 Found 115 hits with Last Name = 'schulte' and Initial = 'ca'
Found 115 hits with Last Name = 'schulte' and Initial = 'ca' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576323
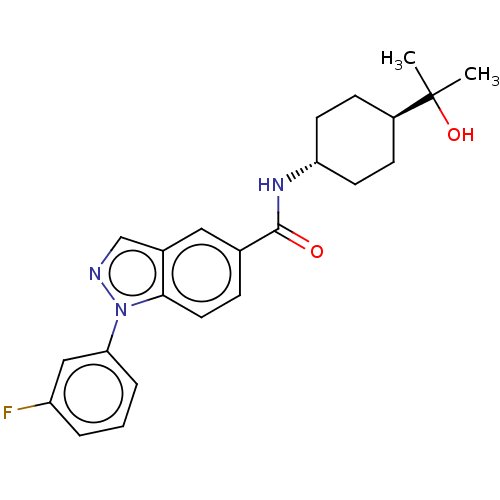
(CHEMBL4866146)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1ccc2n(ncc2c1)-c1cccc(F)c1 |r,wU:7.10,wD:4.3,(17.72,-11.38,;16.96,-12.72,;18.5,-12.71,;16.96,-14.26,;15.63,-11.95,;15.63,-10.41,;14.29,-9.63,;12.97,-10.41,;12.97,-11.95,;14.29,-12.71,;11.64,-9.64,;10.3,-10.41,;8.97,-9.65,;10.31,-11.95,;11.65,-12.72,;11.65,-14.27,;10.31,-15.04,;10,-16.55,;8.46,-16.72,;7.83,-15.31,;8.97,-14.27,;8.98,-12.72,;11.03,-17.69,;10.55,-19.15,;11.59,-20.29,;13.09,-19.97,;13.56,-18.5,;15.07,-18.17,;12.53,-17.36,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576316
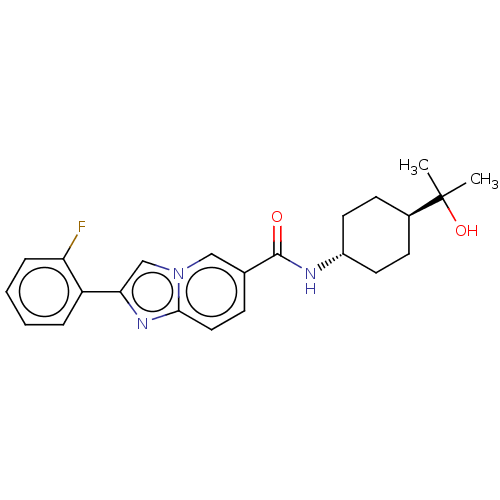
(CHEMBL4870703)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1ccc2nc(cn2c1)-c1ccccc1F |r,wU:7.10,wD:4.3,(2.16,-11.57,;2.98,-12.88,;3.7,-11.51,;1.68,-13.7,;4.35,-13.61,;4.4,-15.15,;5.77,-15.87,;7.07,-15.05,;7.01,-13.51,;5.65,-12.79,;8.44,-15.77,;9.74,-14.95,;9.68,-13.41,;11.1,-15.66,;11.16,-17.2,;12.51,-17.92,;13.81,-17.1,;15.29,-17.52,;16.15,-16.25,;15.2,-15.04,;13.76,-15.57,;12.4,-14.84,;17.68,-16.19,;18.5,-17.5,;20.03,-17.44,;20.76,-16.08,;19.93,-14.77,;18.39,-14.83,;17.57,-13.53,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576314
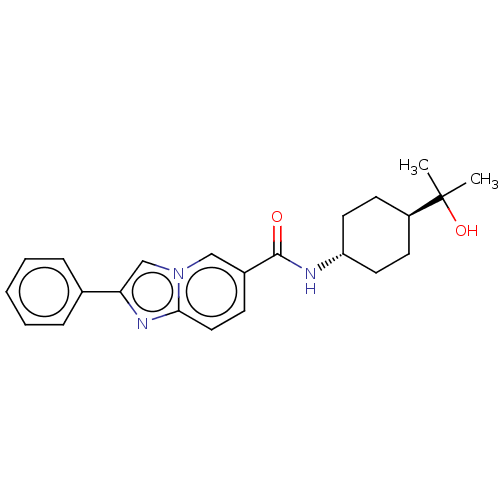
(CHEMBL4864805)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1ccc2nc(cn2c1)-c1ccccc1 |r,wU:7.10,wD:4.3,(2.48,-1.86,;3.3,-3.17,;4.02,-1.8,;2,-3.99,;4.67,-3.9,;4.72,-5.44,;6.09,-6.16,;7.39,-5.34,;7.33,-3.8,;5.97,-3.08,;8.76,-6.06,;10.06,-5.24,;10,-3.7,;11.42,-5.96,;11.48,-7.5,;12.83,-8.21,;14.13,-7.39,;15.61,-7.81,;16.46,-6.54,;15.52,-5.33,;14.08,-5.86,;12.72,-5.13,;18,-6.48,;18.81,-7.79,;20.35,-7.73,;21.08,-6.37,;20.25,-5.06,;18.71,-5.12,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576324
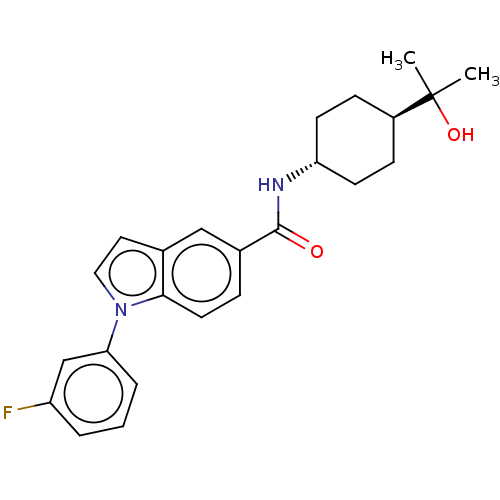
(CHEMBL4868339)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1ccc2n(ccc2c1)-c1cccc(F)c1 |r,wU:7.10,wD:4.3,(32.28,-11.78,;31.52,-13.11,;33.06,-13.1,;31.53,-14.65,;30.2,-12.34,;30.2,-10.8,;28.86,-10.03,;27.54,-10.8,;27.53,-12.34,;28.86,-13.11,;26.2,-10.04,;24.87,-10.81,;23.54,-10.04,;24.88,-12.35,;26.21,-13.11,;26.22,-14.66,;24.88,-15.44,;24.56,-16.95,;23.03,-17.12,;22.39,-15.7,;23.54,-14.67,;23.55,-13.12,;25.6,-18.09,;25.12,-19.55,;26.15,-20.69,;27.66,-20.37,;28.13,-18.89,;29.63,-18.56,;27.09,-17.76,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576322
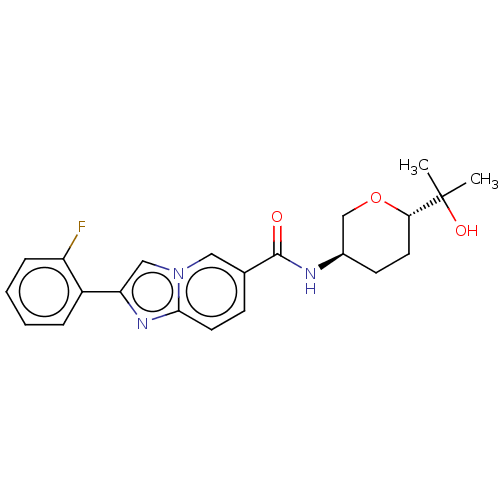
(CHEMBL4859922)Show SMILES CC(C)(O)[C@@H]1CC[C@H](CO1)NC(=O)c1ccc2nc(cn2c1)-c1ccccc1F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576315

