

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50300690 (1-(5-Tert-Butyl-1,2-Oxazol-3-Yl)-3-(4-{7-[2-(Morph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | <0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of human FLT3 using EAIYAAPFAKKK substrate and [gamma-33P]-ATP by radiometric biochemical kinase assay | J Med Chem 62: 9141-9160 (2019) Article DOI: 10.1021/acs.jmedchem.9b01025 BindingDB Entry DOI: 10.7270/Q2H41VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 3 (Homo sapiens (Human)) | BDBM50413752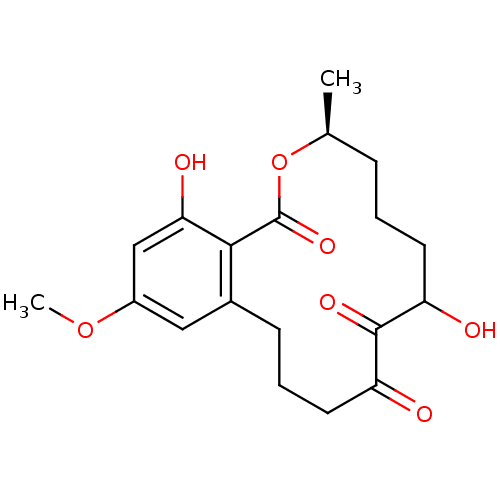 (CHEMBL2012519 | L-783277) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of human VEGFR3 using poly[Glu:Tyr] (4:1) substrate and [gamma-33P]-ATP by radiometric biochemical kinase assay | J Med Chem 62: 9141-9160 (2019) Article DOI: 10.1021/acs.jmedchem.9b01025 BindingDB Entry DOI: 10.7270/Q2H41VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-1B (Homo sapiens (Human)) | BDBM50262079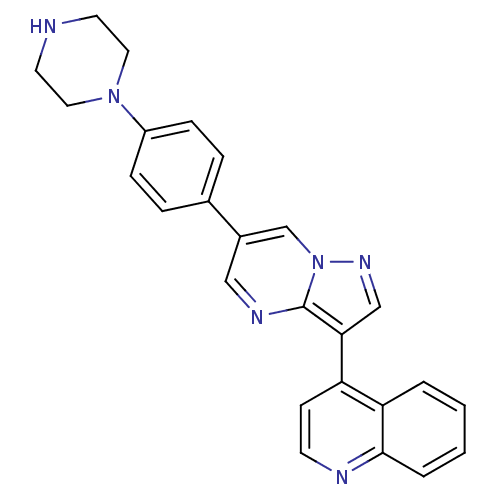 (4-(6-(4-(piperazin-1-yl)phenyl)pyrazolo[1,5-a]pyri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of human ALK6 using casein as substrate in presence of 10 uM ATP by radiometric kinase assay | J Med Chem 60: 1495-1508 (2017) Article DOI: 10.1021/acs.jmedchem.6b01679 BindingDB Entry DOI: 10.7270/Q2TQ63S4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM148383 (US8962637, 108) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Debiopharm S.A.; Aurigene Discovery Technologies Ltd. US Patent | Assay Description Compounds were screened in the TR-FRET assay for JAK2 and c-Src kinase inhibition. Ultra light poly GT (Perkin Elmer) was used as the substrate for J... | US Patent US8962637 (2015) BindingDB Entry DOI: 10.7270/Q2MS3RGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM148382 (US8962637, 107) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Debiopharm S.A.; Aurigene Discovery Technologies Ltd. US Patent | Assay Description Compounds were screened in the TR-FRET assay for JAK2 and c-Src kinase inhibition. Ultra light poly GT (Perkin Elmer) was used as the substrate for J... | US Patent US8962637 (2015) BindingDB Entry DOI: 10.7270/Q2MS3RGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM148373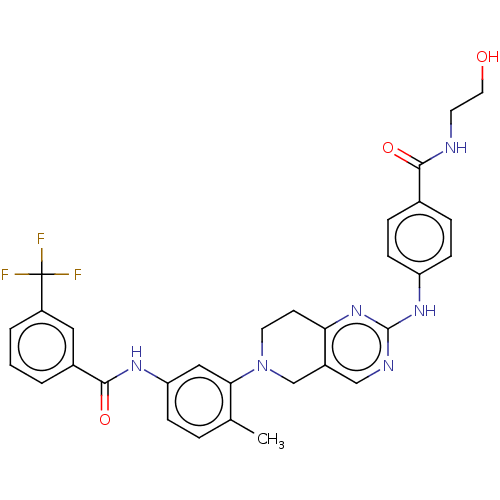 (US8962637, 98) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Debiopharm S.A.; Aurigene Discovery Technologies Ltd. US Patent | Assay Description Compounds were screened in the TR-FRET assay for JAK2 and c-Src kinase inhibition. Ultra light poly GT (Perkin Elmer) was used as the