,(H,28,32)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to human tryptase beta2 |
Bioorg Med Chem Lett 16: 4085-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.088
BindingDB Entry DOI: 10.7270/Q2X929W7 |
More data for this
Ligand-Target Pair | |
Tryptase
(Rattus norvegicus) | BDBM50187800

((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccccc3)cc2)n1 Show InChI InChI=1S/C26H32N4O5/c1-2-33-26(32)28-22(10-6-7-16-27)24(31)25-29-23(35-30-25)18-20-11-13-21(14-12-20)34-17-15-19-8-4-3-5-9-19/h3-5,8-9,11-14,22H,2,6-7,10,15-18,27H2,1H3,(H,28,32)/t22-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to rat tryptase |
Bioorg Med Chem Lett 16: 4085-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.088
BindingDB Entry DOI: 10.7270/Q2X929W7 |
More data for this
Ligand-Target Pair | |
Tryptase
(Mus musculus) | BDBM50187800

((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccccc3)cc2)n1 Show InChI InChI=1S/C26H32N4O5/c1-2-33-26(32)28-22(10-6-7-16-27)24(31)25-29-23(35-30-25)18-20-11-13-21(14-12-20)34-17-15-19-8-4-3-5-9-19/h3-5,8-9,11-14,22H,2,6-7,10,15-18,27H2,1H3,(H,28,32)/t22-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to mouse tryptase |
Bioorg Med Chem Lett 16: 4085-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.088
BindingDB Entry DOI: 10.7270/Q2X929W7 |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187800

((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccccc3)cc2)n1 Show InChI InChI=1S/C26H32N4O5/c1-2-33-26(32)28-22(10-6-7-16-27)24(31)25-29-23(35-30-25)18-20-11-13-21(14-12-20)34-17-15-19-8-4-3-5-9-19/h3-5,8-9,11-14,22H,2,6-7,10,15-18,27H2,1H3,(H,28,32)/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant cynomolgus monkey tryptase |
Bioorg Med Chem Lett 16: 4085-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.088
BindingDB Entry DOI: 10.7270/Q2X929W7 |
More data for this
Ligand-Target Pair | |
Tryptase
(Canis familiaris) | BDBM50187800

((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccccc3)cc2)n1 Show InChI InChI=1S/C26H32N4O5/c1-2-33-26(32)28-22(10-6-7-16-27)24(31)25-29-23(35-30-25)18-20-11-13-21(14-12-20)34-17-15-19-8-4-3-5-9-19/h3-5,8-9,11-14,22H,2,6-7,10,15-18,27H2,1H3,(H,28,32)/t22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to dog tryptase |
Bioorg Med Chem Lett 16: 4085-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.088
BindingDB Entry DOI: 10.7270/Q2X929W7 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50187800

((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccccc3)cc2)n1 Show InChI InChI=1S/C26H32N4O5/c1-2-33-26(32)28-22(10-6-7-16-27)24(31)25-29-23(35-30-25)18-20-11-13-21(14-12-20)34-17-15-19-8-4-3-5-9-19/h3-5,8-9,11-14,22H,2,6-7,10,15-18,27H2,1H3,(H,28,32)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to plasmin |
Bioorg Med Chem Lett 16: 4085-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.088
BindingDB Entry DOI: 10.7270/Q2X929W7 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50187800

((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccccc3)cc2)n1 Show InChI InChI=1S/C26H32N4O5/c1-2-33-26(32)28-22(10-6-7-16-27)24(31)25-29-23(35-30-25)18-20-11-13-21(14-12-20)34-17-15-19-8-4-3-5-9-19/h3-5,8-9,11-14,22H,2,6-7,10,15-18,27H2,1H3,(H,28,32)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to kallikrein |
Bioorg Med Chem Lett 16: 4085-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.088
BindingDB Entry DOI: 10.7270/Q2X929W7 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50187800

((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccccc3)cc2)n1 Show InChI InChI=1S/C26H32N4O5/c1-2-33-26(32)28-22(10-6-7-16-27)24(31)25-29-23(35-30-25)18-20-11-13-21(14-12-20)34-17-15-19-8-4-3-5-9-19/h3-5,8-9,11-14,22H,2,6-7,10,15-18,27H2,1H3,(H,28,32)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to thrombin |
Bioorg Med Chem Lett 16: 4085-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.088
BindingDB Entry DOI: 10.7270/Q2X929W7 |
More data for this
Ligand-Target Pair | |
Granzyme K
(Homo sapiens (Human)) | BDBM50187800

((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccccc3)cc2)n1 Show InChI InChI=1S/C26H32N4O5/c1-2-33-26(32)28-22(10-6-7-16-27)24(31)25-29-23(35-30-25)18-20-11-13-21(14-12-20)34-17-15-19-8-4-3-5-9-19/h3-5,8-9,11-14,22H,2,6-7,10,15-18,27H2,1H3,(H,28,32)/t22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to Granzyme K |
Bioorg Med Chem Lett 16: 4085-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.088
BindingDB Entry DOI: 10.7270/Q2X929W7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50187800

((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccccc3)cc2)n1 Show InChI InChI=1S/C26H32N4O5/c1-2-33-26(32)28-22(10-6-7-16-27)24(31)25-29-23(35-30-25)18-20-11-13-21(14-12-20)34-17-15-19-8-4-3-5-9-19/h3-5,8-9,11-14,22H,2,6-7,10,15-18,27H2,1H3,(H,28,32)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to urokinase |
Bioorg Med Chem Lett 16: 4085-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.088
BindingDB Entry DOI: 10.7270/Q2X929W7 |
More data for this
Ligand-Target Pair | |
Chymase
(Homo sapiens (Human)) | BDBM50187800

((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccccc3)cc2)n1 Show InChI InChI=1S/C26H32N4O5/c1-2-33-26(32)28-22(10-6-7-16-27)24(31)25-29-23(35-30-25)18-20-11-13-21(14-12-20)34-17-15-19-8-4-3-5-9-19/h3-5,8-9,11-14,22H,2,6-7,10,15-18,27H2,1H3,(H,28,32)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to chymase |
Bioorg Med Chem Lett 16: 4085-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.088
BindingDB Entry DOI: 10.7270/Q2X929W7 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50187800

((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccccc3)cc2)n1 Show InChI InChI=1S/C26H32N4O5/c1-2-33-26(32)28-22(10-6-7-16-27)24(31)25-29-23(35-30-25)18-20-11-13-21(14-12-20)34-17-15-19-8-4-3-5-9-19/h3-5,8-9,11-14,22H,2,6-7,10,15-18,27H2,1H3,(H,28,32)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to APC |
Bioorg Med Chem Lett 16: 4085-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.088
BindingDB Entry DOI: 10.7270/Q2X929W7 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM50187800

