 Found 2135 hits with Last Name = 'seal' and Initial = 'j'
Found 2135 hits with Last Name = 'seal' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475465
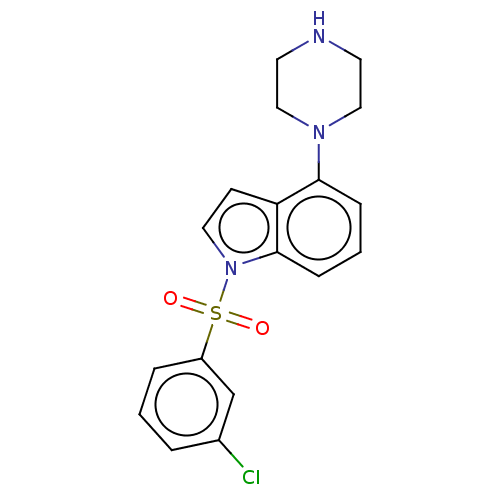
(CHEMBL196410)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C18H18ClN3O2S/c19-14-3-1-4-15(13-14)25(23,24)22-10-7-16-17(5-2-6-18(16)22)21-11-8-20-9-12-21/h1-7,10,13,20H,8-9,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475462
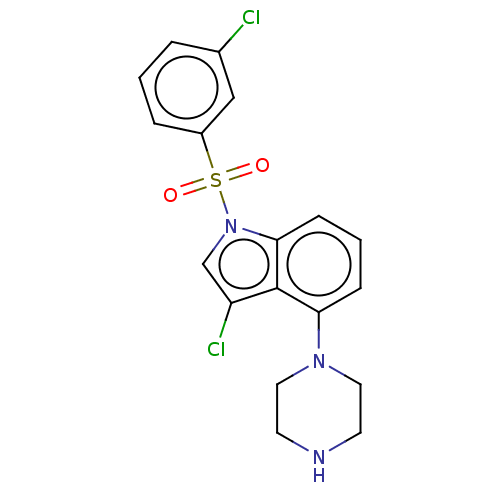
(CHEMBL371375)Show SMILES Clc1cn(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-3-1-4-14(11-13)26(24,25)23-12-15(20)18-16(5-2-6-17(18)23)22-9-7-21-8-10-22/h1-6,11-12,21H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475480
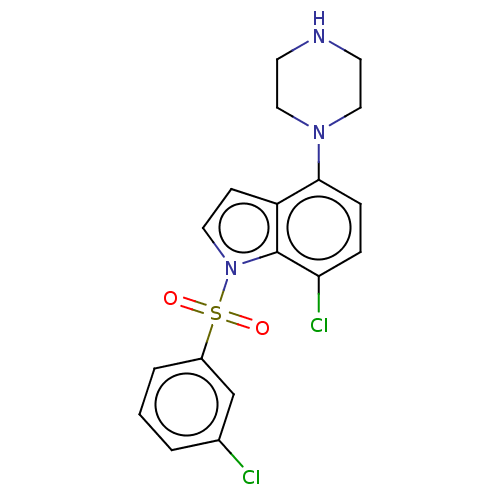
(CHEMBL193629)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(ccc(Cl)c12)N1CCNCC1 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-2-1-3-14(12-13)26(24,25)23-9-6-15-17(5-4-16(20)18(15)23)22-10-7-21-8-11-22/h1-6,9,12,21H,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475467

(CHEMBL425015)Show SMILES Clc1cccc(c1)S(=O)(=O)c1c[nH]c2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C18H18ClN3O2S/c19-13-3-1-4-14(11-13)25(23,24)17-12-21-18-15(17)5-2-6-16(18)22-9-7-20-8-10-22/h1-6,11-12,20-21H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50174269

