 Found 61 hits with Last Name = 'scherman' and Initial = 'd'
Found 61 hits with Last Name = 'scherman' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Probable flavin-dependent thymidylate synthase
(Paramecium bursaria Chlorella virus 1) | BDBM50377324

(CHEMBL257162)Show SMILES CCOC(=O)[C@@H]1CSC(N1C(=O)c1cn(CCCNC(=O)C(F)(F)F)nn1)c1ccccc1 |w:8.28| Show InChI InChI=1S/C20H22F3N5O4S/c1-2-32-18(30)15-12-33-17(13-7-4-3-5-8-13)28(15)16(29)14-11-27(26-25-14)10-6-9-24-19(31)20(21,22)23/h3-5,7-8,11,15,17H,2,6,9-10,12H2,1H3,(H,24,31)/t15-,17?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Paris
Curated by ChEMBL
| Assay Description
Inhibition of PBCV1 Thymidylate synthase X |
Bioorg Med Chem Lett 18: 3628-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.080
BindingDB Entry DOI: 10.7270/Q2N87BNK |
More data for this
Ligand-Target Pair | |
Probable flavin-dependent thymidylate synthase
(Paramecium bursaria Chlorella virus 1) | BDBM50377323
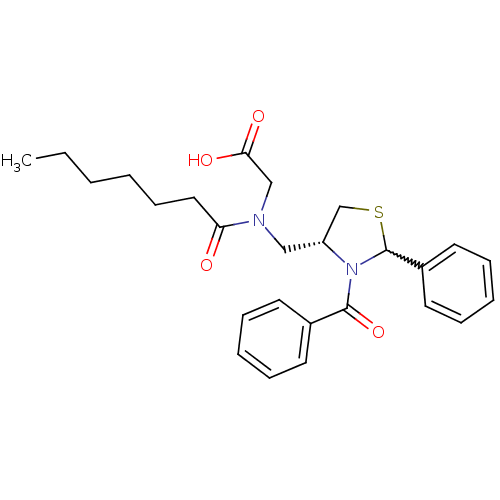
(CHEMBL255717)Show SMILES CCCCCCC(=O)N(C[C@@H]1CSC(N1C(=O)c1ccccc1)c1ccccc1)CC(O)=O |w:13.24| Show InChI InChI=1S/C26H32N2O4S/c1-2-3-4-11-16-23(29)27(18-24(30)31)17-22-19-33-26(21-14-9-6-10-15-21)28(22)25(32)20-12-7-5-8-13-20/h5-10,12-15,22,26H,2-4,11,16-19H2,1H3,(H,30,31)/t22-,26?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Paris
Curated by ChEMBL
| Assay Description
Inhibition of PBCV1 Thymidylate synthase X |
Bioorg Med Chem Lett 18: 3628-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.080
BindingDB Entry DOI: 10.7270/Q2N87BNK |
More data for this
Ligand-Target Pair | |
Probable flavin-dependent thymidylate synthase
(Paramecium bursaria Chlorella virus 1) | BDBM50377326
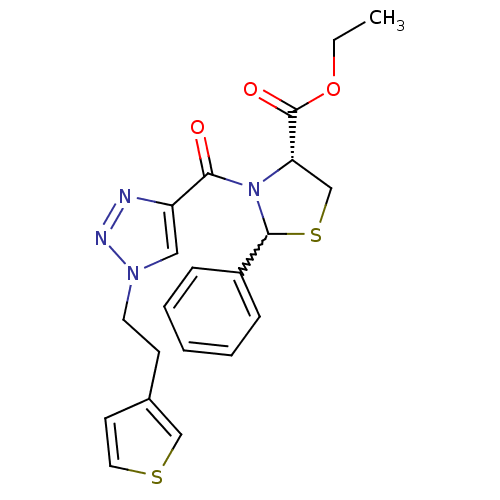
(CHEMBL402780)Show SMILES CCOC(=O)[C@@H]1CSC(N1C(=O)c1cn(CCc2ccsc2)nn1)c1ccccc1 |w:8.26| Show InChI InChI=1S/C21H22N4O3S2/c1-2-28-21(27)18-14-30-20(16-6-4-3-5-7-16)25(18)19(26)17-12-24(23-22-17)10-8-15-9-11-29-13-15/h3-7,9,11-13,18,20H,2,8,10,14H2,1H3/t18-,20?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Paris
Curated by ChEMBL
| Assay Description
Inhibition of PBCV1 Thymidylate synthase X |
Bioorg Med Chem Lett 18: 3628-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.080
BindingDB Entry DOI: 10.7270/Q2N87BNK |
More data for this
Ligand-Target Pair | |
Probable flavin-dependent thymidylate synthase
(Paramecium bursaria Chlorella virus 1) | BDBM50377327
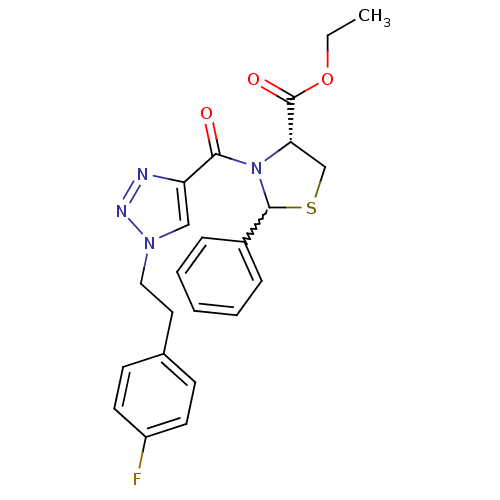
(CHEMBL402148)Show SMILES CCOC(=O)[C@@H]1CSC(N1C(=O)c1cn(CCc2ccc(F)cc2)nn1)c1ccccc1 |w:8.28| Show InChI InChI=1S/C23H23FN4O3S/c1-2-31-23(30)20-15-32-22(17-6-4-3-5-7-17)28(20)21(29)19-14-27(26-25-19)13-12-16-8-10-18(24)11-9-16/h3-11,14,20,22H,2,12-13,15H2,1H3/t20-,22?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Paris
Curated by ChEMBL
| Assay Description
Inhibition of PBCV1 Thymidylate synthase X |
Bioorg Med Chem Lett 18: 3628-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.080
BindingDB Entry DOI: 10.7270/Q2N87BNK |
More data for this
Ligand-Target Pair | |
Probable flavin-dependent thymidylate synthase
(Paramecium bursaria Chlorella virus 1) | BDBM50377325

(CHEMBL257375)Show SMILES CCOC(=O)[C@@H]1CSC(N1C(=O)c1cn(CCCCCCCO)nn1)c1ccccc1 |w:8.26| Show InChI InChI=1S/C22H30N4O4S/c1-2-30-22(29)19-16-31-21(17-11-7-6-8-12-17)26(19)20(28)18-15-25(24-23-18)13-9-4-3-5-10-14-27/h6-8,11-12,15,19,21,27H,2-5,9-10,13-14,16H2,1H3/t19-,21?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Paris
Curated by ChEMBL
| Assay Description
Inhibition of PBCV1 Thymidylate synthase X |
Bioorg Med Chem Lett 18: 3628-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.080
BindingDB Entry DOI: 10.7270/Q2N87BNK |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50058202