(CHEMBL4873385)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1ccc2nc(cn2c1)-c1cccc(F)c1 |r,wU:7.10,wD:4.3,(23.28,-2.13,;24.11,-3.43,;24.83,-2.06,;22.81,-4.26,;25.48,-4.17,;25.53,-5.7,;26.9,-6.43,;28.2,-5.61,;28.14,-4.06,;26.78,-3.35,;29.56,-6.33,;30.87,-5.5,;30.81,-3.96,;32.23,-6.22,;32.29,-7.76,;33.64,-8.47,;34.94,-7.66,;36.42,-8.08,;37.27,-6.8,;36.33,-5.6,;34.89,-6.12,;33.53,-5.4,;38.81,-6.75,;39.62,-8.05,;41.16,-8,;41.88,-6.64,;41.06,-5.33,;41.77,-3.96,;39.52,-5.39,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576330
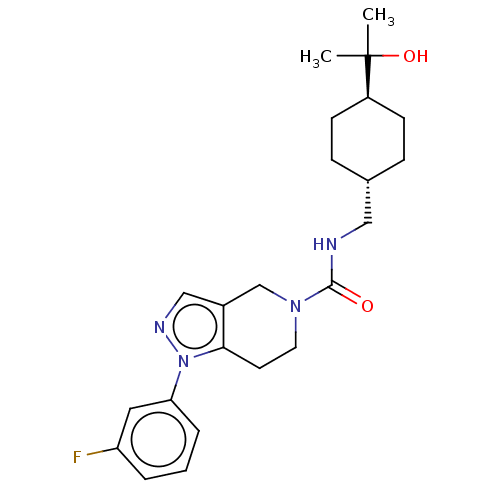
(CHEMBL4874001)Show SMILES CC(C)(O)[C@H]1CC[C@H](CNC(=O)N2CCc3c(C2)cnn3-c2cccc(F)c2)CC1 |r,wU:7.7,wD:4.3,(23.24,-24.76,;24.57,-25.53,;25.37,-24.22,;23.23,-26.3,;25.32,-26.89,;24.52,-28.21,;25.27,-29.56,;26.8,-29.58,;27.55,-30.92,;29.09,-30.95,;29.84,-32.3,;29.04,-33.62,;31.38,-32.32,;32.12,-33.66,;33.66,-33.69,;34.46,-32.37,;33.7,-31.03,;32.16,-31.01,;34.74,-29.9,;36.14,-30.54,;35.97,-32.07,;37.1,-33.11,;36.76,-34.61,;37.9,-35.65,;39.37,-35.19,;39.7,-33.67,;41.19,-33.28,;38.56,-32.64,;27.6,-28.26,;26.86,-26.92,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576320
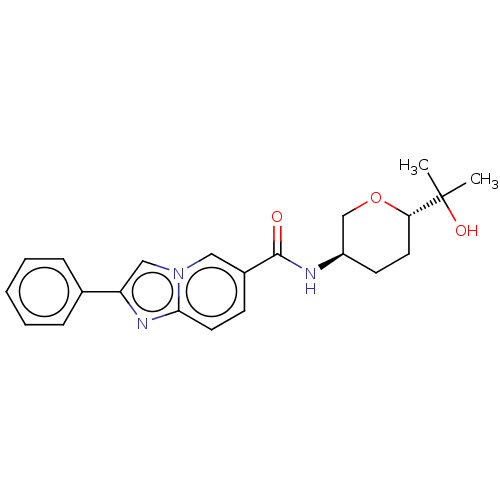
(CHEMBL4847268)Show SMILES CC(C)(O)[C@@H]1CC[C@H](CO1)NC(=O)c1ccc2nc(cn2c1)-c1ccccc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576331
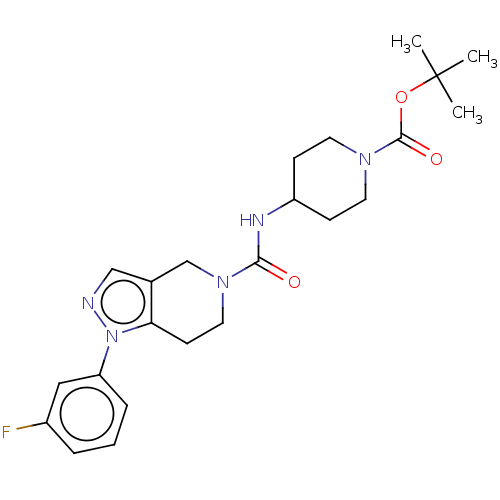
(CHEMBL4853151)Show SMILES CC(C)(C)OC(=O)N1CCC(CC1)NC(=O)N1CCc2c(C1)cnn2-c1cccc(F)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576321
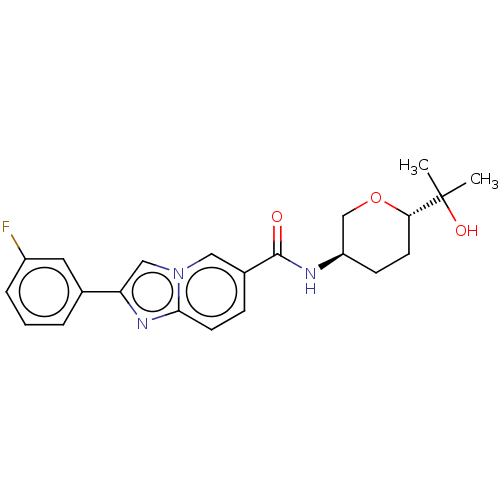
(CHEMBL4872589)Show SMILES CC(C)(O)[C@@H]1CC[C@H](CO1)NC(=O)c1ccc2nc(cn2c1)-c1cccc(F)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576325
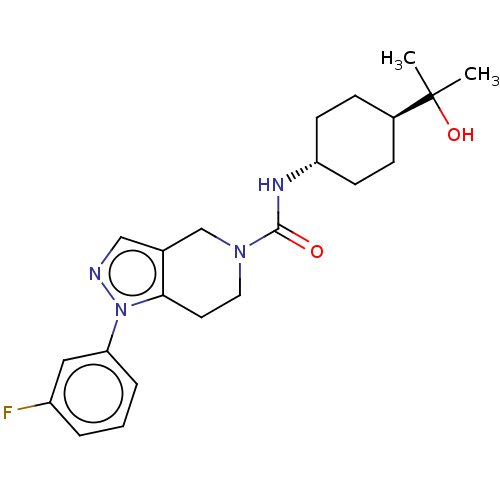
(CHEMBL4852189)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)N1CCc2c(C1)cnn2-c1cccc(F)c1 |r,wU:7.10,wD:4.3,(4.33,-9.7,;4.34,-8.16,;3,-8.92,;3.6,-6.81,;5.88,-8.2,;6.63,-9.55,;8.17,-9.58,;8.96,-8.26,;8.21,-6.91,;6.68,-6.88,;10.5,-8.28,;11.25,-9.63,;10.46,-10.95,;12.79,-9.66,;13.53,-11,;15.07,-11.02,;15.87,-9.71,;15.11,-8.37,;13.57,-8.34,;16.15,-7.24,;17.55,-7.87,;17.38,-9.4,;18.51,-10.44,;18.17,-11.94,;19.31,-12.98,;20.78,-12.52,;21.11,-11.01,;22.58,-10.54,;19.97,-9.98,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Mus musculus) | BDBM50111203