(1-(phenylsulfonyl)-4-(piperazin-1-yl)-1H-indole | ...)Show InChI InChI=1S/C18H19N3O2S/c22-24(23,15-5-2-1-3-6-15)21-12-9-16-17(7-4-8-18(16)21)20-13-10-19-11-14-20/h1-9,12,19H,10-11,13-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475477
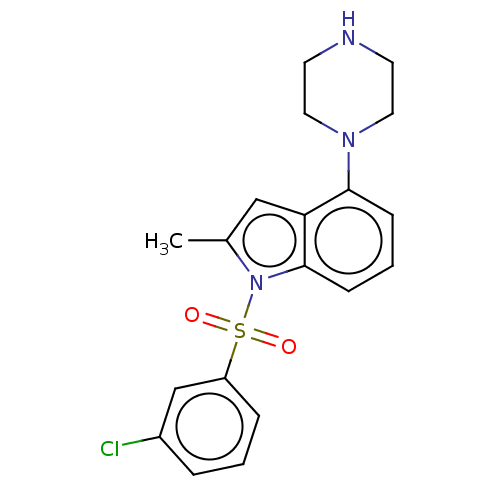
(CHEMBL372929)Show SMILES Cc1cc2c(cccc2n1S(=O)(=O)c1cccc(Cl)c1)N1CCNCC1 Show InChI InChI=1S/C19H20ClN3O2S/c1-14-12-17-18(22-10-8-21-9-11-22)6-3-7-19(17)23(14)26(24,25)16-5-2-4-15(20)13-16/h2-7,12-13,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475475
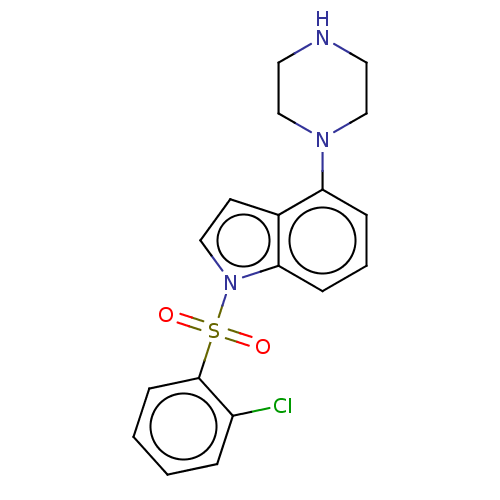
(CHEMBL372513)Show InChI InChI=1S/C18H18ClN3O2S/c19-15-4-1-2-7-18(15)25(23,24)22-11-8-14-16(5-3-6-17(14)22)21-12-9-20-10-13-21/h1-8,11,20H,9-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50044607
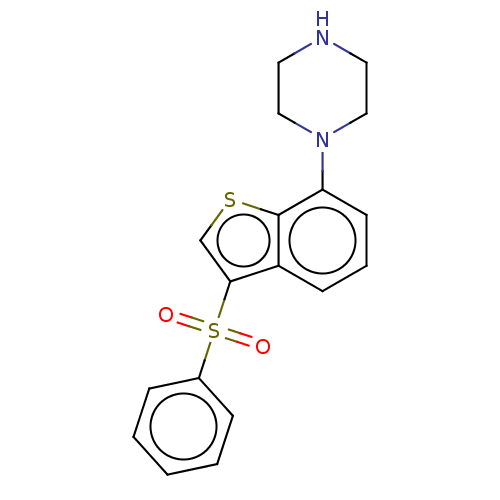
(CHEMBL372537)Show InChI InChI=1S/C18H18N2O2S2/c21-24(22,14-5-2-1-3-6-14)17-13-23-18-15(17)7-4-8-16(18)20-11-9-19-10-12-20/h1-8,13,19H,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475463
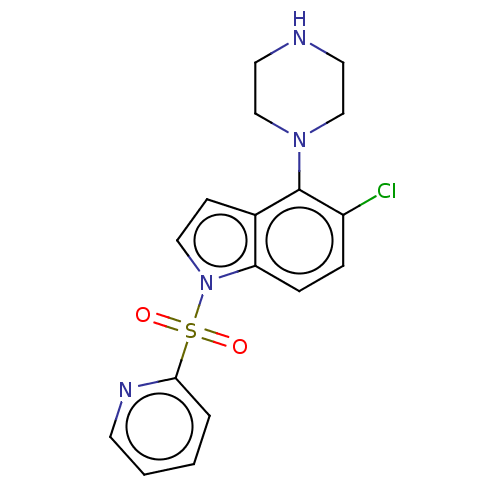
(CHEMBL194915)Show InChI InChI=1S/C17H17ClN4O2S/c18-14-4-5-15-13(17(14)21-11-8-19-9-12-21)6-10-22(15)25(23,24)16-3-1-2-7-20-16/h1-7,10,19H,8-9,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475473
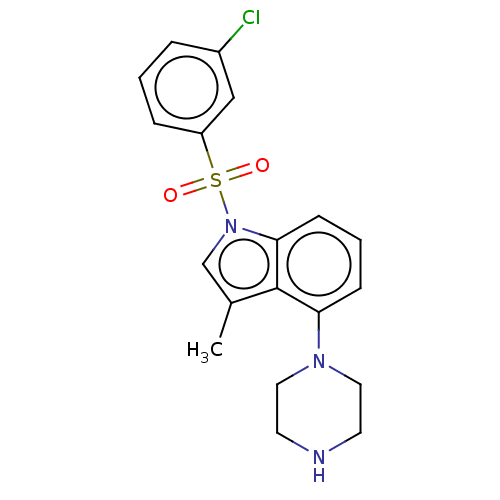
(CHEMBL194039)Show SMILES Cc1cn(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H20ClN3O2S/c1-14-13-23(26(24,25)16-5-2-4-15(20)12-16)18-7-3-6-17(19(14)18)22-10-8-21-9-11-22/h2-7,12-13,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475479
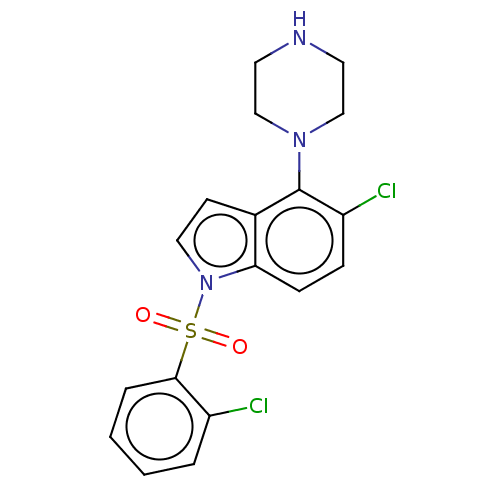
(CHEMBL371176)Show SMILES Clc1ccccc1S(=O)(=O)n1ccc2c(N3CCNCC3)c(Cl)ccc12 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-14-3-1-2-4-17(14)26(24,25)23-10-7-13-16(23)6-5-15(20)18(13)22-11-8-21-9-12-22/h1-7,10,21H,8-9,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475470