((S)-2-[(2-{(S)-2-[((R)-2-Amino-3-mercapto-propiony...)Show SMILES CSCC[C@H](NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)[C@H](C(C)C)N(C)C(=O)[C@@H](N)CS)C(O)=O Show InChI InChI=1S/C24H36N4O5S2/c1-14(2)20(27(3)22(30)17(25)13-34)23(31)28-12-16-8-6-5-7-15(16)11-19(28)21(29)26-18(24(32)33)9-10-35-4/h5-8,14,17-20,34H,9-13,25H2,1-4H3,(H,26,29)(H,32,33)/t17-,18-,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Human farnesyl transferase |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50059852

((S)-2-((S)-2-((S)-2-((R)-2-amino-3-mercaptopropyla...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC[C@@H](NC[C@@H](N)CS)C(C)C)C(O)=O Show InChI InChI=1S/C22H38N4O3S2/c1-15(2)20(24-12-17(23)14-30)13-25-19(11-16-7-5-4-6-8-16)21(27)26-18(22(28)29)9-10-31-3/h4-8,15,17-20,24-25,30H,9-14,23H2,1-3H3,(H,26,27)(H,28,29)/t17-,18+,19+,20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibiion of bovine farnesyl transferase |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50284165
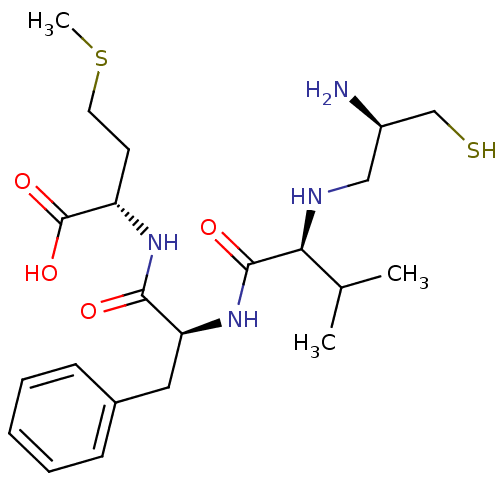
((S)-2-{(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propyl...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC[C@@H](N)CS)C(C)C)C(O)=O Show InChI InChI=1S/C22H36N4O4S2/c1-14(2)19(24-12-16(23)13-31)21(28)26-18(11-15-7-5-4-6-8-15)20(27)25-17(22(29)30)9-10-32-3/h4-8,14,16-19,24,31H,9-13,23H2,1-3H3,(H,25,27)(H,26,28)(H,29,30)/t16-,17+,18+,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of farnesyl transferase in NIH3T3 cell based assay under reducing (+DTT) conditions |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50285838

(CHEMBL89836 | Pseudopeptide derivative)Show SMILES CSCC[C@H](NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)CNCCSSCCNCC(=O)N1Cc2ccccc2C[C@H]1C(=O)N[C@@H](CCSC)C(O)=O)C(O)=O Show InChI InChI=1S/C38H52N6O8S4/c1-53-15-11-29(37(49)50)41-35(47)31-19-25-7-3-5-9-27(25)23-43(31)33(45)21-39-13-17-55-56-18-14-40-22-34(46)44-24-28-10-6-4-8-26(28)20-32(44)36(48)42-30(38(51)52)12-16-54-2/h3-10,29-32,39-40H,11-24H2,1-2H3,(H,41,47)(H,42,48)(H,49,50)(H,51,52)/t29-,30-,31-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of farnesyl transferase |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Probable flavin-dependent thymidylate synthase
(Paramecium bursaria Chlorella virus 1) | BDBM50377324

(CHEMBL257162)Show SMILES CCOC(=O)[C@@H]1CSC(N1C(=O)c1cn(CCCNC(=O)C(F)(F)F)nn1)c1ccccc1 |w:8.28| Show InChI InChI=1S/C20H22F3N5O4S/c1-2-32-18(30)15-12-33-17(13-7-4-3-5-8-13)28(15)16(29)14-11-27(26-25-14)10-6-9-24-19(31)20(21,22)23/h3-5,7-8,11,15,17H,2,6,9-10,12H2,1H3,(H,24,31)/t15-,17?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Paris
Curated by ChEMBL
| Assay Description
Inhibition of PBCV1 Thymidylate synthase X |
Bioorg Med Chem Lett 18: 3628-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.080
BindingDB Entry DOI: 10.7270/Q2N87BNK |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM13373

((2S)-2-[(2S)-2-[(2S)-2-[(2R)-2-amino-3-sulfanylpro...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@@H](N)CS)C(C)C)C(O)=O |r| Show InChI InChI=1S/C22H34N4O5S2/c1-13(2)18(26-19(27)15(23)12-32)21(29)25-17(11-14-7-5-4-6-8-14)20(28)24-16(22(30)31)9-10-33-3/h4-8,13,15-18,32H,9-12,23H2,1-3H3,(H,24,28)(H,25,29)(H,26,27)(H,30,31)/t15-,16-,17-,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibiion of bovine farnesyl transferase |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM13373

((2S)-2-[(2S)-2-[(2S)-2-[(2R)-2-amino-3-sulfanylpro...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@@H](N)CS)C(C)C)C(O)=O |r| Show InChI InChI=1S/C22H34N4O5S2/c1-13(2)18(26-19(27)15(23)12-32)21(29)25-17(11-14-7-5-4-6-8-14)20(28)24-16(22(30)31)9-10-33-3/h4-8,13,15-18,32H,9-12,23H2,1-3H3,(H,24,28)(H,25,29)(H,26,27)(H,30,31)/t15-,16-,17-,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of farnesyl transferase in NIH3T3 cell based assay in non-reducing (-DTT) conditions |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50285846
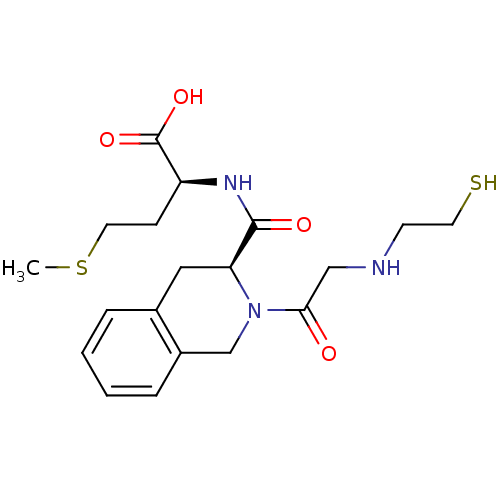
((S)-2-({(S)-2-[2-(2-Mercapto-ethylamino)-acetyl]-1...)Show SMILES CSCC[C@H](NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)CNCCS)C(O)=O Show InChI InChI=1S/C19H27N3O4S2/c1-28-9-6-15(19(25)26)21-18(24)16-10-13-4-2-3-5-14(13)12-22(16)17(23)11-20-7-8-27/h2-5,15-16,20,27H,6-12H2,1H3,(H,21,24)(H,25,26)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of farnesyl transferase |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Probable flavin-dependent thymidylate synthase
(Paramecium bursaria Chlorella virus 1) | BDBM50377323