(CHEMBL3604735)Show SMILES Cc1cc(NCc2c(Cl)ccc(Cl)c2Cl)c2cccc(C(N)=O)c2n1 Show InChI InChI=1S/C18H14Cl3N3O/c1-9-7-15(10-3-2-4-11(18(22)25)17(10)24-9)23-8-12-13(19)5-6-14(20)16(12)21/h2-7H,8H2,1H3,(H2,22,25)(H,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of mouse CD38 |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576332
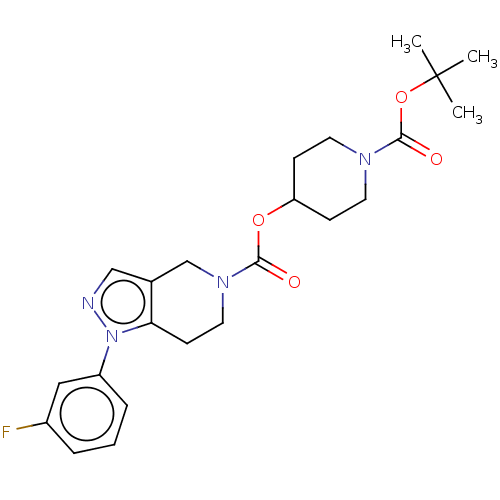
(CHEMBL4849594)Show SMILES CC(C)(C)OC(=O)N1CCC(CC1)OC(=O)N1CCc2c(C1)cnn2-c1cccc(F)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576319
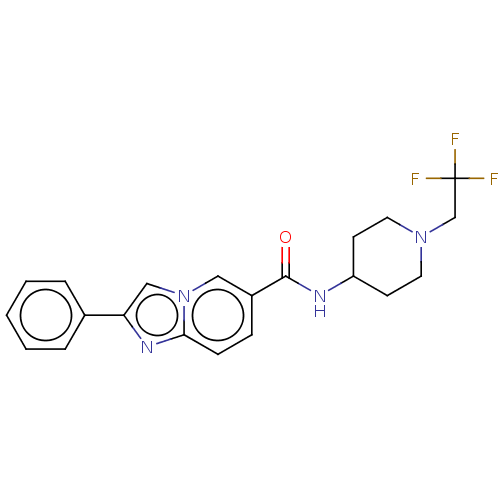
(CHEMBL4868541)Show SMILES FC(F)(F)CN1CCC(CC1)NC(=O)c1ccc2nc(cn2c1)-c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50111135
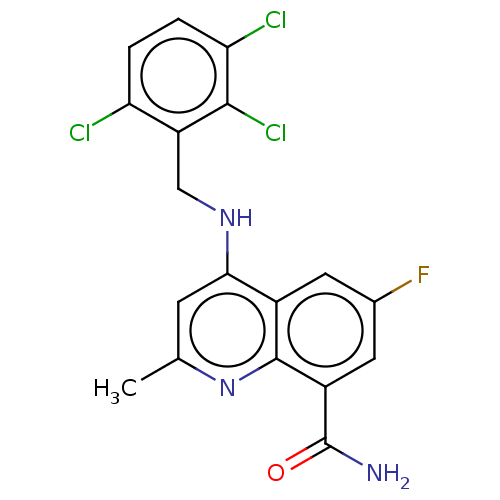
(CHEMBL3604758)Show SMILES Cc1cc(NCc2c(Cl)ccc(Cl)c2Cl)c2cc(F)cc(C(N)=O)c2n1 Show InChI InChI=1S/C18H13Cl3FN3O/c1-8-4-15(24-7-12-13(19)2-3-14(20)16(12)21)10-5-9(22)6-11(18(23)26)17(10)25-8/h2-6H,7H2,1H3,(H2,23,26)(H,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CD38 extracellular domain expressed in Pichia pastoris using CHAPS and NAD by colorimetric-based assay |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hematopoietic prostaglandin D synthase
(Rattus norvegicus) | BDBM50576319