(CHEMBL370209)Show InChI InChI=1S/C17H18N4O2S/c22-24(23,17-6-1-2-8-19-17)21-11-7-14-15(4-3-5-16(14)21)20-12-9-18-10-13-20/h1-8,11,18H,9-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475464
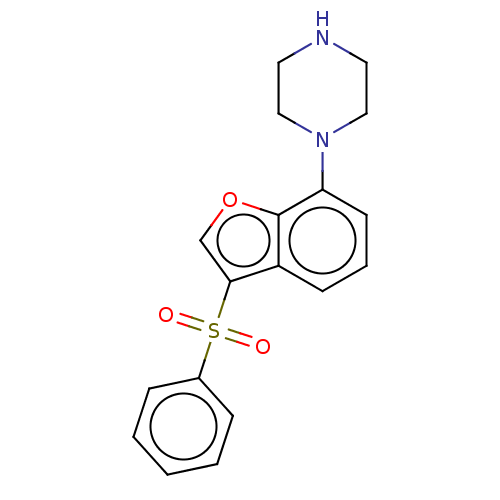
(CHEMBL197574)Show InChI InChI=1S/C18H18N2O3S/c21-24(22,14-5-2-1-3-6-14)17-13-23-18-15(17)7-4-8-16(18)20-11-9-19-10-12-20/h1-8,13,19H,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM28583
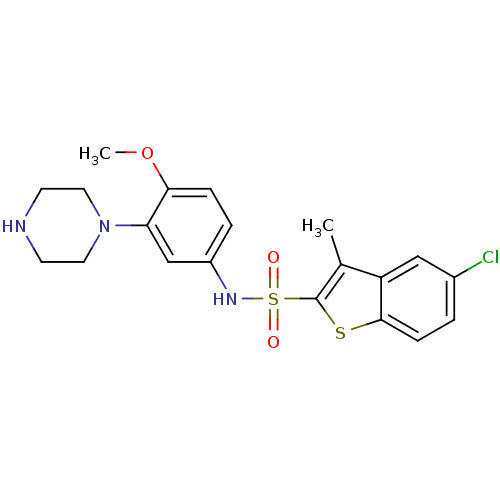
(5-chloro-N-[4-methoxy-3-(piperazin-1-yl)phenyl]-3-...)Show SMILES COc1ccc(NS(=O)(=O)c2sc3ccc(Cl)cc3c2C)cc1N1CCNCC1 Show InChI InChI=1S/C20H22ClN3O3S2/c1-13-16-11-14(21)3-6-19(16)28-20(13)29(25,26)23-15-4-5-18(27-2)17(12-15)24-9-7-22-8-10-24/h3-6,11-12,22-23H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475466
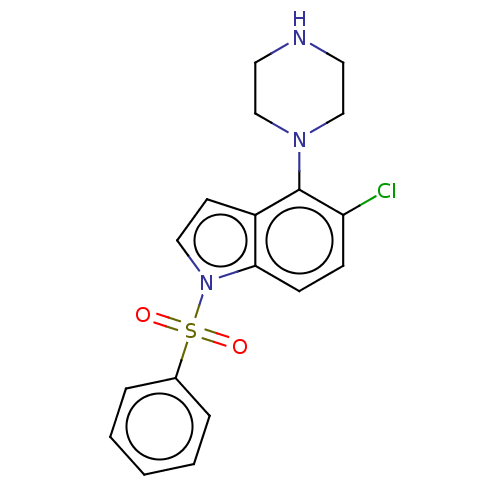
(CHEMBL193665)Show InChI InChI=1S/C18H18ClN3O2S/c19-16-6-7-17-15(18(16)21-12-9-20-10-13-21)8-11-22(17)25(23,24)14-4-2-1-3-5-14/h1-8,11,20H,9-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475471
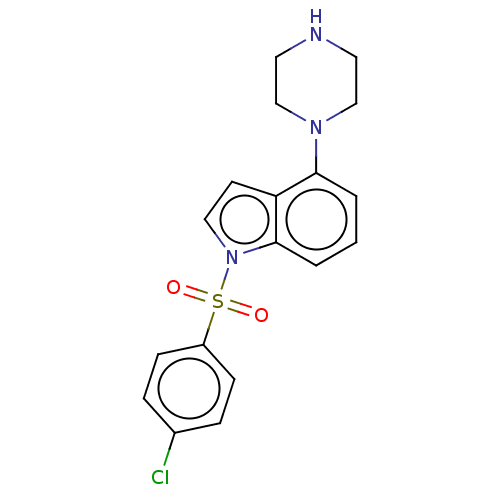
(CHEMBL371876)Show SMILES Clc1ccc(cc1)S(=O)(=O)n1ccc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C18H18ClN3O2S/c19-14-4-6-15(7-5-14)25(23,24)22-11-8-16-17(2-1-3-18(16)22)21-12-9-20-10-13-21/h1-8,11,20H,9-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50044623

(CHEMBL193400)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(N3CCNCC3)c(Cl)ccc12 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-2-1-3-14(12-13)26(24,25)23-9-6-15-17(23)5-4-16(20)18(15)22-10-7-21-8-11-22/h1-6,9,12,21H,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475481

(CHEMBL197297)Show SMILES Cn1cc(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H20ClN3O2S/c1-22-13-18(26(24,25)15-5-2-4-14(20)12-15)16-6-3-7-17(19(16)22)23-10-8-21-9-11-23/h2-7,12-13,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475478
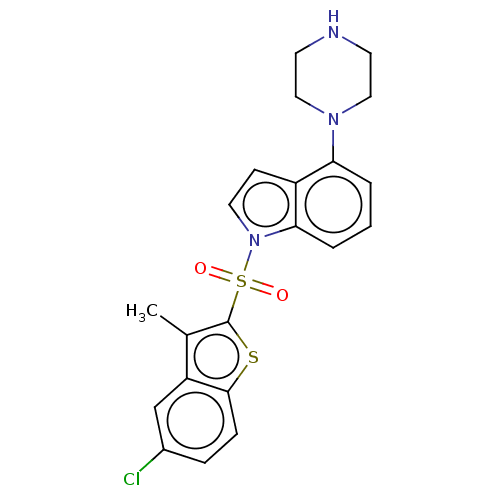
(CHEMBL196644)Show SMILES Cc1c(sc2ccc(Cl)cc12)S(=O)(=O)n1ccc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C21H20ClN3O2S2/c1-14-17-13-15(22)5-6-20(17)28-21(14)29(26,27)25-10-7-16-18(3-2-4-19(16)25)24-11-8-23-9-12-24/h2-7,10,13,23H,8-9,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475482

(CHEMBL193379)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(N3CCNCC3)c(ccc12)C#N Show InChI InChI=1S/C19H17ClN4O2S/c20-15-2-1-3-16(12-15)27(25,26)24-9-6-17-18(24)5-4-14(13-21)19(17)23-10-7-22-8-11-23/h1-6,9,12,22H,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475472