(CHEMBL255717)Show SMILES CCCCCCC(=O)N(C[C@@H]1CSC(N1C(=O)c1ccccc1)c1ccccc1)CC(O)=O |w:13.24| Show InChI InChI=1S/C26H32N2O4S/c1-2-3-4-11-16-23(29)27(18-24(30)31)17-22-19-33-26(21-14-9-6-10-15-21)28(22)25(32)20-12-7-5-8-13-20/h5-10,12-15,22,26H,2-4,11,16-19H2,1H3,(H,30,31)/t22-,26?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Paris
Curated by ChEMBL
| Assay Description
Inhibition of PBCV1 Thymidylate synthase X |
Bioorg Med Chem Lett 18: 3628-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.080
BindingDB Entry DOI: 10.7270/Q2N87BNK |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50285842
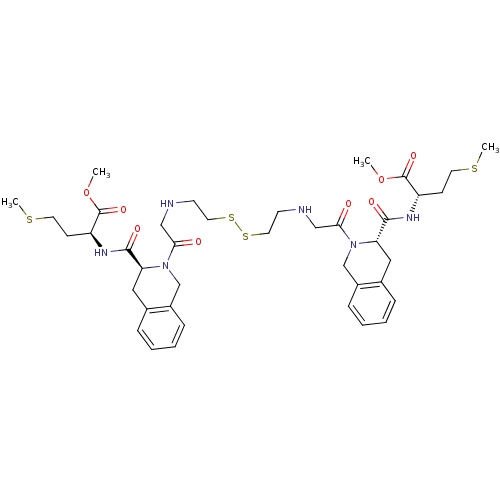
(CHEMBL420801 | Pseudopeptide derivative)Show SMILES COC(=O)[C@H](CCSC)NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)CNCCSSCCNCC(=O)N1Cc2ccccc2C[C@H]1C(=O)N[C@@H](CCSC)C(=O)OC Show InChI InChI=1S/C40H56N6O8S4/c1-53-39(51)31(13-17-55-3)43-37(49)33-21-27-9-5-7-11-29(27)25-45(33)35(47)23-41-15-19-57-58-20-16-42-24-36(48)46-26-30-12-8-6-10-28(30)22-34(46)38(50)44-32(14-18-56-4)40(52)54-2/h5-12,31-34,41-42H,13-26H2,1-4H3,(H,43,49)(H,44,50)/t31-,32-,33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of farnesyl transferase |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50285837
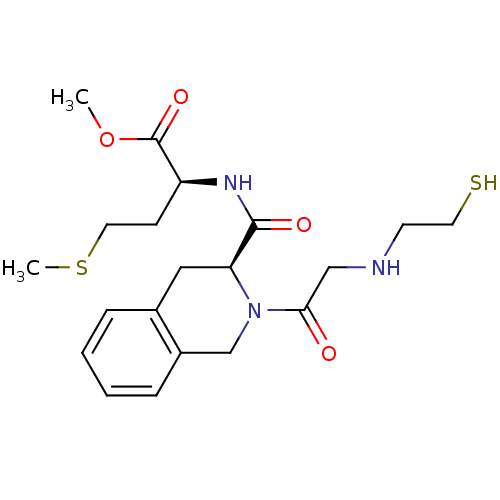
((S)-2-({(S)-2-[2-(2-Mercapto-ethylamino)-acetyl]-1...)Show SMILES COC(=O)[C@H](CCSC)NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)CNCCS Show InChI InChI=1S/C20H29N3O4S2/c1-27-20(26)16(7-10-29-2)22-19(25)17-11-14-5-3-4-6-15(14)13-23(17)18(24)12-21-8-9-28/h3-6,16-17,21,28H,7-13H2,1-2H3,(H,22,25)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of farnesyl transferase |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM13373

((2S)-2-[(2S)-2-[(2S)-2-[(2R)-2-amino-3-sulfanylpro...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@@H](N)CS)C(C)C)C(O)=O |r| Show InChI InChI=1S/C22H34N4O5S2/c1-13(2)18(26-19(27)15(23)12-32)21(29)25-17(11-14-7-5-4-6-8-14)20(28)24-16(22(30)31)9-10-33-3/h4-8,13,15-18,32H,9-12,23H2,1-3H3,(H,24,28)(H,25,29)(H,26,27)(H,30,31)/t15-,16-,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Human farnesyl transferase |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50285845

(2-({2-[(2-Mercapto-ethylcarbamoyl)-methyl]-1,2,3,4...)Show SMILES CSCCC(NC(=O)C1Cc2ccccc2CN1CC(=O)NCCS)C(O)=O Show InChI InChI=1S/C19H27N3O4S2/c1-28-9-6-15(19(25)26)21-18(24)16-10-13-4-2-3-5-14(13)11-22(16)12-17(23)20-7-8-27/h2-5,15-16,27H,6-12H2,1H3,(H,20,23)(H,21,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of farnesyl transferase |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50285843

((S)-2-[((S)-2-{2-[tert-Butoxycarbonyl-(2-mercapto-...)Show SMILES CSCC[C@H](NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)CN(CCS)C(=O)OC(C)(C)C)C(O)=O Show InChI InChI=1S/C24H35N3O6S2/c1-24(2,3)33-23(32)26(10-11-34)15-20(28)27-14-17-8-6-5-7-16(17)13-19(27)21(29)25-18(22(30)31)9-12-35-4/h5-8,18-19,34H,9-15H2,1-4H3,(H,25,29)(H,30,31)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of farnesyl transferase |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50285841