((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccccc3)cc2)n1 Show InChI InChI=1S/C26H32N4O5/c1-2-33-26(32)28-22(10-6-7-16-27)24(31)25-29-23(35-30-25)18-20-11-13-21(14-12-20)34-17-15-19-8-4-3-5-9-19/h3-5,8-9,11-14,22H,2,6-7,10,15-18,27H2,1H3,(H,28,32)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to chymotrypsin |
Bioorg Med Chem Lett 16: 4085-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.088
BindingDB Entry DOI: 10.7270/Q2X929W7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134365
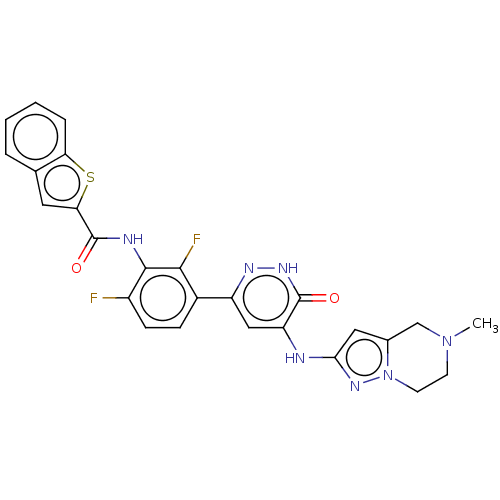
(CHEMBL3745935)Show SMILES CN1CCn2nc(Nc3cc(n[nH]c3=O)-c3ccc(F)c(NC(=O)c4cc5ccccc5s4)c3F)cc2C1 Show InChI InChI=1S/C26H21F2N7O2S/c1-34-8-9-35-15(13-34)11-22(33-35)29-19-12-18(31-32-25(19)36)16-6-7-17(27)24(23(16)28)30-26(37)21-10-14-4-2-3-5-20(14)38-21/h2-7,10-12H,8-9,13H2,1H3,(H,30,37)(H,32,36)(H,29,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM111938

(US8618107, 104)Show SMILES Cn1cc(cc(Nc2ccncn2)c1=O)-c1cccc(N2CCc3c4CCCCc4sc3C2=O)c1CO Show InChI InChI=1S/C28H27N5O3S/c1-32-14-17(13-22(27(32)35)31-25-9-11-29-16-30-25)18-6-4-7-23(21(18)15-34)33-12-10-20-19-5-2-3-8-24(19)37-26(20)28(33)36/h4,6-7,9,11,13-14,16,34H,2-3,5,8,10,12,15H2,1H3,(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human whole blood assessed as suppression of BCR-induced CD69 expression on B cells prein... |
ACS Med Chem Lett 8: 608-613 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00103
BindingDB Entry DOI: 10.7270/Q24M96ZH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50254236
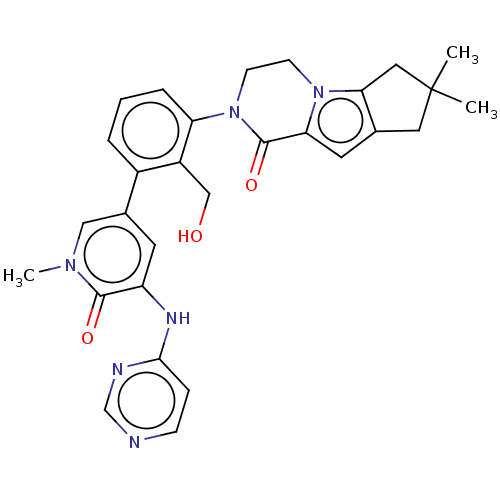
(CHEMBL4090189)Show SMILES Cn1cc(cc(Nc2ccncn2)c1=O)-c1cccc(N2CCn3c4CC(C)(C)Cc4cc3C2=O)c1CO Show InChI InChI=1S/C29H30N6O3/c1-29(2)13-18-12-24-28(38)35(10-9-34(24)25(18)14-29)23-6-4-5-20(21(23)16-36)19-11-22(27(37)33(3)15-19)32-26-7-8-30-17-31-26/h4-8,11-12,15,17,36H,9-10,13-14,16H2,1-3H3,(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human whole blood assessed as suppression of BCR-induced CD69 expression on B cells prein... |
ACS Med Chem Lett 8: 608-613 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00103
BindingDB Entry DOI: 10.7270/Q24M96ZH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM111939