substrate for J... | US Patent US8962637 (2015) BindingDB Entry DOI: 10.7270/Q2MS3RGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM158429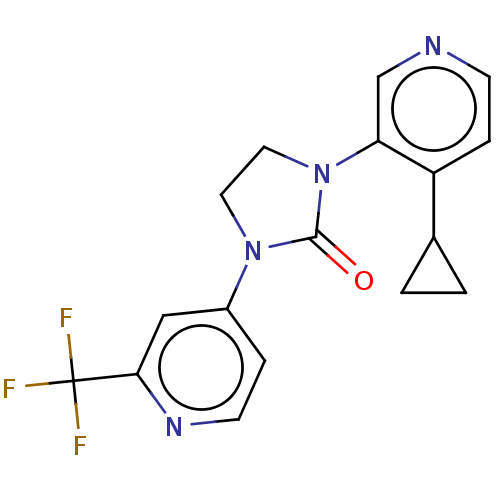 (US9029399, 2A | US9339501, 2A) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description Assay method was adopted from a published protocol with some modifications to suit our requirements (Dmitry N Grigoryev et al, Analytical Biochemistr... | US Patent US9339501 (2016) BindingDB Entry DOI: 10.7270/Q26T0KG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 3 (Homo sapiens (Human)) | BDBM50413752 (CHEMBL2012519 | L-783277) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of human FLT4 using poly[Glu:Tyr] (4:1) as substrate in presence of [gamma-33P]-ATP by scintillation counting method | J Med Chem 60: 1495-1508 (2017) Article DOI: 10.1021/acs.jmedchem.6b01679 BindingDB Entry DOI: 10.7270/Q2TQ63S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM158429 (US9029399, 2A | US9339501, 2A) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG US Patent | Assay Description Assay method was adopted from a published protocol with some modifications to suit our requirements (Dmitry N Grigoryev et al, Analytical Biochemistr... | US Patent US9029399 (2015) BindingDB Entry DOI: 10.7270/Q28G8JFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 3 (Homo sapiens (Human)) | BDBM50509140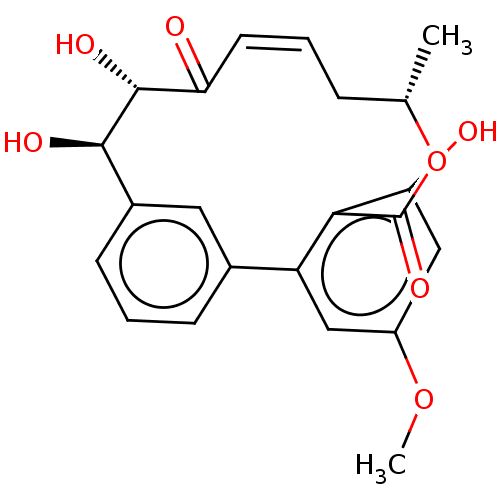 (CHEMBL4453304) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of human VEGFR3 using poly[Glu:Tyr] (4:1) substrate and [gamma-33P]-ATP by radiometric biochemical kinase assay | J Med Chem 62: 9141-9160 (2019) Article DOI: 10.1021/acs.jmedchem.9b01025 BindingDB Entry DOI: 10.7270/Q2H41VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 3 (Homo sapiens (Human)) | BDBM50509146 (CHEMBL4303275) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of human VEGFR3 using poly[Glu:Tyr] (4:1) substrate and [gamma-33P]-ATP by radiometric biochemical kinase assay | J Med Chem 62: 9141-9160 (2019) Article DOI: 10.1021/acs.jmedchem.9b01025 BindingDB Entry DOI: 10.7270/Q2H41VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50413752 (CHEMBL2012519 | L-783277) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of human FLT3 using EAIYAAPFAKKK peptide as substrate in presence of [gamma-33P]-ATP by scintillation counting method | J Med Chem 60: 1495-1508 (2017) Article DOI: 10.1021/acs.jmedchem.6b01679 BindingDB Entry DOI: 10.7270/Q2TQ63S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50413752 (CHEMBL2012519 | L-783277) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of human FLT3 using EAIYAAPFAKKK substrate and [gamma-33P]-ATP by radiometric biochemical kinase assay | J Med Chem 62: 9141-9160 (2019) Article DOI: 10.1021/acs.jmedchem.9b01025 BindingDB Entry DOI: 10.7270/Q2H41VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM158474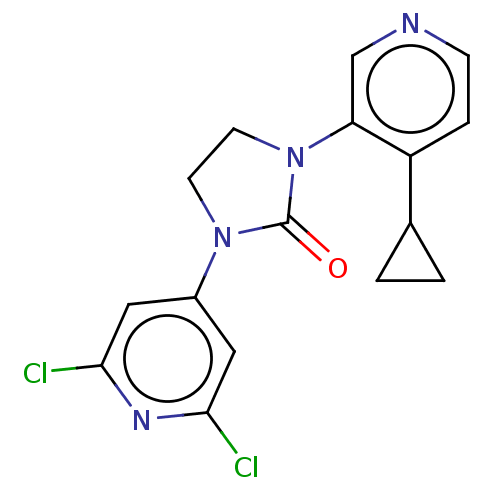 (US9029399, 46A | US9339501, 46A) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG US Patent | Assay Description Assay method was adopted from a published protocol with some modifications to suit our requirements (Dmitry N Grigoryev et al, Analytical Biochemistr... | US Patent US9029399 (2015) BindingDB Entry DOI: 10.7270/Q28G8JFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM158474 (US9029399, 46A | US9339501, 46A) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description Assay method was adopted from a published protocol with some modifications to suit our requirements (Dmitry N Grigoryev et al, Analytical Biochemistr... | US Patent US9339501 (2016) BindingDB Entry DOI: 10.7270/Q26T0KG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM132104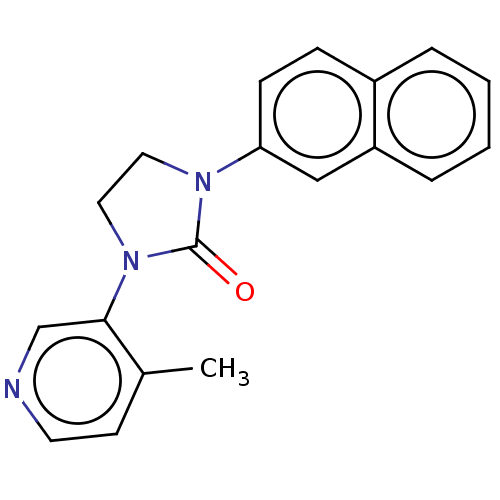 (USRE45173, 14A) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Cytochrome P450, 17-20 lyase (CYP17-lyase) assay development using recombinant human CYP17 enzyme and 17-alpha -hydroxy pregnenolone [21-3H] as the s... | US Patent USRE45173 (2014) BindingDB Entry DOI: 10.7270/Q2MW2FT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM132137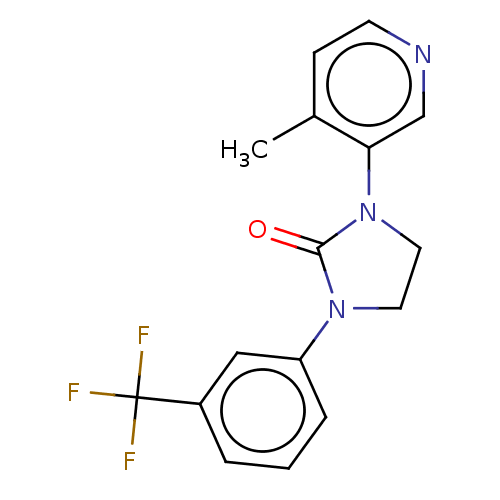 (USRE45173, 70A) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Cytochrome P450, 17-20 lyase (CYP17-lyase) assay development using recombinant human CYP17 enzyme and 17-alpha -hydroxy pregnenolone [21-3H] as the s... | US Patent USRE45173 (2014) BindingDB Entry DOI: 10.7270/Q2MW2FT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM148297 (US8962637, 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Debiopharm S.A.; Aurigene Discovery Technologies Ltd. US Patent | Assay Description Compounds were screened in the TR-FRET assay for JAK2 and c-Src kinase inhibition. Ultra light poly GT (Perkin Elmer) was used as the substrate for J... | US Patent US8962637 (2015) BindingDB Entry DOI: 10.7270/Q2MS3RGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM148424 (US8962637, 155) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Debiopharm S.A.; Aurigene Discovery Technologies Ltd. US Patent | Assay Description Compounds were screened in the TR-FRET assay for JAK2 and c-Src kinase inhibition. Ultra