(CHEMBL4868541)Show SMILES FC(F)(F)CN1CCC(CC1)NC(=O)c1ccc2nc(cn2c1)-c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPGDS in rat RBL cells assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Rattus norvegicus) | BDBM50576314

(CHEMBL4864805)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1ccc2nc(cn2c1)-c1ccccc1 |r,wU:7.10,wD:4.3,(2.48,-1.86,;3.3,-3.17,;4.02,-1.8,;2,-3.99,;4.67,-3.9,;4.72,-5.44,;6.09,-6.16,;7.39,-5.34,;7.33,-3.8,;5.97,-3.08,;8.76,-6.06,;10.06,-5.24,;10,-3.7,;11.42,-5.96,;11.48,-7.5,;12.83,-8.21,;14.13,-7.39,;15.61,-7.81,;16.46,-6.54,;15.52,-5.33,;14.08,-5.86,;12.72,-5.13,;18,-6.48,;18.81,-7.79,;20.35,-7.73,;21.08,-6.37,;20.25,-5.06,;18.71,-5.12,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPGDS in rat RBL cells assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Mus musculus) | BDBM50111218
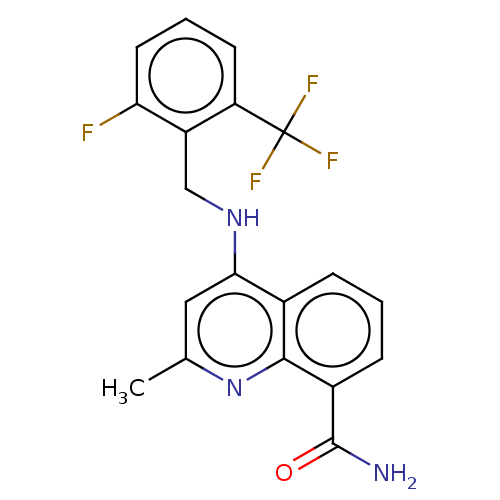
(CHEMBL3604734)Show SMILES Cc1cc(NCc2c(F)cccc2C(F)(F)F)c2cccc(C(N)=O)c2n1 Show InChI InChI=1S/C19H15F4N3O/c1-10-8-16(11-4-2-5-12(18(24)27)17(11)26-10)25-9-13-14(19(21,22)23)6-3-7-15(13)20/h2-8H,9H2,1H3,(H2,24,27)(H,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of mouse CD38 |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Rattus norvegicus) | BDBM50576316

(CHEMBL4870703)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1ccc2nc(cn2c1)-c1ccccc1F |r,wU:7.10,wD:4.3,(2.16,-11.57,;2.98,-12.88,;3.7,-11.51,;1.68,-13.7,;4.35,-13.61,;4.4,-15.15,;5.77,-15.87,;7.07,-15.05,;7.01,-13.51,;5.65,-12.79,;8.44,-15.77,;9.74,-14.95,;9.68,-13.41,;11.1,-15.66,;11.16,-17.2,;12.51,-17.92,;13.81,-17.1,;15.29,-17.52,;16.15,-16.25,;15.2,-15.04,;13.76,-15.57,;12.4,-14.84,;17.68,-16.19,;18.5,-17.5,;20.03,-17.44,;20.76,-16.08,;19.93,-14.77,;18.39,-14.83,;17.57,-13.53,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPGDS in rat RBL cells assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50111158
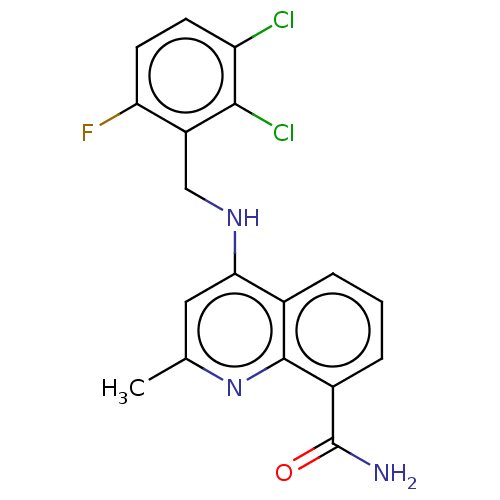
(CHEMBL3604739)Show SMILES Cc1cc(NCc2c(F)ccc(Cl)c2Cl)c2cccc(C(N)=O)c2n1 Show InChI InChI=1S/C18H14Cl2FN3O/c1-9-7-15(10-3-2-4-11(18(22)25)17(10)24-9)23-8-12-14(21)6-5-13(19)16(12)20/h2-7H,8H2,1H3,(H2,22,25)(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CD38 extracellular domain expressed in Pichia pastoris using CHAPS and NAD by colorimetric-based assay |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50111203

(CHEMBL3604735)Show SMILES Cc1cc(NCc2c(Cl)ccc(Cl)c2Cl)c2cccc(C(N)=O)c2n1 Show InChI InChI=1S/C18H14Cl3N3O/c1-9-7-15(10-3-2-4-11(18(22)25)17(10)24-9)23-8-12-13(19)5-6-14(20)16(12)21/h2-7H,8H2,1H3,(H2,22,25)(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CD38 extracellular domain expressed in Pichia pastoris using CHAPS and NAD by colorimetric-based assay |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Rattus norvegicus) | BDBM50576315