(CHEMBL372287)Show SMILES Clc1ccc(cc1)S(=O)(=O)n1ccc2c(N3CCNCC3)c(Cl)ccc12 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-1-3-14(4-2-13)26(24,25)23-10-7-15-17(23)6-5-16(20)18(15)22-11-8-21-9-12-22/h1-7,10,21H,8-9,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475474
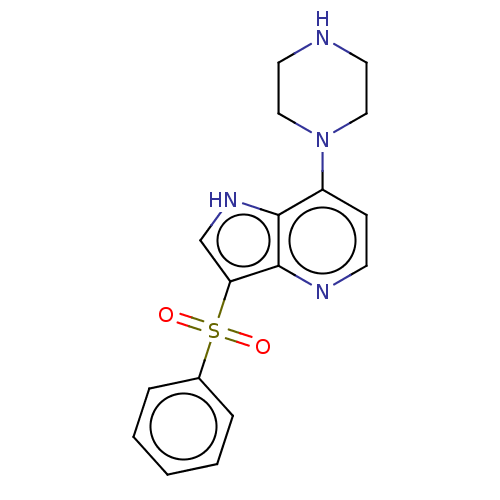
(CHEMBL426640)Show SMILES O=S(=O)(c1c[nH]c2c(ccnc12)N1CCNCC1)c1ccccc1 Show InChI InChI=1S/C17H18N4O2S/c22-24(23,13-4-2-1-3-5-13)15-12-20-16-14(6-7-19-17(15)16)21-10-8-18-9-11-21/h1-7,12,18,20H,8-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50044623

(CHEMBL193400)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(N3CCNCC3)c(Cl)ccc12 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-2-1-3-14(12-13)26(24,25)23-9-6-15-17(23)5-4-16(20)18(15)22-10-7-21-8-11-22/h1-6,9,12,21H,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 6 receptor of human caudate |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475476
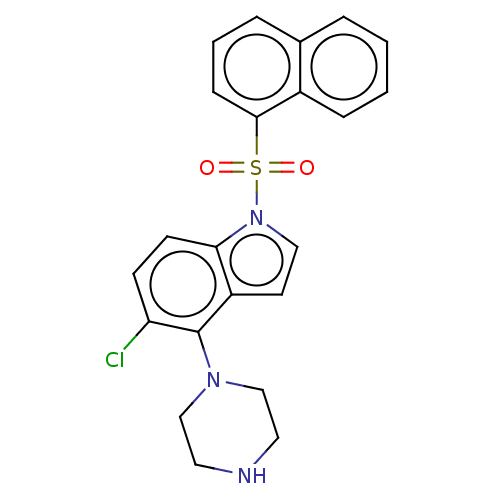
(CHEMBL196103)Show SMILES Clc1ccc2n(ccc2c1N1CCNCC1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C22H20ClN3O2S/c23-19-8-9-20-18(22(19)25-14-11-24-12-15-25)10-13-26(20)29(27,28)21-7-3-5-16-4-1-2-6-17(16)21/h1-10,13,24H,11-12,14-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(RAT) | BDBM50044623

(CHEMBL193400)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(N3CCNCC3)c(Cl)ccc12 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-2-1-3-14(12-13)26(24,25)23-9-6-15-17(23)5-4-16(20)18(15)22-10-7-21-8-11-22/h1-6,9,12,21H,7-8,10-11H2 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity against 5-hydroxytryptamine 6 receptor of rat striatum |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475469