(CHEMBL262383 | Pseudopeptide derivative)Show SMILES CSCC[C@H](NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)CN(CCSSCCN(CC(=O)N1Cc2ccccc2C[C@H]1C(=O)N[C@@H](CCSC)C(O)=O)C(=O)OC(C)(C)C)C(=O)OC(C)(C)C)C(O)=O Show InChI InChI=1S/C48H68N6O12S4/c1-47(2,3)65-45(63)51(29-39(55)53-27-33-15-11-9-13-31(33)25-37(53)41(57)49-35(43(59)60)17-21-67-7)19-23-69-70-24-20-52(46(64)66-48(4,5)6)30-40(56)54-28-34-16-12-10-14-32(34)26-38(54)42(58)50-36(44(61)62)18-22-68-8/h9-16,35-38H,17-30H2,1-8H3,(H,49,57)(H,50,58)(H,59,60)(H,61,62)/t35-,36-,37-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of farnesyl transferase |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50285840
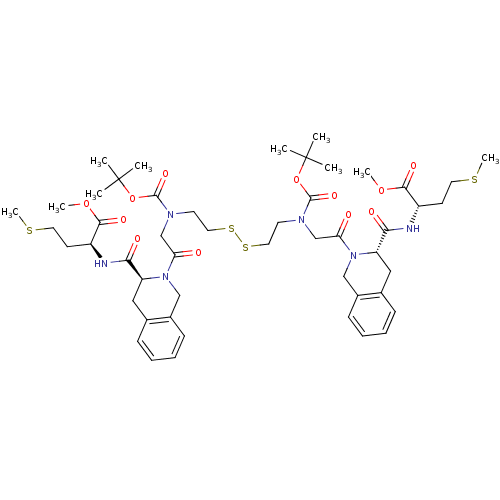
(CHEMBL412576 | Pseudopeptide derivative)Show SMILES COC(=O)[C@H](CCSC)NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)CN(CCSSCCN(CC(=O)N1Cc2ccccc2C[C@H]1C(=O)N[C@@H](CCSC)C(=O)OC)C(=O)OC(C)(C)C)C(=O)OC(C)(C)C Show InChI InChI=1S/C50H72N6O12S4/c1-49(2,3)67-47(63)53(31-41(57)55-29-35-17-13-11-15-33(35)27-39(55)43(59)51-37(19-23-69-9)45(61)65-7)21-25-71-72-26-22-54(48(64)68-50(4,5)6)32-42(58)56-30-36-18-14-12-16-34(36)28-40(56)44(60)52-38(20-24-70-10)46(62)66-8/h11-18,37-40H,19-32H2,1-10H3,(H,51,59)(H,52,60)/t37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of farnesyl transferase |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Mus musculus) | BDBM50285842

(CHEMBL420801 | Pseudopeptide derivative)Show SMILES COC(=O)[C@H](CCSC)NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)CNCCSSCCNCC(=O)N1Cc2ccccc2C[C@H]1C(=O)N[C@@H](CCSC)C(=O)OC Show InChI InChI=1S/C40H56N6O8S4/c1-53-39(51)31(13-17-55-3)43-37(49)33-21-27-9-5-7-11-29(27)25-45(33)35(47)23-41-15-19-57-58-20-16-42-24-36(48)46-26-30-12-8-6-10-28(30)22-34(46)38(50)44-32(14-18-56-4)40(52)54-2/h5-12,31-34,41-42H,13-26H2,1-4H3,(H,43,49)(H,44,50)/t31-,32-,33-,34-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of farnesyl transferase in NIH3T3 cell based assay in non-reducing (-DTT) conditions |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Mus musculus) | BDBM50285842

(CHEMBL420801 | Pseudopeptide derivative)Show SMILES COC(=O)[C@H](CCSC)NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)CNCCSSCCNCC(=O)N1Cc2ccccc2C[C@H]1C(=O)N[C@@H](CCSC)C(=O)OC Show InChI InChI=1S/C40H56N6O8S4/c1-53-39(51)31(13-17-55-3)43-37(49)33-21-27-9-5-7-11-29(27)25-45(33)35(47)23-41-15-19-57-58-20-16-42-24-36(48)46-26-30-12-8-6-10-28(30)22-34(46)38(50)44-32(14-18-56-4)40(52)54-2/h5-12,31-34,41-42H,13-26H2,1-4H3,(H,43,49)(H,44,50)/t31-,32-,33-,34-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of farnesyl transferase in NIH3T3 cell based assay under reducing (+DTT) conditions |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50285839
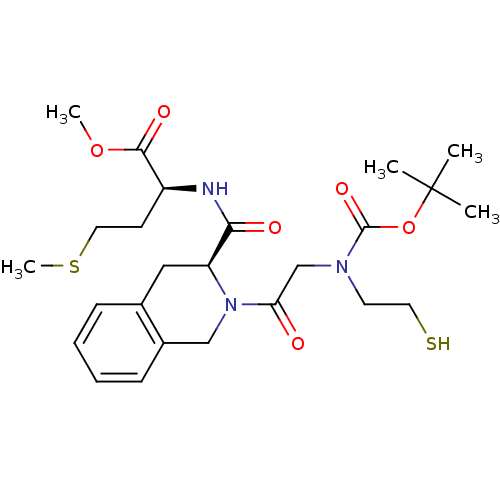
((S)-2-[((S)-2-{2-[tert-Butoxycarbonyl-(2-mercapto-...)Show SMILES COC(=O)[C@H](CCSC)NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)CN(CCS)C(=O)OC(C)(C)C Show InChI InChI=1S/C25H37N3O6S2/c1-25(2,3)34-24(32)27(11-12-35)16-21(29)28-15-18-9-7-6-8-17(18)14-20(28)22(30)26-19(10-13-36-5)23(31)33-4/h6-9,19-20,35H,10-16H2,1-5H3,(H,26,30)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of farnesyl transferase |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
E-selectin
(Homo sapiens (Human)) | BDBM50313785
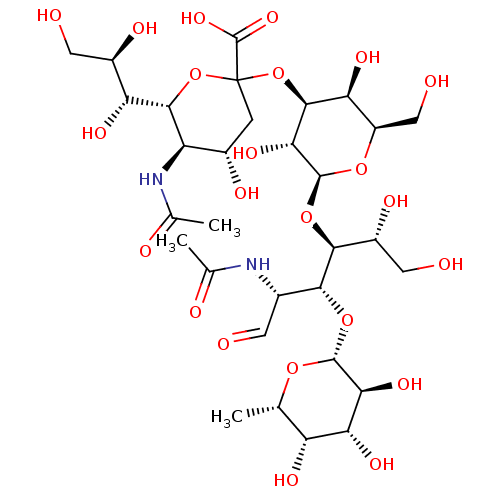
((2S,4S,5R,6R)-5-acetamido-2-((2S,3R,4S,5S,6R)-2-((...)Show SMILES C[C@@H]1O[C@H](O[C@H]([C@@H](NC(C)=O)C=O)[C@@H](O[C@@H]2O[C@H](CO)[C@H](O)[C@H](OC3(C[C@H](O)[C@@H](NC(C)=O)[C@@H](O3)[C@H](O)[C@H](O)CO)C(O)=O)[C@H]2O)[C@H](O)CO)[C@@H](O)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C31H52N2O23/c1-9-18(43)21(46)22(47)28(51-9)53-24(12(5-34)32-10(2)38)25(15(42)7-36)54-29-23(48)27(20(45)16(8-37)52-29)56-31(30(49)50)4-13(40)17(33-11(3)39)26(55-31)19(44)14(41)6-35/h5,9,12-29,35-37,40-48H,4,6-8H2,1-3H3,(H,32,38)(H,33,39)(H,49,50)/t9-,12-,13-,14+,15+,16+,17+,18+,19+,20-,21+,22-,23+,24+,25-,26+,27-,28+,29-,31?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ et INSA de Rouen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant E-Selectin assessed as inhibition of binding of HL-60 cells expressing tetrasaccharide sialyl Lewis x to selectin coa... |
Bioorg Med Chem Lett 20: 1957-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.141
BindingDB Entry DOI: 10.7270/Q22Z15P9 |
More data for this
Ligand-Target Pair | |
E-selectin
(Homo sapiens (Human)) | BDBM50168310