(US8618107, 105)Show SMILES Cn1cc(cc(Nc2ccncn2)c1=O)-c1cccc(N2CCc3c4CC(C)(C)Cc4sc3C2=O)c1CO Show InChI InChI=1S/C29H29N5O3S/c1-29(2)12-20-19-8-10-34(28(37)26(19)38-24(20)13-29)23-6-4-5-18(21(23)15-35)17-11-22(27(36)33(3)14-17)32-25-7-9-30-16-31-25/h4-7,9,11,14,16,35H,8,10,12-13,15H2,1-3H3,(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human whole blood assessed as suppression of BCR-induced CD69 expression on B cells prein... |
ACS Med Chem Lett 8: 608-613 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00103
BindingDB Entry DOI: 10.7270/Q24M96ZH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM111951
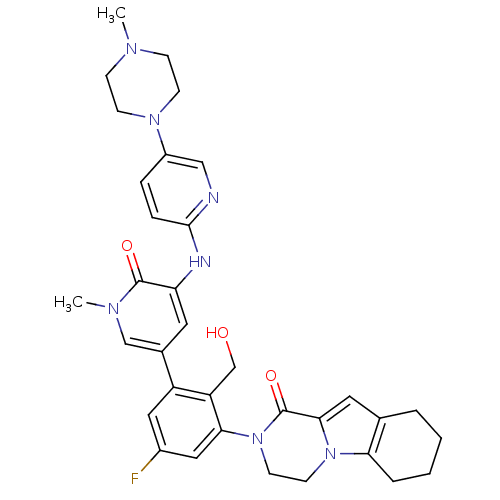
(US8618107, 197)Show SMILES CN1CCN(CC1)c1ccc(Nc2cc(cn(C)c2=O)-c2cc(F)cc(N3CCn4c5CCCCc5cc4C3=O)c2CO)nc1 Show InChI InChI=1S/C34H38FN7O3/c1-38-9-11-40(12-10-38)25-7-8-32(36-19-25)37-28-15-23(20-39(2)33(28)44)26-17-24(35)18-30(27(26)21-43)42-14-13-41-29-6-4-3-5-22(29)16-31(41)34(42)45/h7-8,15-20,43H,3-6,9-14,21H2,1-2H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human whole blood assessed as suppression of BCR-induced CD69 expression on B cells prein... |
ACS Med Chem Lett 8: 608-613 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00103
BindingDB Entry DOI: 10.7270/Q24M96ZH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50254270
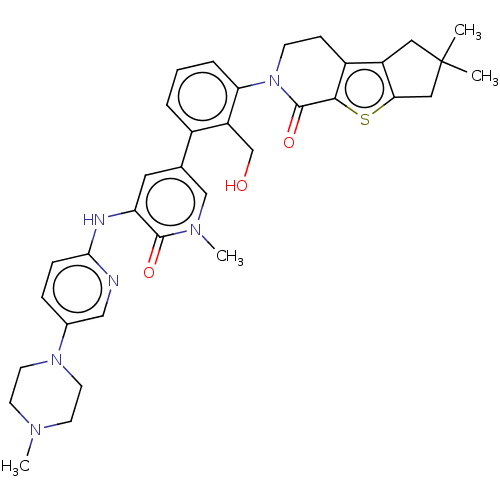
(CHEMBL4080943)Show SMILES CN1CCN(CC1)c1ccc(Nc2cc(cn(C)c2=O)-c2cccc(N3CCc4c5CC(C)(C)Cc5sc4C3=O)c2CO)nc1 Show InChI InChI=1S/C35H40N6O3S/c1-35(2)17-26-25-10-11-41(34(44)32(25)45-30(26)18-35)29-7-5-6-24(27(29)21-42)22-16-28(33(43)39(4)20-22)37-31-9-8-23(19-36-31)40-14-12-38(3)13-15-40/h5-9,16,19-20,42H,10-15,17-18,21H2,1-4H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human whole blood assessed as suppression of BCR-induced CD69 expression on B cells prein... |
ACS Med Chem Lett 8: 608-613 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00103
BindingDB Entry DOI: 10.7270/Q24M96ZH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50254268
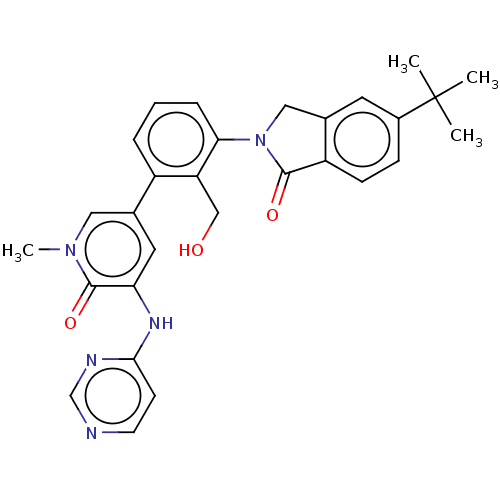
(CHEMBL4077625)Show SMILES Cn1cc(cc(Nc2ccncn2)c1=O)-c1cccc(N2Cc3cc(ccc3C2=O)C(C)(C)C)c1CO Show InChI InChI=1S/C29H29N5O3/c1-29(2,3)20-8-9-22-18(12-20)15-34(27(22)36)25-7-5-6-21(23(25)16-35)19-13-24(28(37)33(4)14-19)32-26-10-11-30-17-31-26/h5-14,17,35H,15-16H2,1-4H3,(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human whole blood assessed as suppression of BCR-induced CD69 expression on B cells prein... |
ACS Med Chem Lett 8: 608-613 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00103
BindingDB Entry DOI: 10.7270/Q24M96ZH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134377
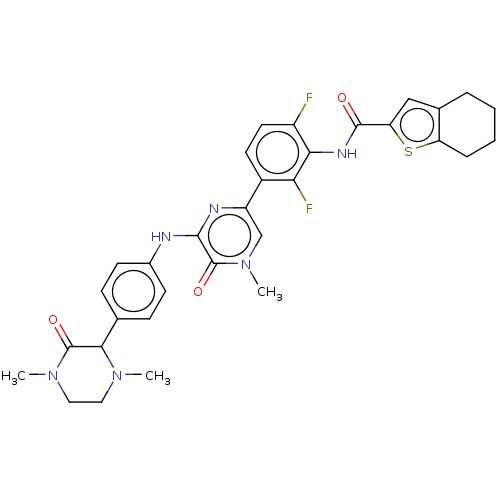
(CHEMBL3745934)Show SMILES CN1CCN(C)C(=O)C1c1ccc(Nc2nc(cn(C)c2=O)-c2ccc(F)c(NC(=O)c3cc4CCCCc4s3)c2F)cc1 Show InChI InChI=1S/C32H32F2N6O3S/c1-38-14-15-39(2)31(42)28(38)18-8-10-20(11-9-18)35-29-32(43)40(3)17-23(36-29)21-12-13-22(33)27(26(21)34)37-30(41)25-16-19-6-4-5-7-24(19)44-25/h8-13,16-17,28H,4-7,14-15H2,1-3H3,(H,35,36)(H,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134320
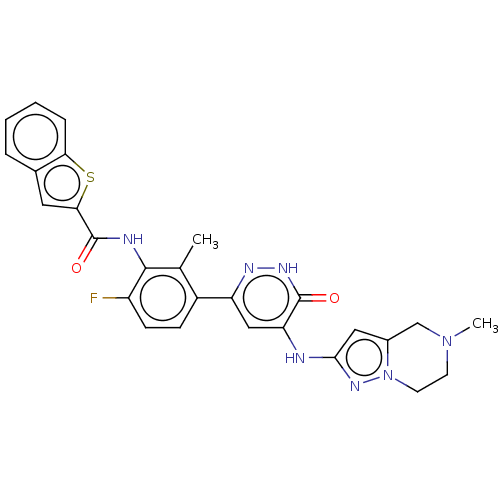
(CHEMBL3746293)Show SMILES CN1CCn2nc(Nc3cc(n[nH]c3=O)-c3ccc(F)c(NC(=O)c4cc5ccccc5s4)c3C)cc2C1 Show InChI InChI=1S/C27H24FN7O2S/c1-15-18(7-8-19(28)25(15)30-27(37)23-11-16-5-3-4-6-22(16)38-23)20-13-21(26(36)32-31-20)29-24-12-17-14-34(2)9-10-35(17)33-24/h3-8,11-13H,9-10,14H2,1-2H3,(H,30,37)(H,32,36)(H,29,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50254277
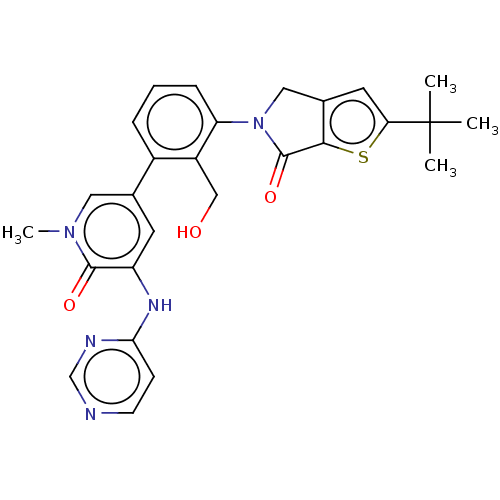
(CHEMBL4067714)Show SMILES Cn1cc(cc(Nc2ccncn2)c1=O)-c1cccc(N2Cc3cc(sc3C2=O)C(C)(C)C)c1CO Show InChI InChI=1S/C27H27N5O3S/c1-27(2,3)22-11-17-13-32(26(35)24(17)36-22)21-7-5-6-18(19(21)14-33)16-10-20(25(34)31(4)12-16)30-23-8-9-28-15-29-23/h5-12,15,33H,13-14H2,1-4H3,(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human whole blood assessed as suppression of BCR-induced CD69 expression on B cells prein... |
ACS Med Chem Lett 8: 608-613 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00103
BindingDB Entry DOI: 10.7270/Q24M96ZH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134377