(CHEMBL196524)Show SMILES Clc1ccc2n(ccc2c1N1CCNCC1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C22H20ClN3O2S/c23-20-7-8-21-19(22(20)25-13-10-24-11-14-25)9-12-26(21)29(27,28)18-6-5-16-3-1-2-4-17(16)15-18/h1-9,12,15,24H,10-11,13-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475468
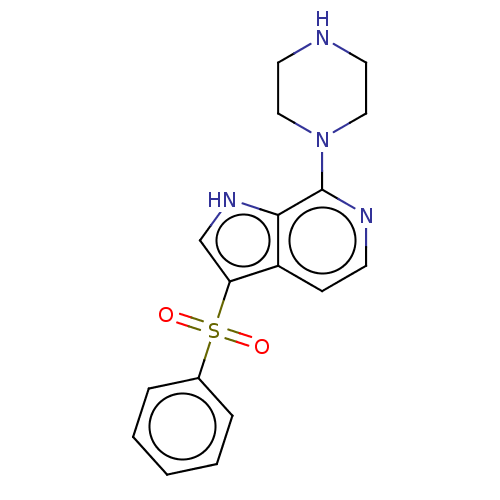
(CHEMBL366248)Show SMILES O=S(=O)(c1c[nH]c2c(nccc12)N1CCNCC1)c1ccccc1 Show InChI InChI=1S/C17H18N4O2S/c22-24(23,13-4-2-1-3-5-13)15-12-20-16-14(15)6-7-19-17(16)21-10-8-18-9-11-21/h1-7,12,18,20H,8-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50352620
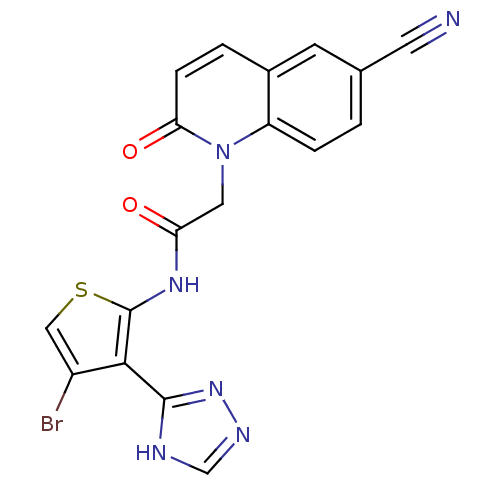
(CHEMBL1822151)Show SMILES Brc1csc(NC(=O)Cn2c3ccc(cc3ccc2=O)C#N)c1-c1nnc[nH]1 Show InChI InChI=1S/C18H11BrN6O2S/c19-12-8-28-18(16(12)17-21-9-22-24-17)23-14(26)7-25-13-3-1-10(6-20)5-11(13)2-4-15(25)27/h1-5,8-9H,7H2,(H,23,26)(H,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50352624
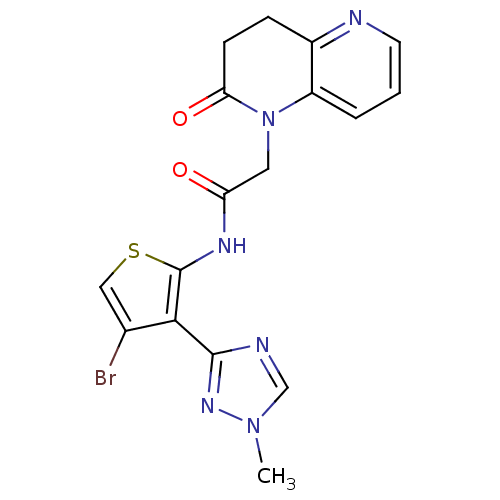
(CHEMBL1822305 | US9796706, Compound 139)Show SMILES Cn1cnc(n1)-c1c(Br)csc1NC(=O)CN1C(=O)CCc2ncccc12 Show InChI InChI=1S/C17H15BrN6O2S/c1-23-9-20-16(22-23)15-10(18)8-27-17(15)21-13(25)7-24-12-3-2-6-19-11(12)4-5-14(24)26/h2-3,6,8-9H,4-5,7H2,1H3,(H,21,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50352621
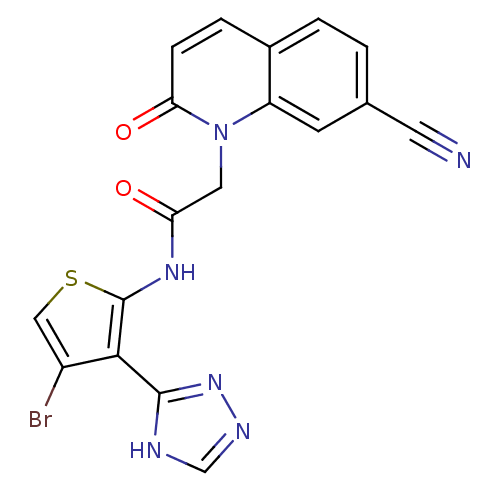
(CHEMBL1822152)Show SMILES Brc1csc(NC(=O)Cn2c3cc(ccc3ccc2=O)C#N)c1-c1nnc[nH]1 Show InChI InChI=1S/C18H11BrN6O2S/c19-12-8-28-18(16(12)17-21-9-22-24-17)23-14(26)7-25-13-5-10(6-20)1-2-11(13)3-4-15(25)27/h1-5,8-9H,7H2,(H,23,26)(H,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM16016
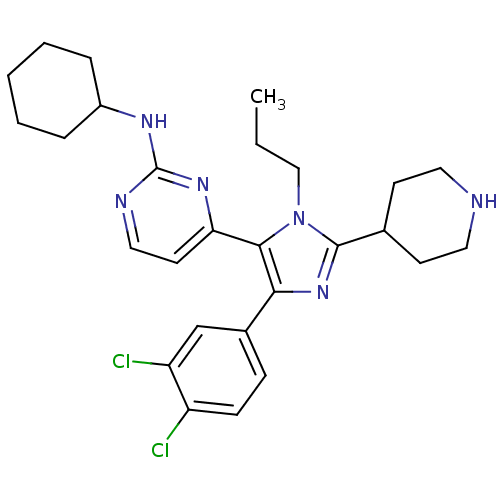
(CHEMBL437747 | N-cyclohexyl-4-[4-(3,4-dichlorophen...)Show SMILES CCCn1c(nc(c1-c1ccnc(NC2CCCCC2)n1)-c1ccc(Cl)c(Cl)c1)C1CCNCC1 Show InChI InChI=1S/C27H34Cl2N6/c1-2-16-35-25(23-12-15-31-27(33-23)32-20-6-4-3-5-7-20)24(19-8-9-21(28)22(29)17-19)34-26(35)18-10-13-30-14-11-18/h8-9,12,15,17-18,20,30H,2-7,10-11,13-14,16H2,1H3,(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK3 after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 315-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.010
BindingDB Entry DOI: 10.7270/Q24F1R0T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50352620

(CHEMBL1822151)Show SMILES Brc1csc(NC(=O)Cn2c3ccc(cc3ccc2=O)C#N)c1-c1nnc[nH]1 Show InChI InChI=1S/C18H11BrN6O2S/c19-12-8-28-18(16(12)17-21-9-22-24-17)23-14(26)7-25-13-3-1-10(6-20)5-11(13)2-4-15(25)27/h1-5,8-9H,7H2,(H,23,26)(H,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK3 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50352621