(CHEMBL4873385)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1ccc2nc(cn2c1)-c1cccc(F)c1 |r,wU:7.10,wD:4.3,(23.28,-2.13,;24.11,-3.43,;24.83,-2.06,;22.81,-4.26,;25.48,-4.17,;25.53,-5.7,;26.9,-6.43,;28.2,-5.61,;28.14,-4.06,;26.78,-3.35,;29.56,-6.33,;30.87,-5.5,;30.81,-3.96,;32.23,-6.22,;32.29,-7.76,;33.64,-8.47,;34.94,-7.66,;36.42,-8.08,;37.27,-6.8,;36.33,-5.6,;34.89,-6.12,;33.53,-5.4,;38.81,-6.75,;39.62,-8.05,;41.16,-8,;41.88,-6.64,;41.06,-5.33,;41.77,-3.96,;39.52,-5.39,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPGDS in rat RBL cells assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576328
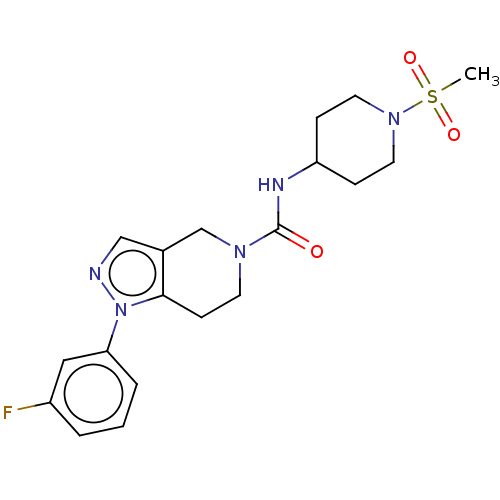
(CHEMBL4864256)Show SMILES CS(=O)(=O)N1CCC(CC1)NC(=O)N1CCc2c(C1)cnn2-c1cccc(F)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50111177

(CHEMBL3604737)Show SMILES Cc1cc(NCc2c(F)ccc(F)c2Cl)c2cccc(C(N)=O)c2n1 Show InChI InChI=1S/C18H14ClF2N3O/c1-9-7-15(10-3-2-4-11(18(22)25)17(10)24-9)23-8-12-13(20)5-6-14(21)16(12)19/h2-7H,8H2,1H3,(H2,22,25)(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CD38 extracellular domain expressed in Pichia pastoris using CHAPS and NAD by colorimetric-based assay |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Rattus norvegicus) | BDBM50576322

(CHEMBL4859922)Show SMILES CC(C)(O)[C@@H]1CC[C@H](CO1)NC(=O)c1ccc2nc(cn2c1)-c1ccccc1F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPGDS in rat RBL cells assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576329
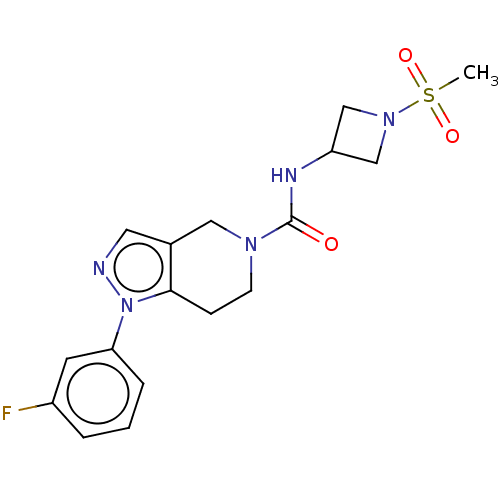
(CHEMBL4859293)Show SMILES CS(=O)(=O)N1CC(C1)NC(=O)N1CCc2c(C1)cnn2-c1cccc(F)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576317
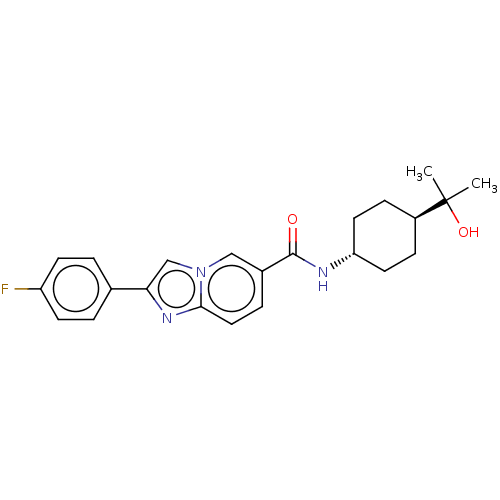
(CHEMBL4849005)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1ccc2nc(cn2c1)-c1ccc(F)cc1 |r,wU:7.10,wD:4.3,(24.97,-11.42,;25.8,-12.73,;26.52,-11.36,;24.5,-13.55,;27.17,-13.46,;27.22,-15,;28.58,-15.72,;29.89,-14.9,;29.83,-13.36,;28.47,-12.64,;31.25,-15.62,;32.55,-14.8,;32.49,-13.26,;33.92,-15.52,;33.97,-17.06,;35.33,-17.77,;36.63,-16.95,;38.1,-17.37,;38.96,-16.1,;38.01,-14.89,;36.57,-15.42,;35.22,-14.69,;40.5,-16.04,;41.31,-17.35,;42.85,-17.29,;43.57,-15.93,;45.11,-15.87,;42.74,-14.62,;41.21,-14.69,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50111153
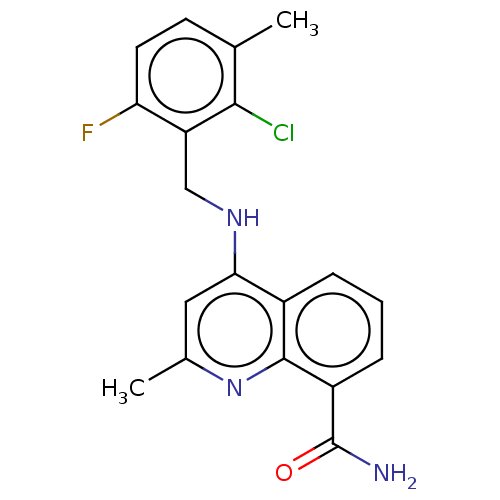
(CHEMBL3604740)Show SMILES Cc1cc(NCc2c(F)ccc(C)c2Cl)c2cccc(C(N)=O)c2n1 Show InChI InChI=1S/C19H17ClFN3O/c1-10-6-7-15(21)14(17(10)20)9-23-16-8-11(2)24-18-12(16)4-3-5-13(18)19(22)25/h3-8H,9H2,1-2H3,(H2,22,25)(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CD38 extracellular domain expressed in Pichia pastoris using CHAPS and NAD by colorimetric-based assay |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Rattus norvegicus) | BDBM50576323