(CHEMBL3745934)Show SMILES CN1CCN(C)C(=O)C1c1ccc(Nc2nc(cn(C)c2=O)-c2ccc(F)c(NC(=O)c3cc4CCCCc4s3)c2F)cc1 Show InChI InChI=1S/C32H32F2N6O3S/c1-38-14-15-39(2)31(42)28(38)18-8-10-20(11-9-18)35-29-32(43)40(3)17-23(36-29)21-12-13-22(33)27(26(21)34)37-30(41)25-16-19-6-4-5-7-24(19)44-25/h8-13,16-17,28H,4-7,14-15H2,1-3H3,(H,35,36)(H,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM111952
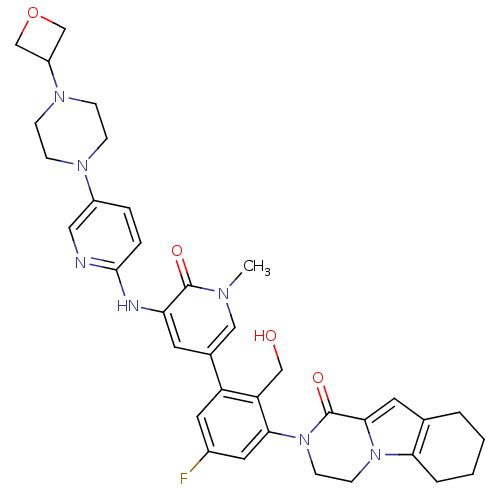
(US8618107, 210)Show SMILES Cn1cc(cc(Nc2ccc(cn2)N2CCN(CC2)C2COC2)c1=O)-c1cc(F)cc(N2CCn3c4CCCCc4cc3C2=O)c1CO Show InChI InChI=1S/C36H40FN7O4/c1-40-19-24(14-30(35(40)46)39-34-7-6-26(18-38-34)41-8-10-42(11-9-41)27-21-48-22-27)28-16-25(37)17-32(29(28)20-45)44-13-12-43-31-5-3-2-4-23(31)15-33(43)36(44)47/h6-7,14-19,27,45H,2-5,8-13,20-22H2,1H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human whole blood assessed as suppression of BCR-induced CD69 expression on B cells prein... |
ACS Med Chem Lett 8: 608-613 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00103
BindingDB Entry DOI: 10.7270/Q24M96ZH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
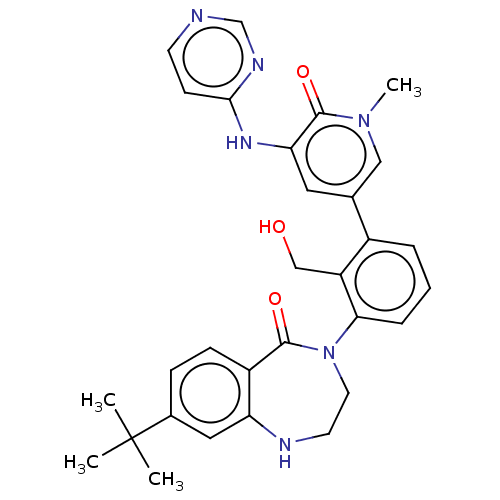
(Homo sapiens (Human)) | BDBM50254278
(CHEMBL4069030)Show SMILES Cn1cc(cc(Nc2ccncn2)c1=O)-c1cccc(N2CCNc3cc(ccc3C2=O)C(C)(C)C)c1CO Show InChI InChI=1S/C30H32N6O3/c1-30(2,3)20-8-9-22-24(15-20)32-12-13-36(28(22)38)26-7-5-6-21(23(26)17-37)19-14-25(29(39)35(4)16-19)34-27-10-11-31-18-33-27/h5-11,14-16,18,32,37H,12-13,17H2,1-4H3,(H,31,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human whole blood assessed as suppression of BCR-induced CD69 expression on B cells prein... |
ACS Med Chem Lett 8: 608-613 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00103
BindingDB Entry DOI: 10.7270/Q24M96ZH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
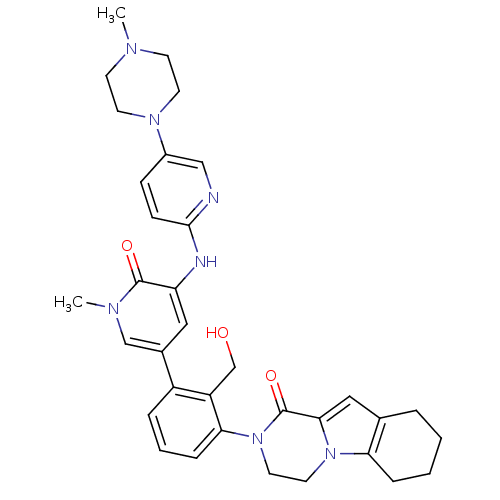
(Homo sapiens (Human)) | BDBM111945
(US8618107, 113)Show SMILES CN1CCN(CC1)c1ccc(Nc2cc(cn(C)c2=O)-c2cccc(N3CCn4c5CCCCc5cc4C3=O)c2CO)nc1 Show InChI InChI=1S/C34H39N7O3/c1-37-12-14-39(15-13-37)25-10-11-32(35-20-25)36-28-18-24(21-38(2)33(28)43)26-7-5-9-30(27(26)22-42)41-17-16-40-29-8-4-3-6-23(29)19-31(40)34(41)44/h5,7,9-11,18-21,42H,3-4,6,8,12-17,22H2,1-2H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length His-tagged BTK expressed in baculovirus expression system by Z-LYTE assay |
ACS Med Chem Lett 8: 608-613 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00103
BindingDB Entry DOI: 10.7270/Q24M96ZH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134368