(CHEMBL1822152)Show SMILES Brc1csc(NC(=O)Cn2c3cc(ccc3ccc2=O)C#N)c1-c1nnc[nH]1 Show InChI InChI=1S/C18H11BrN6O2S/c19-12-8-28-18(16(12)17-21-9-22-24-17)23-14(26)7-25-13-5-10(6-20)1-2-11(13)3-4-15(25)27/h1-5,8-9H,7H2,(H,23,26)(H,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK3 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50352618
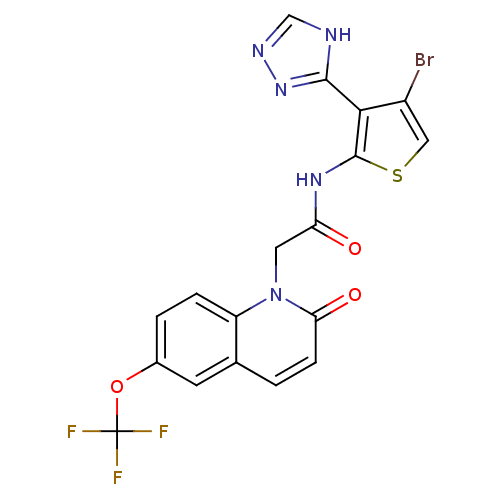
(CHEMBL1822149)Show SMILES FC(F)(F)Oc1ccc2n(CC(=O)Nc3scc(Br)c3-c3nnc[nH]3)c(=O)ccc2c1 Show InChI InChI=1S/C18H11BrF3N5O3S/c19-11-7-31-17(15(11)16-23-8-24-26-16)25-13(28)6-27-12-3-2-10(30-18(20,21)22)5-9(12)1-4-14(27)29/h1-5,7-8H,6H2,(H,25,28)(H,23,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50352615
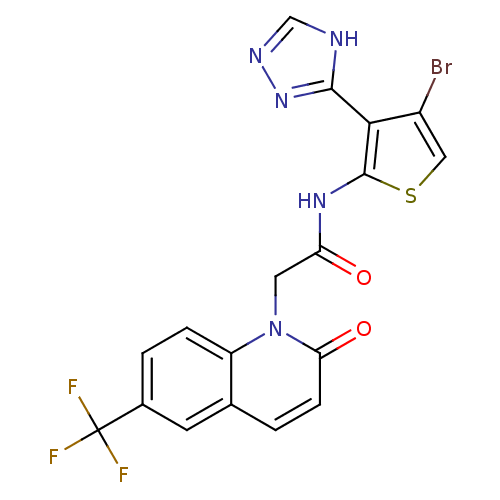
(CHEMBL1822146)Show SMILES FC(F)(F)c1ccc2n(CC(=O)Nc3scc(Br)c3-c3nnc[nH]3)c(=O)ccc2c1 Show InChI InChI=1S/C18H11BrF3N5O2S/c19-11-7-30-17(15(11)16-23-8-24-26-16)25-13(28)6-27-12-3-2-10(18(20,21)22)5-9(12)1-4-14(27)29/h1-5,7-8H,6H2,(H,25,28)(H,23,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50352628
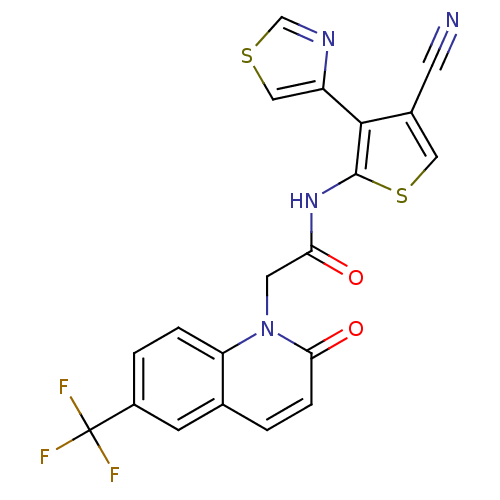
(CHEMBL1822309 | US9796706, Compound 136)Show SMILES FC(F)(F)c1ccc2n(CC(=O)Nc3scc(C#N)c3-c3cscn3)c(=O)ccc2c1 Show InChI InChI=1S/C20H11F3N4O2S2/c21-20(22,23)13-2-3-15-11(5-13)1-4-17(29)27(15)7-16(28)26-19-18(12(6-24)8-31-19)14-9-30-10-25-14/h1-5,8-10H,7H2,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50352624