(CHEMBL425373 | [ alpha-Neu5Ac-(2,3)-beta-D-Gal-(1,...)Show SMILES CC1OC(OC2C(NC(C)=O)C(O)OC(CO)C2OC2OC(CO)C(O)C(OC3(CC(O)C(NC(C)=O)C(C3)[C@H](O)C(O)CO)C(O)=O)C2O)C(O)C(O)C1O Show InChI InChI=1S/C32H54N2O22/c1-9-19(42)22(45)23(46)29(51-9)55-26-18(34-11(3)39)28(48)52-16(8-37)25(26)54-30-24(47)27(21(44)15(7-36)53-30)56-32(31(49)50)4-12(20(43)14(41)6-35)17(13(40)5-32)33-10(2)38/h9,12-30,35-37,40-48H,4-8H2,1-3H3,(H,33,38)(H,34,39)(H,49,50)/t9?,12?,13?,14?,15?,16?,17?,18?,19?,20-,21?,22?,23?,24?,25?,26?,27?,28?,29?,30?,32?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of HL-60 cell adhesion to recombinant human Selectin E |
Bioorg Med Chem Lett 15: 3224-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.004
BindingDB Entry DOI: 10.7270/Q23N22WB |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Mus musculus) | BDBM50285838

(CHEMBL89836 | Pseudopeptide derivative)Show SMILES CSCC[C@H](NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)CNCCSSCCNCC(=O)N1Cc2ccccc2C[C@H]1C(=O)N[C@@H](CCSC)C(O)=O)C(O)=O Show InChI InChI=1S/C38H52N6O8S4/c1-53-15-11-29(37(49)50)41-35(47)31-19-25-7-3-5-9-27(25)23-43(31)33(45)21-39-13-17-55-56-18-14-40-22-34(46)44-24-28-10-6-4-8-26(28)20-32(44)36(48)42-30(38(51)52)12-16-54-2/h3-10,29-32,39-40H,11-24H2,1-2H3,(H,41,47)(H,42,48)(H,49,50)(H,51,52)/t29-,30-,31-,32-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibiion of bovine farnesyl transferase |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Mus musculus) | BDBM50285840

(CHEMBL412576 | Pseudopeptide derivative)Show SMILES COC(=O)[C@H](CCSC)NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)CN(CCSSCCN(CC(=O)N1Cc2ccccc2C[C@H]1C(=O)N[C@@H](CCSC)C(=O)OC)C(=O)OC(C)(C)C)C(=O)OC(C)(C)C Show InChI InChI=1S/C50H72N6O12S4/c1-49(2,3)67-47(63)53(31-41(57)55-29-35-17-13-11-15-33(35)27-39(55)43(59)51-37(19-23-69-9)45(61)65-7)21-25-71-72-26-22-54(48(64)68-50(4,5)6)32-42(58)56-30-36-18-14-12-16-34(36)28-40(56)44(60)52-38(20-24-70-10)46(62)66-8/h11-18,37-40H,19-32H2,1-10H3,(H,51,59)(H,52,60)/t37-,38-,39-,40-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of farnesyl transferase in NIH3T3 cell based assay under reducing (+DTT) conditions |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Mus musculus) | BDBM50285841

(CHEMBL262383 | Pseudopeptide derivative)Show SMILES CSCC[C@H](NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)CN(CCSSCCN(CC(=O)N1Cc2ccccc2C[C@H]1C(=O)N[C@@H](CCSC)C(O)=O)C(=O)OC(C)(C)C)C(=O)OC(C)(C)C)C(O)=O Show InChI InChI=1S/C48H68N6O12S4/c1-47(2,3)65-45(63)51(29-39(55)53-27-33-15-11-9-13-31(33)25-37(53)41(57)49-35(43(59)60)17-21-67-7)19-23-69-70-24-20-52(46(64)66-48(4,5)6)30-40(56)54-28-34-16-12-10-14-32(34)26-38(54)42(58)50-36(44(61)62)18-22-68-8/h9-16,35-38H,17-30H2,1-8H3,(H,49,57)(H,50,58)(H,59,60)(H,61,62)/t35-,36-,37-,38-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of farnesyl transferase in NIH3T3 cell based assay in non-reducing (-DTT) conditions |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Mus musculus) | BDBM50285840

(CHEMBL412576 | Pseudopeptide derivative)Show SMILES COC(=O)[C@H](CCSC)NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)CN(CCSSCCN(CC(=O)N1Cc2ccccc2C[C@H]1C(=O)N[C@@H](CCSC)C(=O)OC)C(=O)OC(C)(C)C)C(=O)OC(C)(C)C Show InChI InChI=1S/C50H72N6O12S4/c1-49(2,3)67-47(63)53(31-41(57)55-29-35-17-13-11-15-33(35)27-39(55)43(59)51-37(19-23-69-9)45(61)65-7)21-25-71-72-26-22-54(48(64)68-50(4,5)6)32-42(58)56-30-36-18-14-12-16-34(36)28-40(56)44(60)52-38(20-24-70-10)46(62)66-8/h11-18,37-40H,19-32H2,1-10H3,(H,51,59)(H,52,60)/t37-,38-,39-,40-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of farnesyl transferase in NIH3T3 cell based assay in non-reducing (-DTT) conditions |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Mus musculus) | BDBM50285841

(CHEMBL262383 | Pseudopeptide derivative)Show SMILES CSCC[C@H](NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)CN(CCSSCCN(CC(=O)N1Cc2ccccc2C[C@H]1C(=O)N[C@@H](CCSC)C(O)=O)C(=O)OC(C)(C)C)C(=O)OC(C)(C)C)C(O)=O Show InChI InChI=1S/C48H68N6O12S4/c1-47(2,3)65-45(63)51(29-39(55)53-27-33-15-11-9-13-31(33)25-37(53)41(57)49-35(43(59)60)17-21-67-7)19-23-69-70-24-20-52(46(64)66-48(4,5)6)30-40(56)54-28-34-16-12-10-14-32(34)26-38(54)42(58)50-36(44(61)62)18-22-68-8/h9-16,35-38H,17-30H2,1-8H3,(H,49,57)(H,50,58)(H,59,60)(H,61,62)/t35-,36-,37-,38-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibiion of bovine farnesyl transferase |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Mus musculus) | BDBM50285838

(CHEMBL89836 | Pseudopeptide derivative)Show SMILES CSCC[C@H](NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)CNCCSSCCNCC(=O)N1Cc2ccccc2C[C@H]1C(=O)N[C@@H](CCSC)C(O)=O)C(O)=O Show InChI InChI=1S/C38H52N6O8S4/c1-53-15-11-29(37(49)50)41-35(47)31-19-25-7-3-5-9-27(25)23-43(31)33(45)21-39-13-17-55-56-18-14-40-22-34(46)44-24-28-10-6-4-8-26(28)20-32(44)36(48)42-30(38(51)52)12-16-54-2/h3-10,29-32,39-40H,11-24H2,1-2H3,(H,41,47)(H,42,48)(H,49,50)(H,51,52)/t29-,30-,31-,32-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of farnesyl transferase in NIH3T3 cell based assay under reducing (+DTT) conditions |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
E-selectin
(Homo sapiens (Human)) | BDBM50313791