(CHEMBL3746333)Show SMILES CN1CCn2nc(Nc3cc(n[nH]c3=O)-c3ccc(F)c(NC(=O)c4cnc(cn4)C(C)(C)C)c3C)cc2C1 Show InChI InChI=1S/C27H30FN9O2/c1-15-17(6-7-18(28)24(15)32-25(38)21-12-30-22(13-29-21)27(2,3)4)19-11-20(26(39)34-33-19)31-23-10-16-14-36(5)8-9-37(16)35-23/h6-7,10-13H,8-9,14H2,1-5H3,(H,32,38)(H,34,39)(H,31,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
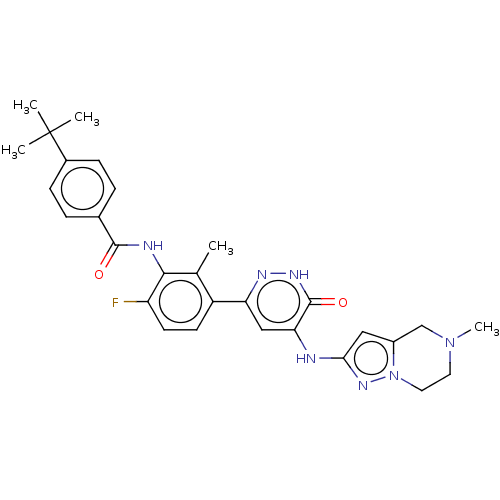
(Homo sapiens (Human)) | BDBM50134369
(CHEMBL3747417)Show SMILES CN1CCn2nc(Nc3cc(n[nH]c3=O)-c3ccc(F)c(NC(=O)c4ccc(cc4)C(C)(C)C)c3C)cc2C1 Show InChI InChI=1S/C29H32FN7O2/c1-17-21(10-11-22(30)26(17)32-27(38)18-6-8-19(9-7-18)29(2,3)4)23-15-24(28(39)34-33-23)31-25-14-20-16-36(5)12-13-37(20)35-25/h6-11,14-15H,12-13,16H2,1-5H3,(H,32,38)(H,34,39)(H,31,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | |
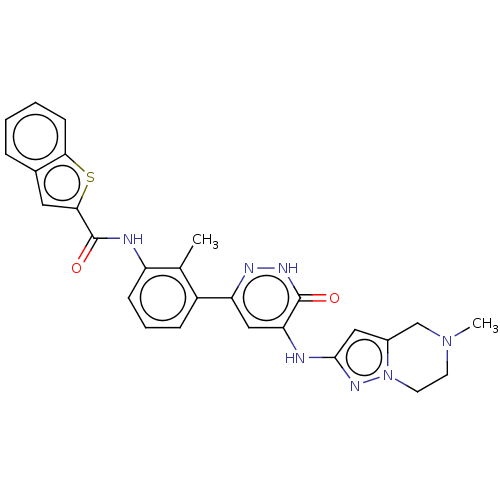
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134366
(CHEMBL3746115)Show SMILES CN1CCn2nc(Nc3cc(n[nH]c3=O)-c3cccc(NC(=O)c4cc5ccccc5s4)c3C)cc2C1 Show InChI InChI=1S/C27H25N7O2S/c1-16-19(7-5-8-20(16)29-27(36)24-12-17-6-3-4-9-23(17)37-24)21-14-22(26(35)31-30-21)28-25-13-18-15-33(2)10-11-34(18)32-25/h3-9,12-14H,10-11,15H2,1-2H3,(H,29,36)(H,31,35)(H,28,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | |
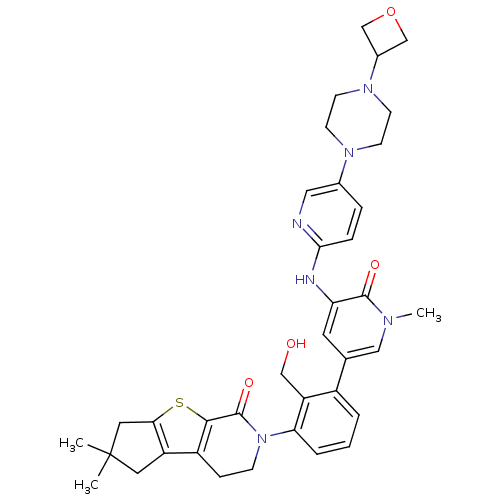
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM111998
(US8618107, 312)Show SMILES Cn1cc(cc(Nc2ccc(cn2)N2CCN(CC2)C2COC2)c1=O)-c1cccc(N2CCc3c4CC(C)(C)Cc4sc3C2=O)c1CO Show InChI InChI=1S/C37H42N6O4S/c1-37(2)16-28-27-9-10-43(36(46)34(27)48-32(28)17-37)31-6-4-5-26(29(31)20-44)23-15-30(35(45)40(3)19-23)39-33-8-7-24(18-38-33)41-11-13-42(14-12-41)25-21-47-22-25/h4-8,15,18-19,25,44H,9-14,16-17,20-22H2,1-3H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human whole blood assessed as suppression of BCR-induced CD69 expression on B cells prein... |
ACS Med Chem Lett 8: 608-613 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00103
BindingDB Entry DOI: 10.7270/Q24M96ZH |
More data for this
Ligand-Target Pair | |
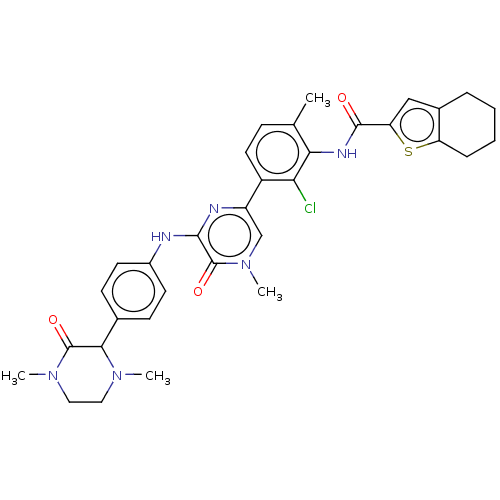
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134371
(CHEMBL3747742)Show SMILES CN1CCN(C)C(=O)C1c1ccc(Nc2nc(cn(C)c2=O)-c2ccc(C)c(NC(=O)c3cc4CCCCc4s3)c2Cl)cc1 Show InChI InChI=1S/C33H35ClN6O3S/c1-19-9-14-23(27(34)28(19)37-31(41)26-17-21-7-5-6-8-25(21)44-26)24-18-40(4)33(43)30(36-24)35-22-12-10-20(11-13-22)29-32(42)39(3)16-15-38(29)2/h9-14,17-18,29H,5-8,15-16H2,1-4H3,(H,35,36)(H,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134371