(CHEMBL1822305 | US9796706, Compound 139)Show SMILES Cn1cnc(n1)-c1c(Br)csc1NC(=O)CN1C(=O)CCc2ncccc12 Show InChI InChI=1S/C17H15BrN6O2S/c1-23-9-20-16(22-23)15-10(18)8-27-17(15)21-13(25)7-24-12-3-2-6-19-11(12)4-5-14(24)26/h2-3,6,8-9H,4-5,7H2,1H3,(H,21,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK3 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50352614
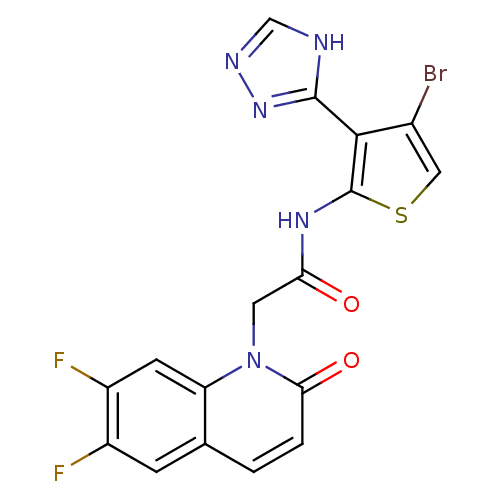
(CHEMBL1822145)Show SMILES Fc1cc2ccc(=O)n(CC(=O)Nc3scc(Br)c3-c3nnc[nH]3)c2cc1F Show InChI InChI=1S/C17H10BrF2N5O2S/c18-9-6-28-17(15(9)16-21-7-22-24-16)23-13(26)5-25-12-4-11(20)10(19)3-8(12)1-2-14(25)27/h1-4,6-7H,5H2,(H,23,26)(H,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50352613
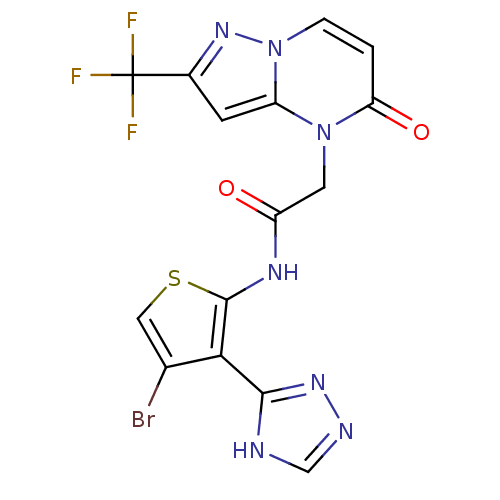
(CHEMBL1822144)Show SMILES FC(F)(F)c1cc2n(CC(=O)Nc3scc(Br)c3-c3nnc[nH]3)c(=O)ccn2n1 Show InChI InChI=1S/C15H9BrF3N7O2S/c16-7-5-29-14(12(7)13-20-6-21-23-13)22-9(27)4-25-10-3-8(15(17,18)19)24-26(10)2-1-11(25)28/h1-3,5-6H,4H2,(H,22,27)(H,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50302846
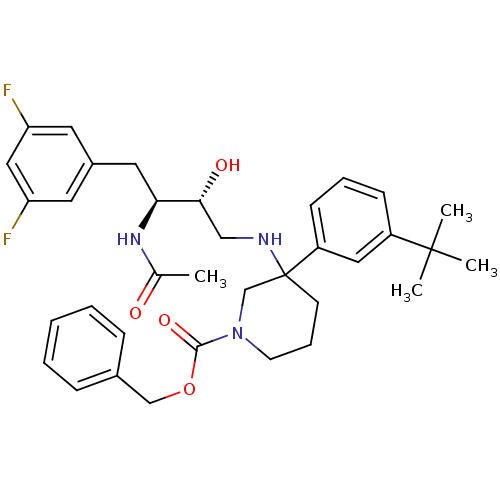
(CHEMBL570165 | benzyl 3-((2R,3S)-3-acetamido-4-(3,...)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNC1(CCCN(C1)C(=O)OCc1ccccc1)c1cccc(c1)C(C)(C)C |r| Show InChI InChI=1S/C35H43F2N3O4/c1-24(41)39-31(18-26-16-29(36)20-30(37)17-26)32(42)21-38-35(28-13-8-12-27(19-28)34(2,3)4)14-9-15-40(23-35)33(43)44-22-25-10-6-5-7-11-25/h5-8,10-13,16-17,19-20,31-32,38,42H,9,14-15,18,21-23H2,1-4H3,(H,39,41)/t31-,32+,35?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin D assessed as reduction in polarization after 110 mins by oregon green based fluorescence polarization assay |
Bioorg Med Chem Lett 19: 6386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.061
BindingDB Entry DOI: 10.7270/Q23779NV |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50328778
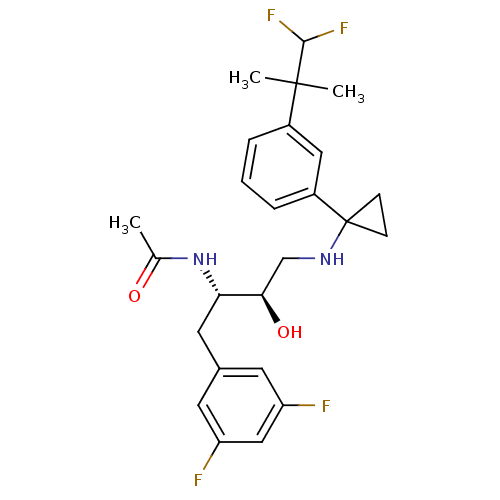
(CHEMBL1270362 | N-((2S,3R)-4-(1-(3-(1,1-difluoro-2...)Show SMILES CC(=O)N[C@@H](Cc1cc(F)cc(F)c1)[C@H](O)CNC1(CC1)c1cccc(c1)C(C)(C)C(F)F |r| Show InChI InChI=1S/C25H30F4N2O2/c1-15(32)31-21(11-16-9-19(26)13-20(27)10-16)22(33)14-30-25(7-8-25)18-6-4-5-17(12-18)24(2,3)23(28)29/h4-6,9-10,12-13,21-23,30,33H,7-8,11,14H2,1-3H3,(H,31,32)/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in presence of NADPH |
Bioorg Med Chem Lett 20: 6231-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.102
BindingDB Entry DOI: 10.7270/Q2NZ87V9 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM16016

(CHEMBL437747 | N-cyclohexyl-4-[4-(3,4-dichlorophen...)Show SMILES CCCn1c(nc(c1-c1ccnc(NC2CCCCC2)n1)-c1ccc(Cl)c(Cl)c1)C1CCNCC1 Show InChI InChI=1S/C27H34Cl2N6/c1-2-16-35-25(23-12-15-31-27(33-23)32-20-6-4-3-5-7-20)24(19-8-9-21(28)22(29)17-19)34-26(35)18-10-13-30-14-11-18/h8-9,12,15,17-18,20,30H,2-7,10-11,13-14,16H2,1H3,(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant p38alpha after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 315-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.010
BindingDB Entry DOI: 10.7270/Q24F1R0T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50352626
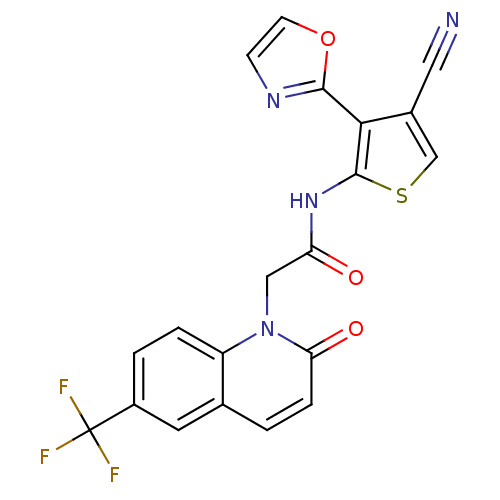
(CHEMBL1822307 | US9796706, Compound 131)Show SMILES FC(F)(F)c1ccc2n(CC(=O)Nc3scc(C#N)c3-c3ncco3)c(=O)ccc2c1 Show InChI InChI=1S/C20H11F3N4O3S/c21-20(22,23)13-2-3-14-11(7-13)1-4-16(29)27(14)9-15(28)26-19-17(12(8-24)10-31-19)18-25-5-6-30-18/h1-7,10H,9H2,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50352610
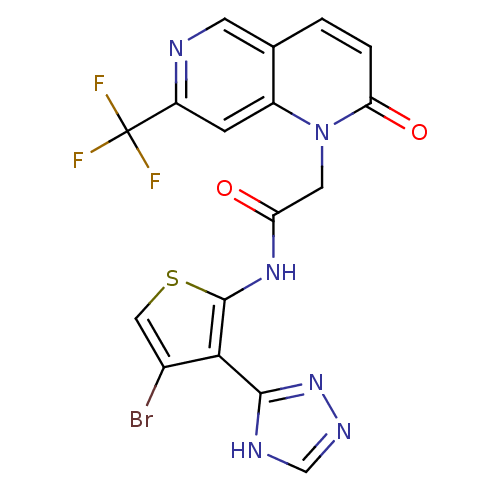
(CHEMBL1822141)Show SMILES FC(F)(F)c1cc2n(CC(=O)Nc3scc(Br)c3-c3nnc[nH]3)c(=O)ccc2cn1 Show InChI InChI=1S/C17H10BrF3N6O2S/c18-9-6-30-16(14(9)15-23-7-24-26-15)25-12(28)5-27-10-3-11(17(19,20)21)22-4-8(10)1-2-13(27)29/h1-4,6-7H,5H2,(H,25,28)(H,23,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50352609
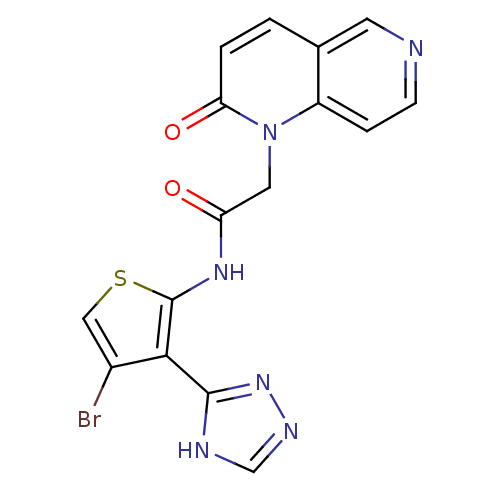
(CHEMBL1822140)Show SMILES Brc1csc(NC(=O)Cn2c3ccncc3ccc2=O)c1-c1nnc[nH]1 Show InChI InChI=1S/C16H11BrN6O2S/c17-10-7-26-16(14(10)15-19-8-20-22-15)21-12(24)6-23-11-3-4-18-5-9(11)1-2-13(23)25/h1-5,7-8H,6H2,(H,21,24)(H,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50352615