(3-(2,2-difluoro-2-((2R,3S,4S,5S,6R)-3,4,5-trihydro...)Show SMILES OC[C@H]1O[C@H]([C@@H](O)[C@@H](O)[C@@H]1O)C(F)(F)C(=O)Nc1cccc(c1)C(O)=O |r| Show InChI InChI=1S/C15H17F2NO8/c16-15(17,12-11(22)10(21)9(20)8(5-19)26-12)14(25)18-7-3-1-2-6(4-7)13(23)24/h1-4,8-12,19-22H,5H2,(H,18,25)(H,23,24)/t8-,9-,10+,11+,12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ et INSA de Rouen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant E-Selectin assessed as inhibition of binding of HL-60 cells expressing tetrasaccharide sialyl Lewis x to selectin coa... |
Bioorg Med Chem Lett 20: 1957-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.141
BindingDB Entry DOI: 10.7270/Q22Z15P9 |
More data for this
Ligand-Target Pair | |
P-selectin
(Homo sapiens (Human)) | BDBM50313791

(3-(2,2-difluoro-2-((2R,3S,4S,5S,6R)-3,4,5-trihydro...)Show SMILES OC[C@H]1O[C@H]([C@@H](O)[C@@H](O)[C@@H]1O)C(F)(F)C(=O)Nc1cccc(c1)C(O)=O |r| Show InChI InChI=1S/C15H17F2NO8/c16-15(17,12-11(22)10(21)9(20)8(5-19)26-12)14(25)18-7-3-1-2-6(4-7)13(23)24/h1-4,8-12,19-22H,5H2,(H,18,25)(H,23,24)/t8-,9-,10+,11+,12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ et INSA de Rouen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant P-Selectin assessed as inhibition of binding of HL-60 cells expressing tetrasaccharide sialyl Lewis x to selectin coa... |
Bioorg Med Chem Lett 20: 1957-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.141
BindingDB Entry DOI: 10.7270/Q22Z15P9 |
More data for this
Ligand-Target Pair | |
E-selectin
(Homo sapiens (Human)) | BDBM50313788

((S)-2-(2,2-difluoro-2-((2S,3S,4S,5S,6R)-3,4,5-trih...)Show SMILES OC[C@H]1O[C@@H]([C@@H](O)[C@@H](O)[C@@H]1O)C(F)(F)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C13H19F2NO10/c14-13(15,10-9(22)8(21)7(20)5(3-17)26-10)12(25)16-4(11(23)24)1-2-6(18)19/h4-5,7-10,17,20-22H,1-3H2,(H,16,25)(H,18,19)(H,23,24)/t4-,5+,7+,8-,9-,10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ et INSA de Rouen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant E-Selectin assessed as inhibition of binding of HL-60 cells expressing tetrasaccharide sialyl Lewis x to selectin coa... |
Bioorg Med Chem Lett 20: 1957-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.141
BindingDB Entry DOI: 10.7270/Q22Z15P9 |
More data for this
Ligand-Target Pair | |
P-selectin
(Homo sapiens (Human)) | BDBM50313788

((S)-2-(2,2-difluoro-2-((2S,3S,4S,5S,6R)-3,4,5-trih...)Show SMILES OC[C@H]1O[C@@H]([C@@H](O)[C@@H](O)[C@@H]1O)C(F)(F)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C13H19F2NO10/c14-13(15,10-9(22)8(21)7(20)5(3-17)26-10)12(25)16-4(11(23)24)1-2-6(18)19/h4-5,7-10,17,20-22H,1-3H2,(H,16,25)(H,18,19)(H,23,24)/t4-,5+,7+,8-,9-,10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ et INSA de Rouen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant P-Selectin assessed as inhibition of binding of HL-60 cells expressing tetrasaccharide sialyl Lewis x to selectin coa... |
Bioorg Med Chem Lett 20: 1957-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.141
BindingDB Entry DOI: 10.7270/Q22Z15P9 |
More data for this
Ligand-Target Pair | |
P-selectin
(Homo sapiens (Human)) | BDBM50313789

(3-(2,2-difluoro-2-((2S,3S,4S,5S,6R)-3,4,5-trihydro...)Show SMILES OC[C@H]1O[C@@H]([C@@H](O)[C@@H](O)[C@@H]1O)C(F)(F)C(=O)Nc1cccc(c1)C(O)=O |r| Show InChI InChI=1S/C15H17F2NO8/c16-15(17,12-11(22)10(21)9(20)8(5-19)26-12)14(25)18-7-3-1-2-6(4-7)13(23)24/h1-4,8-12,19-22H,5H2,(H,18,25)(H,23,24)/t8-,9-,10+,11+,12+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ et INSA de Rouen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant P-Selectin assessed as inhibition of binding of HL-60 cells expressing tetrasaccharide sialyl Lewis x to selectin coa... |
Bioorg Med Chem Lett 20: 1957-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.141
BindingDB Entry DOI: 10.7270/Q22Z15P9 |
More data for this
Ligand-Target Pair | |
E-selectin
(Homo sapiens (Human)) | BDBM50313787

(3-(2,2-difluoro-2-((2R,3S,4S,5S,6R)-2,3,4,5-tetrah...)Show SMILES OC[C@H]1O[C@](O)([C@@H](O)[C@@H](O)[C@@H]1O)C(F)(F)C(=O)Nc1cccc(c1)C(O)=O |r| Show InChI InChI=1S/C15H17F2NO9/c16-14(17,13(25)18-7-3-1-2-6(4-7)12(23)24)15(26)11(22)10(21)9(20)8(5-19)27-15/h1-4,8-11,19-22,26H,5H2,(H,18,25)(H,23,24)/t8-,9-,10+,11+,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ et INSA de Rouen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant E-Selectin assessed as inhibition of binding of HL-60 cells expressing tetrasaccharide sialyl Lewis x to selectin coa... |
Bioorg Med Chem Lett 20: 1957-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.141
BindingDB Entry DOI: 10.7270/Q22Z15P9 |
More data for this
Ligand-Target Pair | |
P-selectin
(Homo sapiens (Human)) | BDBM50313785