(CHEMBL3747742)Show SMILES CN1CCN(C)C(=O)C1c1ccc(Nc2nc(cn(C)c2=O)-c2ccc(C)c(NC(=O)c3cc4CCCCc4s3)c2Cl)cc1 Show InChI InChI=1S/C33H35ClN6O3S/c1-19-9-14-23(27(34)28(19)37-31(41)26-17-21-7-5-6-8-25(21)44-26)24-18-40(4)33(43)30(36-24)35-22-12-10-20(11-13-22)29-32(42)39(3)16-15-38(29)2/h9-14,17-18,29H,5-8,15-16H2,1-4H3,(H,35,36)(H,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | |
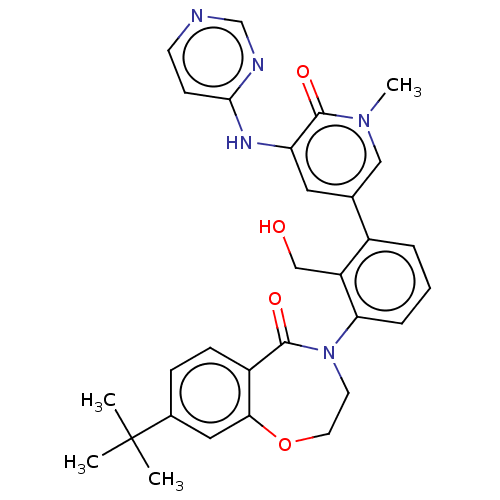
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50254269
(CHEMBL4096404)Show SMILES Cn1cc(cc(Nc2ccncn2)c1=O)-c1cccc(N2CCOc3cc(ccc3C2=O)C(C)(C)C)c1CO Show InChI InChI=1S/C30H31N5O4/c1-30(2,3)20-8-9-22-26(15-20)39-13-12-35(28(22)37)25-7-5-6-21(23(25)17-36)19-14-24(29(38)34(4)16-19)33-27-10-11-31-18-32-27/h5-11,14-16,18,36H,12-13,17H2,1-4H3,(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human whole blood assessed as suppression of BCR-induced CD69 expression on B cells prein... |
ACS Med Chem Lett 8: 608-613 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00103
BindingDB Entry DOI: 10.7270/Q24M96ZH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Mus musculus) | BDBM111998

(US8618107, 312)Show SMILES Cn1cc(cc(Nc2ccc(cn2)N2CCN(CC2)C2COC2)c1=O)-c1cccc(N2CCc3c4CC(C)(C)Cc4sc3C2=O)c1CO Show InChI InChI=1S/C37H42N6O4S/c1-37(2)16-28-27-9-10-43(36(46)34(27)48-32(28)17-37)31-6-4-5-26(29(31)20-44)23-15-30(35(45)40(3)19-23)39-33-8-7-24(18-38-33)41-11-13-42(14-12-41)25-21-47-22-25/h4-8,15,18-19,25,44H,9-14,16-17,20-22H2,1-3H3,(H,38,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Inhibition of BTK in goat F(ab')2anti-mouse IgM-stimulated Balb/c mouse splenocyte B cells assessed as suppression of BCR-mediated CD86 induction pre... |
ACS Med Chem Lett 8: 608-613 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00103
BindingDB Entry DOI: 10.7270/Q24M96ZH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50388183
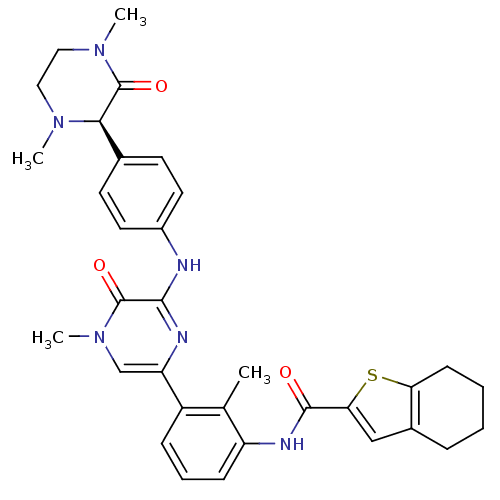
(CHEMBL2057915)Show SMILES CN1CCN(C)C(=O)[C@H]1c1ccc(Nc2nc(cn(C)c2=O)-c2cccc(NC(=O)c3cc4CCCCc4s3)c2C)cc1 |r| Show InChI InChI=1S/C33H36N6O3S/c1-20-24(9-7-10-25(20)36-31(40)28-18-22-8-5-6-11-27(22)43-28)26-19-39(4)33(42)30(35-26)34-23-14-12-21(13-15-23)29-32(41)38(3)17-16-37(29)2/h7,9-10,12-15,18-19,29H,5-6,8,11,16-17H2,1-4H3,(H,34,35)(H,36,40)/t29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen method |
Bioorg Med Chem Lett 25: 1333-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.032
BindingDB Entry DOI: 10.7270/Q26Q1ZXS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134373
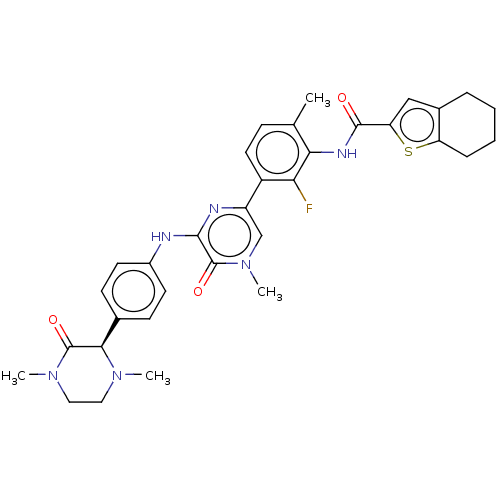
(CHEMBL3747315)Show SMILES CN1CCN(C)C(=O)[C@H]1c1ccc(Nc2nc(cn(C)c2=O)-c2ccc(C)c(NC(=O)c3cc4CCCCc4s3)c2F)cc1 |r| Show InChI InChI=1S/C33H35FN6O3S/c1-19-9-14-23(27(34)28(19)37-31(41)26-17-21-7-5-6-8-25(21)44-26)24-18-40(4)33(43)30(36-24)35-22-12-10-20(11-13-22)29-32(42)39(3)16-15-38(29)2/h9-14,17-18,29H,5-8,15-16H2,1-4H3,(H,35,36)(H,37,41)/t29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134374

(CHEMBL3747161)Show SMILES CN1CCN(C)C(=O)[C@@H]1c1ccc(Nc2nc(cn(C)c2=O)-c2ccc(C)c(NC(=O)c3cc4CCCCc4s3)c2F)cc1 |r| Show InChI InChI=1S/C33H35FN6O3S/c1-19-9-14-23(27(34)28(19)37-31(41)26-17-21-7-5-6-8-25(21)44-26)24-18-40(4)33(43)30(36-24)35-22-12-10-20(11-13-22)29-32(42)39(3)16-15-38(29)2/h9-14,17-18,29H,5-8,15-16H2,1-4H3,(H,35,36)(H,37,41)/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134383