(CHEMBL4866146)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1ccc2n(ncc2c1)-c1cccc(F)c1 |r,wU:7.10,wD:4.3,(17.72,-11.38,;16.96,-12.72,;18.5,-12.71,;16.96,-14.26,;15.63,-11.95,;15.63,-10.41,;14.29,-9.63,;12.97,-10.41,;12.97,-11.95,;14.29,-12.71,;11.64,-9.64,;10.3,-10.41,;8.97,-9.65,;10.31,-11.95,;11.65,-12.72,;11.65,-14.27,;10.31,-15.04,;10,-16.55,;8.46,-16.72,;7.83,-15.31,;8.97,-14.27,;8.98,-12.72,;11.03,-17.69,;10.55,-19.15,;11.59,-20.29,;13.09,-19.97,;13.56,-18.5,;15.07,-18.17,;12.53,-17.36,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPGDS in rat RBL cells assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576326
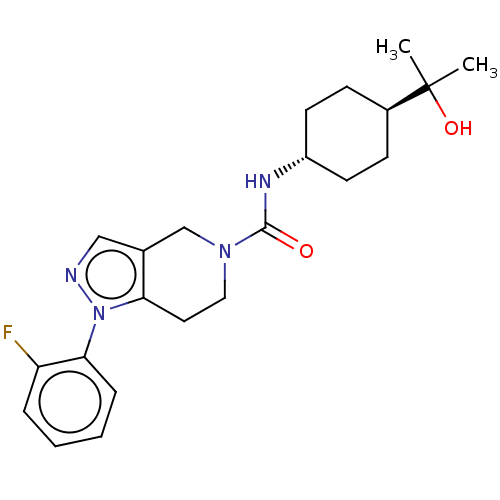
(CHEMBL4878511)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)N1CCc2c(C1)cnn2-c1ccccc1F |r,wU:7.10,wD:4.3,(24.54,-7.55,;24.55,-6,;23.21,-6.77,;23.81,-4.66,;26.09,-6.04,;26.84,-7.39,;28.38,-7.42,;29.17,-6.1,;28.42,-4.75,;26.89,-4.72,;30.71,-6.13,;31.46,-7.48,;30.66,-8.8,;33,-7.5,;33.74,-8.84,;35.28,-8.87,;36.08,-7.55,;35.32,-6.21,;33.78,-6.19,;36.36,-5.08,;37.76,-5.72,;37.58,-7.25,;38.72,-8.29,;38.38,-9.79,;39.52,-10.83,;40.99,-10.37,;41.32,-8.85,;40.18,-7.82,;40.51,-6.32,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50111218

(CHEMBL3604734)Show SMILES Cc1cc(NCc2c(F)cccc2C(F)(F)F)c2cccc(C(N)=O)c2n1 Show InChI InChI=1S/C19H15F4N3O/c1-10-8-16(11-4-2-5-12(18(24)27)17(11)26-10)25-9-13-14(19(21,22)23)6-3-7-15(13)20/h2-8H,9H2,1H3,(H2,24,27)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Rattus norvegicus) | BDBM50576320

(CHEMBL4847268)Show SMILES CC(C)(O)[C@@H]1CC[C@H](CO1)NC(=O)c1ccc2nc(cn2c1)-c1ccccc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPGDS in rat RBL cells assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
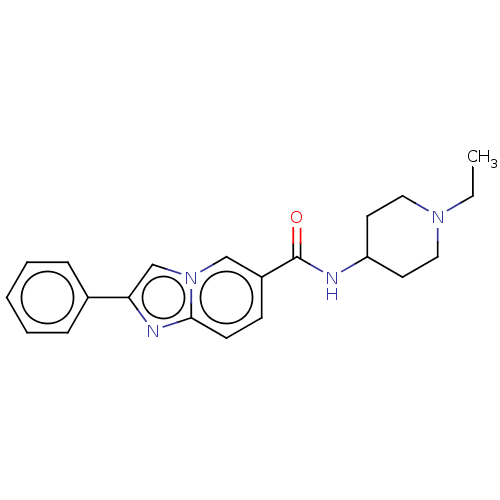
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50110915
(CHEMBL3604701)Show InChI InChI=1S/C20H21N3O/c1-12-6-4-7-13(2)17(12)11-22-18-10-14(3)23-19-15(18)8-5-9-16(19)20(21)24/h4-10H,11H2,1-3H3,(H2,21,24)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50111203

(CHEMBL3604735)Show SMILES Cc1cc(NCc2c(Cl)ccc(Cl)c2Cl)c2cccc(C(N)=O)c2n1 Show InChI InChI=1S/C18H14Cl3N3O/c1-9-7-15(10-3-2-4-11(18(22)25)17(10)24-9)23-8-12-13(19)5-6-14(20)16(12)21/h2-7H,8H2,1H3,(H2,22,25)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Rattus norvegicus) | BDBM50576325

(CHEMBL4852189)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)N1CCc2c(C1)cnn2-c1cccc(F)c1 |r,wU:7.10,wD:4.3,(4.33,-9.7,;4.34,-8.16,;3,-8.92,;3.6,-6.81,;5.88,-8.2,;6.63,-9.55,;8.17,-9.58,;8.96,-8.26,;8.21,-6.91,;6.68,-6.88,;10.5,-8.28,;11.25,-9.63,;10.46,-10.95,;12.79,-9.66,;13.53,-11,;15.07,-11.02,;15.87,-9.71,;15.11,-8.37,;13.57,-8.34,;16.15,-7.24,;17.55,-7.87,;17.38,-9.4,;18.51,-10.44,;18.17,-11.94,;19.31,-12.98,;20.78,-12.52,;21.11,-11.01,;22.58,-10.54,;19.97,-9.98,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPGDS in rat RBL cells assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50111218