((2S,4S,5R,6R)-5-acetamido-2-((2S,3R,4S,5S,6R)-2-((...)Show SMILES C[C@@H]1O[C@H](O[C@H]([C@@H](NC(C)=O)C=O)[C@@H](O[C@@H]2O[C@H](CO)[C@H](O)[C@H](OC3(C[C@H](O)[C@@H](NC(C)=O)[C@@H](O3)[C@H](O)[C@H](O)CO)C(O)=O)[C@H]2O)[C@H](O)CO)[C@@H](O)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C31H52N2O23/c1-9-18(43)21(46)22(47)28(51-9)53-24(12(5-34)32-10(2)38)25(15(42)7-36)54-29-23(48)27(20(45)16(8-37)52-29)56-31(30(49)50)4-13(40)17(33-11(3)39)26(55-31)19(44)14(41)6-35/h5,9,12-29,35-37,40-48H,4,6-8H2,1-3H3,(H,32,38)(H,33,39)(H,49,50)/t9-,12-,13-,14+,15+,16+,17+,18+,19+,20-,21+,22-,23+,24+,25-,26+,27-,28+,29-,31?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ et INSA de Rouen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant P-Selectin assessed as inhibition of binding of HL-60 cells expressing tetrasaccharide sialyl Lewis x to selectin coa... |
Bioorg Med Chem Lett 20: 1957-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.141
BindingDB Entry DOI: 10.7270/Q22Z15P9 |
More data for this
Ligand-Target Pair | |
E-selectin
(Homo sapiens (Human)) | BDBM50313789

(3-(2,2-difluoro-2-((2S,3S,4S,5S,6R)-3,4,5-trihydro...)Show SMILES OC[C@H]1O[C@@H]([C@@H](O)[C@@H](O)[C@@H]1O)C(F)(F)C(=O)Nc1cccc(c1)C(O)=O |r| Show InChI InChI=1S/C15H17F2NO8/c16-15(17,12-11(22)10(21)9(20)8(5-19)26-12)14(25)18-7-3-1-2-6(4-7)13(23)24/h1-4,8-12,19-22H,5H2,(H,18,25)(H,23,24)/t8-,9-,10+,11+,12+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ et INSA de Rouen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant E-Selectin assessed as inhibition of binding of HL-60 cells expressing tetrasaccharide sialyl Lewis x to selectin coa... |
Bioorg Med Chem Lett 20: 1957-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.141
BindingDB Entry DOI: 10.7270/Q22Z15P9 |
More data for this
Ligand-Target Pair | |
E-selectin
(Homo sapiens (Human)) | BDBM50313792

(CHEMBL1088214 | beta-mannosylglutamate)Show SMILES OC[C@H]1O[C@H](N[C@@H](CCC(O)=O)C(O)=O)[C@@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C11H19NO9/c13-3-5-7(16)8(17)9(18)10(21-5)12-4(11(19)20)1-2-6(14)15/h4-5,7-10,12-13,16-18H,1-3H2,(H,14,15)(H,19,20)/t4-,5+,7+,8-,9-,10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ et INSA de Rouen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant E-Selectin assessed as inhibition of binding of HL-60 cells expressing tetrasaccharide sialyl Lewis x to selectin coa... |
Bioorg Med Chem Lett 20: 1957-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.141
BindingDB Entry DOI: 10.7270/Q22Z15P9 |
More data for this
Ligand-Target Pair | |
P-selectin
(Homo sapiens (Human)) | BDBM50313787

(3-(2,2-difluoro-2-((2R,3S,4S,5S,6R)-2,3,4,5-tetrah...)Show SMILES OC[C@H]1O[C@](O)([C@@H](O)[C@@H](O)[C@@H]1O)C(F)(F)C(=O)Nc1cccc(c1)C(O)=O |r| Show InChI InChI=1S/C15H17F2NO9/c16-14(17,13(25)18-7-3-1-2-6(4-7)12(23)24)15(26)11(22)10(21)9(20)8(5-19)27-15/h1-4,8-11,19-22,26H,5H2,(H,18,25)(H,23,24)/t8-,9-,10+,11+,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ et INSA de Rouen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant P-Selectin assessed as inhibition of binding of HL-60 cells expressing tetrasaccharide sialyl Lewis x to selectin coa... |
Bioorg Med Chem Lett 20: 1957-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.141
BindingDB Entry DOI: 10.7270/Q22Z15P9 |
More data for this
Ligand-Target Pair | |
P-selectin
(Homo sapiens (Human)) | BDBM50313786

((S)-2-(2,2-difluoro-2-((2R,3S,4S,5S,6R)-2,3,4,5-te...)Show SMILES OC[C@H]1O[C@](O)([C@@H](O)[C@@H](O)[C@@H]1O)C(F)(F)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C13H19F2NO11/c14-12(15,11(25)16-4(10(23)24)1-2-6(18)19)13(26)9(22)8(21)7(20)5(3-17)27-13/h4-5,7-9,17,20-22,26H,1-3H2,(H,16,25)(H,18,19)(H,23,24)/t4-,5+,7+,8-,9-,13+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ et INSA de Rouen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant P-Selectin assessed as inhibition of binding of HL-60 cells expressing tetrasaccharide sialyl Lewis x to selectin coa... |
Bioorg Med Chem Lett 20: 1957-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.141
BindingDB Entry DOI: 10.7270/Q22Z15P9 |
More data for this
Ligand-Target Pair | |
E-selectin
(Homo sapiens (Human)) | BDBM50313786

((S)-2-(2,2-difluoro-2-((2R,3S,4S,5S,6R)-2,3,4,5-te...)Show SMILES OC[C@H]1O[C@](O)([C@@H](O)[C@@H](O)[C@@H]1O)C(F)(F)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C13H19F2NO11/c14-12(15,11(25)16-4(10(23)24)1-2-6(18)19)13(26)9(22)8(21)7(20)5(3-17)27-13/h4-5,7-9,17,20-22,26H,1-3H2,(H,16,25)(H,18,19)(H,23,24)/t4-,5+,7+,8-,9-,13+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ et INSA de Rouen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant E-Selectin assessed as inhibition of binding of HL-60 cells expressing tetrasaccharide sialyl Lewis x to selectin coa... |
Bioorg Med Chem Lett 20: 1957-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.141
BindingDB Entry DOI: 10.7270/Q22Z15P9 |
More data for this
Ligand-Target Pair | |
P-selectin
(Homo sapiens (Human)) | BDBM50313790

((S)-2-(2,2-difluoro-2-((2R,3S,4S,5S,6R)-3,4,5-trih...)Show SMILES OC[C@H]1O[C@H]([C@@H](O)[C@@H](O)[C@@H]1O)C(F)(F)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C13H19F2NO10/c14-13(15,10-9(22)8(21)7(20)5(3-17)26-10)12(25)16-4(11(23)24)1-2-6(18)19/h4-5,7-10,17,20-22H,1-3H2,(H,16,25)(H,18,19)(H,23,24)/t4-,5+,7+,8-,9-,10+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ et INSA de Rouen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant P-Selectin assessed as inhibition of binding of HL-60 cells expressing tetrasaccharide sialyl Lewis x to selectin coa... |
Bioorg Med Chem Lett 20: 1957-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.141
BindingDB Entry DOI: 10.7270/Q22Z15P9 |
More data for this
Ligand-Target Pair | |
P-selectin
(Homo sapiens (Human)) | BDBM50168310