(CHEMBL3747554)Show SMILES CN1CCN(C)C(=O)C1c1ccc(Nc2nc(cn(C)c2=O)-c2cccc(NC(=O)c3cc4CCCCc4s3)c2Cl)cc1 Show InChI InChI=1S/C32H33ClN6O3S/c1-37-15-16-38(2)31(41)28(37)19-11-13-21(14-12-19)34-29-32(42)39(3)18-24(35-29)22-8-6-9-23(27(22)33)36-30(40)26-17-20-7-4-5-10-25(20)43-26/h6,8-9,11-14,17-18,28H,4-5,7,10,15-16H2,1-3H3,(H,34,35)(H,36,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134385
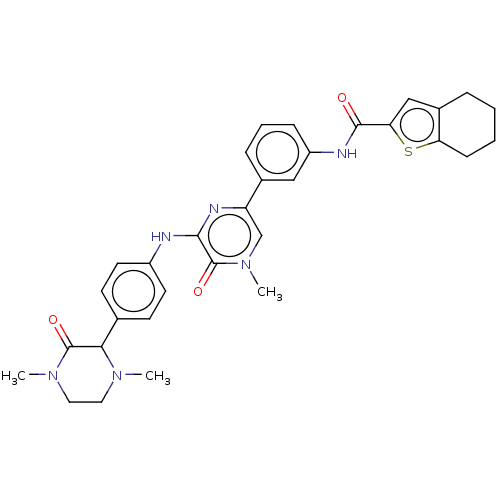
(CHEMBL3745795)Show SMILES CN1CCN(C)C(=O)C1c1ccc(Nc2nc(cn(C)c2=O)-c2cccc(NC(=O)c3cc4CCCCc4s3)c2)cc1 Show InChI InChI=1S/C32H34N6O3S/c1-36-15-16-37(2)31(40)28(36)20-11-13-23(14-12-20)33-29-32(41)38(3)19-25(35-29)21-8-6-9-24(17-21)34-30(39)27-18-22-7-4-5-10-26(22)42-27/h6,8-9,11-14,17-19,28H,4-5,7,10,15-16H2,1-3H3,(H,33,35)(H,34,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM111943
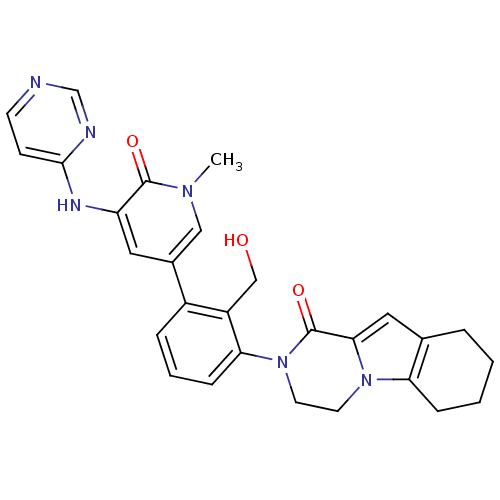
(US8618107, 109)Show SMILES Cn1cc(cc(Nc2ccncn2)c1=O)-c1cccc(N2CCn3c4CCCCc4cc3C2=O)c1CO Show InChI InChI=1S/C28H28N6O3/c1-32-15-19(13-22(27(32)36)31-26-9-10-29-17-30-26)20-6-4-8-24(21(20)16-35)34-12-11-33-23-7-3-2-5-18(23)14-25(33)28(34)37/h4,6,8-10,13-15,17,35H,2-3,5,7,11-12,16H2,1H3,(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human whole blood assessed as suppression of BCR-induced CD69 expression on B cells prein... |
ACS Med Chem Lett 8: 608-613 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00103
BindingDB Entry DOI: 10.7270/Q24M96ZH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50254279
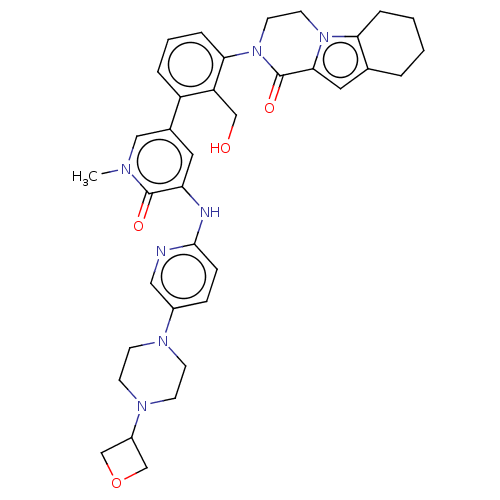
(CHEMBL4060453)Show SMILES Cn1cc(cc(Nc2ccc(cn2)N2CCN(CC2)C2COC2)c1=O)-c1cccc(N2CCn3c4CCCCc4cc3C2=O)c1CO Show InChI InChI=1S/C36H41N7O4/c1-39-20-25(17-30(35(39)45)38-34-10-9-26(19-37-34)40-11-13-41(14-12-40)27-22-47-23-27)28-6-4-8-32(29(28)21-44)43-16-15-42-31-7-3-2-5-24(31)18-33(42)36(43)46/h4,6,8-10,17-20,27,44H,2-3,5,7,11-16,21-23H2,1H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Inhibition of BTK in goat anti-human IgM F(ab')2-stimulated human whole blood assessed as suppression of BCR-induced CD69 expression on B cells prein... |
ACS Med Chem Lett 8: 608-613 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00103
BindingDB Entry DOI: 10.7270/Q24M96ZH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM36516
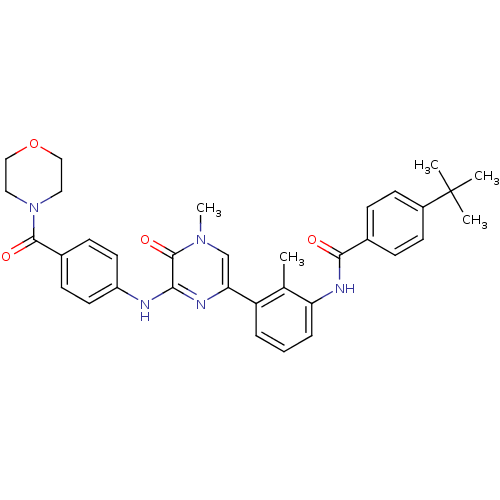
(4-(tert-Butyl)-N-(2-methyl-3-(4-methyl-6-((4-(morp...)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C(C)(C)C)cccc1-c1cn(C)c(=O)c(Nc2ccc(cc2)C(=O)N2CCOCC2)n1 Show InChI InChI=1S/C34H37N5O4/c1-22-27(7-6-8-28(22)37-31(40)23-9-13-25(14-10-23)34(2,3)4)29-21-38(5)33(42)30(36-29)35-26-15-11-24(12-16-26)32(41)39-17-19-43-20-18-39/h6-16,21H,17-20H2,1-5H3,(H,35,36)(H,37,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) |
Bioorg Med Chem Lett 25: 1333-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.032
BindingDB Entry DOI: 10.7270/Q26Q1ZXS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50064869
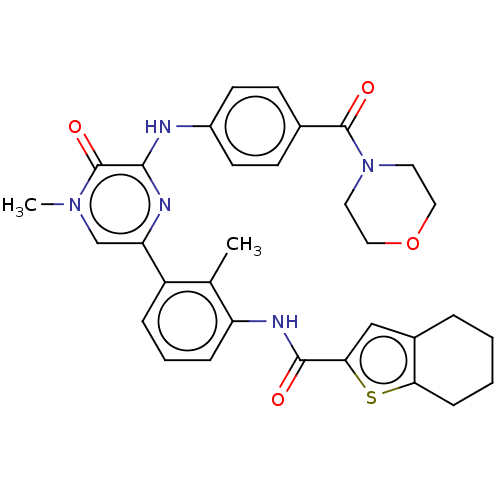
(CHEMBL3401277)Show SMILES Cc1c(NC(=O)c2cc3CCCCc3s2)cccc1-c1cn(C)c(=O)c(Nc2ccc(cc2)C(=O)N2CCOCC2)n1 Show InChI InChI=1S/C32H33N5O4S/c1-20-24(7-5-8-25(20)35-30(38)28-18-22-6-3-4-9-27(22)42-28)26-19-36(2)32(40)29(34-26)33-23-12-10-21(11-13-23)31(39)37-14-16-41-17-15-37/h5,7-8,10-13,18-19H,3-4,6,9,14-17H2,1-2H3,(H,33,34)(H,35,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen method |
Bioorg Med Chem Lett 25: 1333-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.032
BindingDB Entry DOI: 10.7270/Q26Q1ZXS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134384
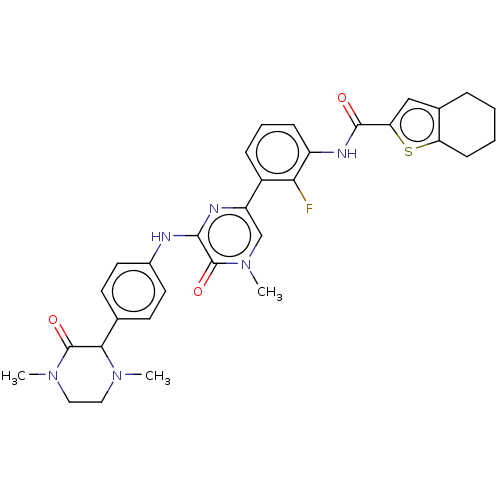
(CHEMBL3746128)Show SMILES CN1CCN(C)C(=O)C1c1ccc(Nc2nc(cn(C)c2=O)-c2cccc(NC(=O)c3cc4CCCCc4s3)c2F)cc1 Show InChI InChI=1S/C32H33FN6O3S/c1-37-15-16-38(2)31(41)28(37)19-11-13-21(14-12-19)34-29-32(42)39(3)18-24(35-29)22-8-6-9-23(27(22)33)36-30(40)26-17-20-7-4-5-10-25(20)43-26/h6,8-9,11-14,17-18,28H,4-5,7,10,15-16H2,1-3H3,(H,34,35)(H,36,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134372