(CHEMBL3604734)Show SMILES Cc1cc(NCc2c(F)cccc2C(F)(F)F)c2cccc(C(N)=O)c2n1 Show InChI InChI=1S/C19H15F4N3O/c1-10-8-16(11-4-2-5-12(18(24)27)17(11)26-10)25-9-13-14(19(21,22)23)6-3-7-15(13)20/h2-8H,9H2,1H3,(H2,24,27)(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CD38 extracellular domain expressed in Pichia pastoris using CHAPS and NAD by colorimetric-based assay |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50111011
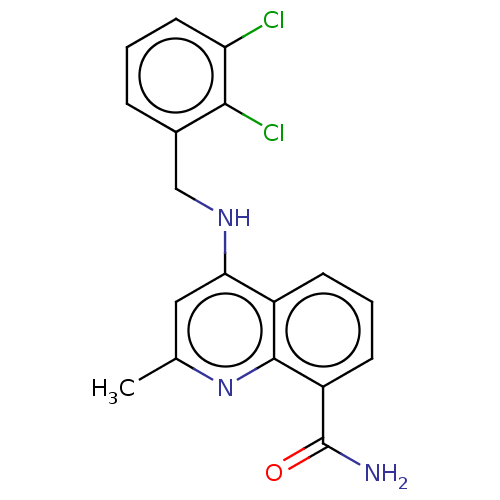
(CHEMBL3604717)Show InChI InChI=1S/C18H15Cl2N3O/c1-10-8-15(22-9-11-4-2-7-14(19)16(11)20)12-5-3-6-13(18(21)24)17(12)23-10/h2-8H,9H2,1H3,(H2,21,24)(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CD38 extracellular domain expressed in Pichia pastoris using CHAPS and NAD by colorimetric-based assay |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Rattus norvegicus) | BDBM50576321

(CHEMBL4872589)Show SMILES CC(C)(O)[C@@H]1CC[C@H](CO1)NC(=O)c1ccc2nc(cn2c1)-c1cccc(F)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPGDS in rat RBL cells assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Rattus norvegicus) | BDBM50576324

(CHEMBL4868339)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1ccc2n(ccc2c1)-c1cccc(F)c1 |r,wU:7.10,wD:4.3,(32.28,-11.78,;31.52,-13.11,;33.06,-13.1,;31.53,-14.65,;30.2,-12.34,;30.2,-10.8,;28.86,-10.03,;27.54,-10.8,;27.53,-12.34,;28.86,-13.11,;26.2,-10.04,;24.87,-10.81,;23.54,-10.04,;24.88,-12.35,;26.21,-13.11,;26.22,-14.66,;24.88,-15.44,;24.56,-16.95,;23.03,-17.12,;22.39,-15.7,;23.54,-14.67,;23.55,-13.12,;25.6,-18.09,;25.12,-19.55,;26.15,-20.69,;27.66,-20.37,;28.13,-18.89,;29.63,-18.56,;27.09,-17.76,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPGDS in rat RBL cells assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50111263
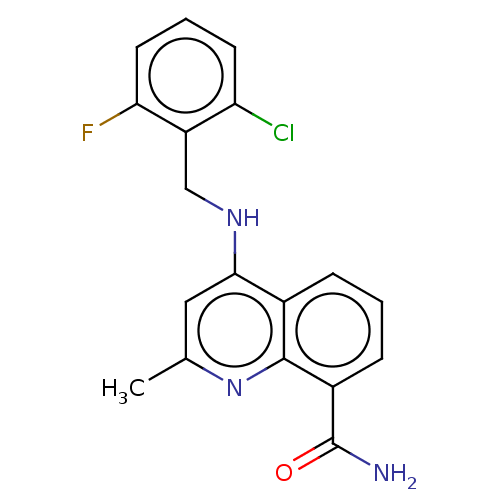
(CHEMBL3604732)Show InChI InChI=1S/C18H15ClFN3O/c1-10-8-16(22-9-13-14(19)6-3-7-15(13)20)11-4-2-5-12(18(21)24)17(11)23-10/h2-8H,9H2,1H3,(H2,21,24)(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CD38 extracellular domain expressed in Pichia pastoris using CHAPS and NAD by colorimetric-based assay |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50110922

(CHEMBL3604722)Show InChI InChI=1S/C18H15Cl2N3O/c1-10-8-16(22-9-13-14(19)6-3-7-15(13)20)11-4-2-5-12(18(21)24)17(11)23-10/h2-8H,9H2,1H3,(H2,21,24)(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CD38 extracellular domain expressed in Pichia pastoris using CHAPS and NAD by colorimetric-based assay |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | |
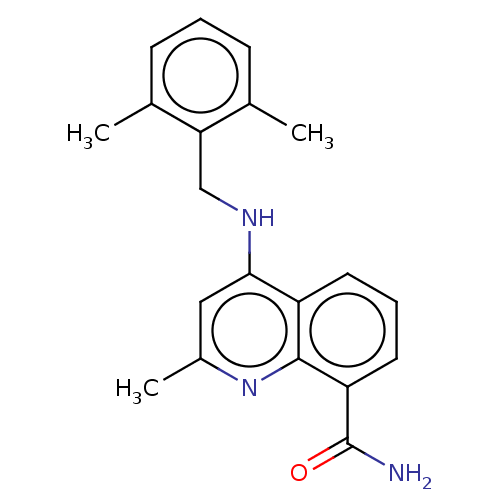
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576318
(CHEMBL4868975) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50111262

(CHEMBL3604733)Show InChI InChI=1S/C19H18ClN3O/c1-11-5-3-8-16(20)15(11)10-22-17-9-12(2)23-18-13(17)6-4-7-14(18)19(21)24/h3-9H,10H2,1-2H3,(H2,21,24)(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CD38 extracellular domain expressed in Pichia pastoris using CHAPS and NAD by colorimetric-based assay |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576327
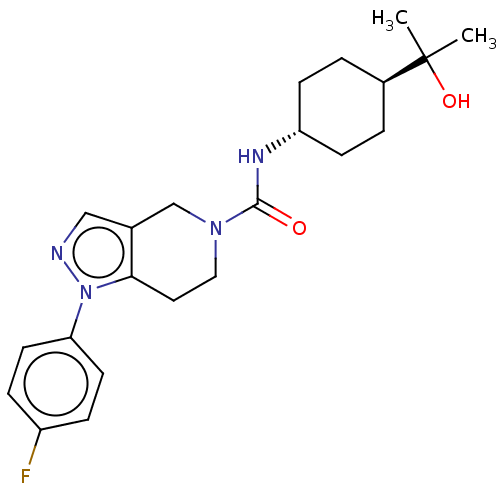
(CHEMBL4851328)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)N1CCc2c(C1)cnn2-c1ccc(F)cc1 |r,wU:7.10,wD:4.3,(1.68,-20.07,;1.69,-18.53,;.35,-19.29,;.95,-17.18,;3.23,-18.56,;3.97,-19.91,;5.52,-19.95,;6.31,-18.63,;5.56,-17.28,;4.03,-17.25,;7.85,-18.65,;8.6,-20,;7.8,-21.32,;10.14,-20.03,;10.88,-21.37,;12.42,-21.39,;13.22,-20.08,;12.46,-18.74,;10.92,-18.71,;13.5,-17.6,;14.9,-18.24,;14.72,-19.77,;15.86,-20.81,;15.52,-22.31,;16.66,-23.35,;18.13,-22.89,;19.26,-23.93,;18.46,-21.38,;17.32,-20.34,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Rattus norvegicus) | BDBM50576317