(CHEMBL425373 | [ alpha-Neu5Ac-(2,3)-beta-D-Gal-(1,...)Show SMILES CC1OC(OC2C(NC(C)=O)C(O)OC(CO)C2OC2OC(CO)C(O)C(OC3(CC(O)C(NC(C)=O)C(C3)[C@H](O)C(O)CO)C(O)=O)C2O)C(O)C(O)C1O Show InChI InChI=1S/C32H54N2O22/c1-9-19(42)22(45)23(46)29(51-9)55-26-18(34-11(3)39)28(48)52-16(8-37)25(26)54-30-24(47)27(21(44)15(7-36)53-30)56-32(31(49)50)4-12(20(43)14(41)6-35)17(13(40)5-32)33-10(2)38/h9,12-30,35-37,40-48H,4-8H2,1-3H3,(H,33,38)(H,34,39)(H,49,50)/t9?,12?,13?,14?,15?,16?,17?,18?,19?,20-,21?,22?,23?,24?,25?,26?,27?,28?,29?,30?,32?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of HL-60 cell adhesion to recombinant human Selectin P |
Bioorg Med Chem Lett 15: 3224-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.004
BindingDB Entry DOI: 10.7270/Q23N22WB |
More data for this
Ligand-Target Pair | |
E-selectin
(Homo sapiens (Human)) | BDBM50313790

((S)-2-(2,2-difluoro-2-((2R,3S,4S,5S,6R)-3,4,5-trih...)Show SMILES OC[C@H]1O[C@H]([C@@H](O)[C@@H](O)[C@@H]1O)C(F)(F)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C13H19F2NO10/c14-13(15,10-9(22)8(21)7(20)5(3-17)26-10)12(25)16-4(11(23)24)1-2-6(18)19/h4-5,7-10,17,20-22H,1-3H2,(H,16,25)(H,18,19)(H,23,24)/t4-,5+,7+,8-,9-,10+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ et INSA de Rouen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant E-Selectin assessed as inhibition of binding of HL-60 cells expressing tetrasaccharide sialyl Lewis x to selectin coa... |
Bioorg Med Chem Lett 20: 1957-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.141
BindingDB Entry DOI: 10.7270/Q22Z15P9 |
More data for this
Ligand-Target Pair | |
P-selectin
(Homo sapiens (Human)) | BDBM50168307

(3-Methyl-2-[((3R,5R)-1,3,5-trihydroxy-4-(S)-hydrox...)Show SMILES CCC(C)[C@H](NC(=O)[C@@]1(O)C[C@@H](O)[C@H](O)[C@H](O)C1)C(O)=O |wU:8.8,13.13,15.15,wD:8.7,4.4,11.11,(-2.14,2.27,;-.81,3.04,;.52,2.27,;1.85,3.04,;.52,.74,;1.85,-.03,;3.17,.74,;3.17,2.27,;4.52,-.03,;5.85,.74,;3.19,-.8,;3.19,-2.35,;1.85,-3.13,;4.52,-3.12,;4.52,-4.66,;5.85,-2.35,;7.19,-3.13,;5.85,-.8,;-.81,-.03,;-2.14,.74,;-.81,-1.58,)| Show InChI InChI=1S/C13H23NO7/c1-3-6(2)9(11(18)19)14-12(20)13(21)4-7(15)10(17)8(16)5-13/h6-10,15-17,21H,3-5H2,1-2H3,(H,14,20)(H,18,19)/t6?,7-,8-,9+,10-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of HL-60 cell adhesion to recombinant human Selectin P |
Bioorg Med Chem Lett 15: 3224-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.004
BindingDB Entry DOI: 10.7270/Q23N22WB |
More data for this
Ligand-Target Pair | |
P-selectin
(Homo sapiens (Human)) | BDBM50168303
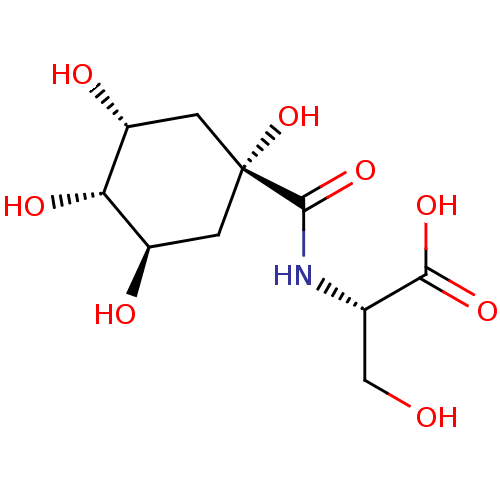
(3-Hydroxy-2-[((3R,5R)-1,3,5-trihydroxy-4-(S)-hydro...)Show SMILES OC[C@H](NC(=O)[C@@]1(O)C[C@@H](O)[C@H](O)[C@H](O)C1)C(O)=O |wU:6.6,11.11,13.13,wD:6.5,2.2,9.9,(1.73,1.74,;.4,.97,;.4,-.57,;1.73,-1.34,;3.06,-.57,;3.06,.97,;4.39,-1.34,;5.74,-.57,;3.06,-2.11,;3.06,-3.66,;1.73,-4.44,;4.39,-4.43,;4.39,-5.97,;5.74,-3.66,;7.08,-4.44,;5.74,-2.11,;-.93,-1.34,;-2.28,-.57,;-.93,-2.89,)| Show InChI InChI=1S/C10H17NO8/c12-3-4(8(16)17)11-9(18)10(19)1-5(13)7(15)6(14)2-10/h4-7,12-15,19H,1-3H2,(H,11,18)(H,16,17)/t4-,5+,6+,7-,10+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.95E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of HL-60 cell adhesion to recombinant human Selectin P |
Bioorg Med Chem Lett 15: 3224-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.004
BindingDB Entry DOI: 10.7270/Q23N22WB |
More data for this
Ligand-Target Pair | |
P-selectin
(Homo sapiens (Human)) | BDBM50168306

((S)-2-[((3R,5R)-1,3,4,5-Tetrahydroxy-cyclohexaneca...)Show SMILES O[C@@H]1C[C@@](O)(C[C@@H](O)[C@H]1O)C(=O)N[C@@H](CCC(O)=O)C(O)=O |wU:13.13,3.3,8.9,6.6,wD:3.10,1.0,(2.22,-3.74,;3.55,-2.96,;3.55,-1.41,;4.88,-.64,;6.23,.13,;6.23,-1.41,;6.23,-2.96,;7.57,-3.74,;4.88,-3.73,;4.88,-5.27,;3.55,.13,;3.55,1.67,;2.22,-.64,;.89,.13,;-.44,-.64,;-1.79,.13,;-3.12,-.64,;-4.46,.13,;-3.12,-2.19,;.89,1.67,;-.46,2.44,;2.22,2.44,)| Show InChI InChI=1S/C12H19NO9/c14-6-3-12(22,4-7(15)9(6)18)11(21)13-5(10(19)20)1-2-8(16)17/h5-7,9,14-15,18,22H,1-4H2,(H,13,21)(H,16,17)(H,19,20)/t5-,6+,7+,9-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of HL-60 cell adhesion to recombinant human Selectin P |
Bioorg Med Chem Lett 15: 3224-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.004
BindingDB Entry DOI: 10.7270/Q23N22WB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data