(CHEMBL3747268)Show SMILES CN1CCN(C)C(=O)C1c1ccc(Nc2nc(cn(C)c2=O)-c2ccc(Cl)c(NC(=O)c3cc4CCCCc4s3)c2F)cc1 Show InChI InChI=1S/C32H32ClFN6O3S/c1-38-14-15-39(2)31(42)28(38)18-8-10-20(11-9-18)35-29-32(43)40(3)17-23(36-29)21-12-13-22(33)27(26(21)34)37-30(41)25-16-19-6-4-5-7-24(19)44-25/h8-13,16-17,28H,4-7,14-15H2,1-3H3,(H,35,36)(H,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134372

(CHEMBL3747268)Show SMILES CN1CCN(C)C(=O)C1c1ccc(Nc2nc(cn(C)c2=O)-c2ccc(Cl)c(NC(=O)c3cc4CCCCc4s3)c2F)cc1 Show InChI InChI=1S/C32H32ClFN6O3S/c1-38-14-15-39(2)31(42)28(38)18-8-10-20(11-9-18)35-29-32(43)40(3)17-23(36-29)21-12-13-22(33)27(26(21)34)37-30(41)25-16-19-6-4-5-7-24(19)44-25/h8-13,16-17,28H,4-7,14-15H2,1-3H3,(H,35,36)(H,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134380
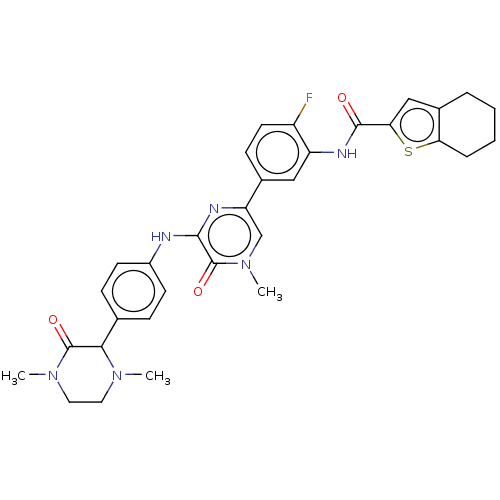
(CHEMBL3747551)Show SMILES CN1CCN(C)C(=O)C1c1ccc(Nc2nc(cn(C)c2=O)-c2ccc(F)c(NC(=O)c3cc4CCCCc4s3)c2)cc1 Show InChI InChI=1S/C32H33FN6O3S/c1-37-14-15-38(2)31(41)28(37)19-8-11-22(12-9-19)34-29-32(42)39(3)18-25(35-29)20-10-13-23(33)24(16-20)36-30(40)27-17-21-6-4-5-7-26(21)43-27/h8-13,16-18,28H,4-7,14-15H2,1-3H3,(H,34,35)(H,36,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50134380

(CHEMBL3747551)Show SMILES CN1CCN(C)C(=O)C1c1ccc(Nc2nc(cn(C)c2=O)-c2ccc(F)c(NC(=O)c3cc4CCCCc4s3)c2)cc1 Show InChI InChI=1S/C32H33FN6O3S/c1-37-14-15-38(2)31(41)28(37)19-8-11-22(12-9-19)34-29-32(42)39(3)18-25(35-29)20-10-13-23(33)24(16-20)36-30(40)27-17-21-6-4-5-7-26(21)43-27/h8-13,16-18,28H,4-7,14-15H2,1-3H3,(H,34,35)(H,36,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of BTK (unknown origin) by Lanthascreen assay |
Bioorg Med Chem Lett 26: 575-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.076
BindingDB Entry DOI: 10.7270/Q2222WMH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data