(CHEMBL1822146)Show SMILES FC(F)(F)c1ccc2n(CC(=O)Nc3scc(Br)c3-c3nnc[nH]3)c(=O)ccc2c1 Show InChI InChI=1S/C18H11BrF3N5O2S/c19-11-7-30-17(15(11)16-23-8-24-26-16)25-13(28)6-27-12-3-2-10(18(20,21)22)5-9(12)1-4-14(27)29/h1-5,7-8H,6H2,(H,25,28)(H,23,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK2 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50352608
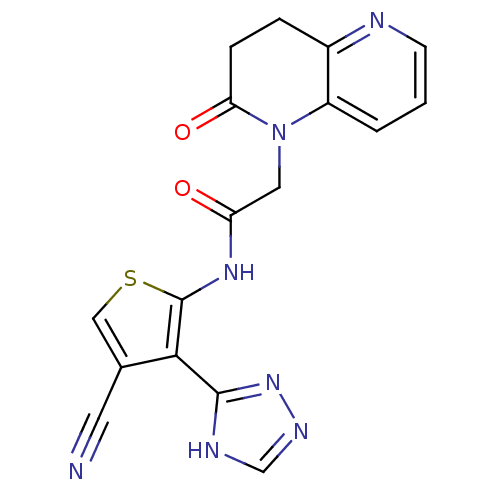
(CHEMBL1822139)Show SMILES O=C(CN1C(=O)CCc2ncccc12)Nc1scc(C#N)c1-c1nnc[nH]1 Show InChI InChI=1S/C17H13N7O2S/c18-6-10-8-27-17(15(10)16-20-9-21-23-16)22-13(25)7-24-12-2-1-5-19-11(12)3-4-14(24)26/h1-2,5,8-9H,3-4,7H2,(H,22,25)(H,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK1 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK2
(Homo sapiens (Human)) | BDBM50436720

(CHEMBL2401963)Show SMILES CC[C@H]1N(C2CCCC2)c2nc(ncc2N(C)C1=O)-n1ccnc1-c1ccccc1 |r| Show InChI InChI=1S/C23H26N6O/c1-3-18-22(30)27(2)19-15-25-23(26-21(19)29(18)17-11-7-8-12-17)28-14-13-24-20(28)16-9-5-4-6-10-16/h4-6,9-10,13-15,17-18H,3,7-8,11-12H2,1-2H3/t18-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged Plk2 (unknown origin) assessed as inhibition of DEKTDDED phosphorylation at Thr1342 after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 23: 2743-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.065
BindingDB Entry DOI: 10.7270/Q28G8N4M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50352614

(CHEMBL1822145)Show SMILES Fc1cc2ccc(=O)n(CC(=O)Nc3scc(Br)c3-c3nnc[nH]3)c2cc1F Show InChI InChI=1S/C17H10BrF2N5O2S/c18-9-6-28-17(15(9)16-21-7-22-24-16)23-13(26)5-25-12-4-11(20)10(19)3-8(12)1-2-14(25)27/h1-4,6-7H,5H2,(H,23,26)(H,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK3 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50352618

(CHEMBL1822149)Show SMILES FC(F)(F)Oc1ccc2n(CC(=O)Nc3scc(Br)c3-c3nnc[nH]3)c(=O)ccc2c1 Show InChI InChI=1S/C18H11BrF3N5O3S/c19-11-7-31-17(15(11)16-23-8-24-26-16)25-13(28)6-27-12-3-2-10(30-18(20,21)22)5-9(12)1-4-14(27)29/h1-5,7-8H,6H2,(H,25,28)(H,23,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JNK3 using ser73 of c-jun as substrate preincubated for 15 mins measured after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 21: 5521-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.100
BindingDB Entry DOI: 10.7270/Q29Z959K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data