(CHEMBL4849005)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1ccc2nc(cn2c1)-c1ccc(F)cc1 |r,wU:7.10,wD:4.3,(24.97,-11.42,;25.8,-12.73,;26.52,-11.36,;24.5,-13.55,;27.17,-13.46,;27.22,-15,;28.58,-15.72,;29.89,-14.9,;29.83,-13.36,;28.47,-12.64,;31.25,-15.62,;32.55,-14.8,;32.49,-13.26,;33.92,-15.52,;33.97,-17.06,;35.33,-17.77,;36.63,-16.95,;38.1,-17.37,;38.96,-16.1,;38.01,-14.89,;36.57,-15.42,;35.22,-14.69,;40.5,-16.04,;41.31,-17.35,;42.85,-17.29,;43.57,-15.93,;45.11,-15.87,;42.74,-14.62,;41.21,-14.69,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPGDS in rat RBL cells assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50111149
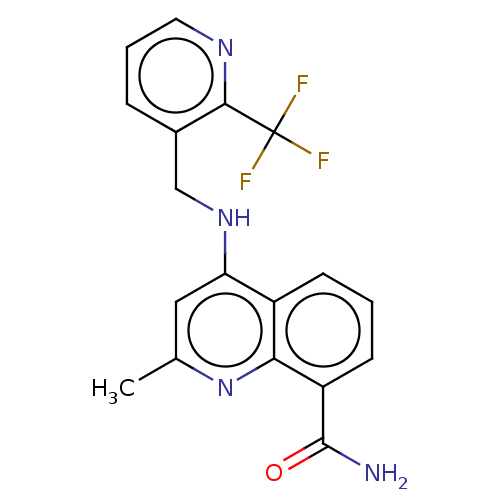
(CHEMBL3604744)Show SMILES Cc1cc(NCc2cccnc2C(F)(F)F)c2cccc(C(N)=O)c2n1 Show InChI InChI=1S/C18H15F3N4O/c1-10-8-14(12-5-2-6-13(17(22)26)15(12)25-10)24-9-11-4-3-7-23-16(11)18(19,20)21/h2-8H,9H2,1H3,(H2,22,26)(H,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CD38 extracellular domain expressed in Pichia pastoris using CHAPS and NAD by colorimetric-based assay |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50111014
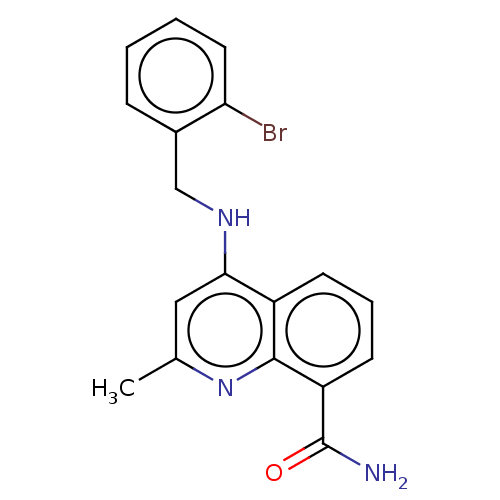
(CHEMBL3604714)Show InChI InChI=1S/C18H16BrN3O/c1-11-9-16(21-10-12-5-2-3-8-15(12)19)13-6-4-7-14(18(20)23)17(13)22-11/h2-9H,10H2,1H3,(H2,20,23)(H,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CD38 extracellular domain expressed in Pichia pastoris using CHAPS and NAD by colorimetric-based assay |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50111140
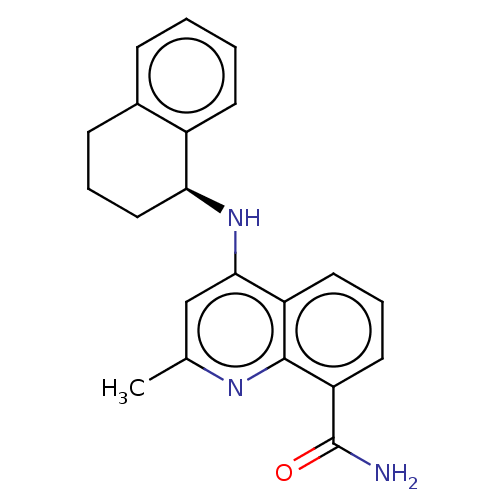
(CHEMBL3604753)Show SMILES Cc1cc(N[C@H]2CCCc3ccccc23)c2cccc(C(N)=O)c2n1 |r| Show InChI InChI=1S/C21H21N3O/c1-13-12-19(16-9-5-10-17(21(22)25)20(16)23-13)24-18-11-4-7-14-6-2-3-8-15(14)18/h2-3,5-6,8-10,12,18H,4,7,11H2,1H3,(H2,22,25)(H,23,24)/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CD38 extracellular domain expressed in Pichia pastoris using CHAPS and NAD by colorimetric-based assay |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576333
(CHEMBL4853862) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS by fluorescence polarization assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50111015
(CHEMBL3604713)Show SMILES Cc1cc(NCc2ccccc2C(F)(F)F)c2cccc(C(N)=O)c2n1 Show InChI InChI=1S/C19H16F3N3O/c1-11-9-16(13-6-4-7-14(18(23)26)17(13)25-11)24-10-12-5-2-3-8-15(12)19(20,21)22/h2-9H,10H2,1H3,(H2,23,26)(H,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CD38 extracellular domain expressed in Pichia pastoris using CHAPS and NAD by colorimetric-based assay |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data