light poly GT (Perkin Elmer) was used as the substrate for J... | US Patent US8962637 (2015) BindingDB Entry DOI: 10.7270/Q2MS3RGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM148381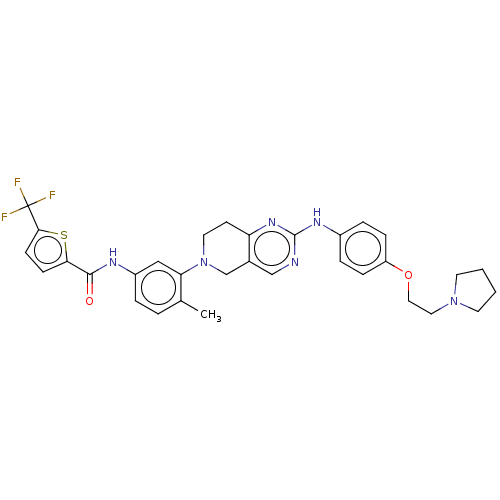 (US8962637, 106) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Debiopharm S.A.; Aurigene Discovery Technologies Ltd. US Patent | Assay Description Compounds were screened in the TR-FRET assay for JAK2 and c-Src kinase inhibition. Ultra light poly GT (Perkin Elmer) was used as the substrate for J... | US Patent US8962637 (2015) BindingDB Entry DOI: 10.7270/Q2MS3RGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM148369 (US8962637, 186 | US8962637, 94) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Debiopharm S.A.; Aurigene Discovery Technologies Ltd. US Patent | Assay Description Compounds were screened in the TR-FRET assay for JAK2 and c-Src kinase inhibition. Ultra light poly GT (Perkin Elmer) was used as the substrate for J... | US Patent US8962637 (2015) BindingDB Entry DOI: 10.7270/Q2MS3RGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM148367 (US8962637, 92) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Debiopharm S.A.; Aurigene Discovery Technologies Ltd. US Patent | Assay Description Compounds were screened in the TR-FRET assay for JAK2 and c-Src kinase inhibition. Ultra light poly GT (Perkin Elmer) was used as the substrate for J... | US Patent US8962637 (2015) BindingDB Entry DOI: 10.7270/Q2MS3RGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-1A (Homo sapiens (Human)) | BDBM50262079 (4-(6-(4-(piperazin-1-yl)phenyl)pyrazolo[1,5-a]pyri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of human ALK3 using casein as substrate in presence of 10 uM ATP by radiometric kinase assay | J Med Chem 60: 1495-1508 (2017) Article DOI: 10.1021/acs.jmedchem.6b01679 BindingDB Entry DOI: 10.7270/Q2TQ63S4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM148419 (US8962637, 148) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Debiopharm S.A.; Aurigene Discovery Technologies Ltd. US Patent | Assay Description Compounds were screened in the TR-FRET assay for JAK2 and c-Src kinase inhibition. Ultra light poly GT (Perkin Elmer) was used as the substrate for J... | US Patent US8962637 (2015) BindingDB Entry DOI: 10.7270/Q2MS3RGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM132182 (USRE45173, 121A) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Cytochrome P450, 17-20 lyase (CYP17-lyase) assay development using recombinant human CYP17 enzyme and 17-alpha -hydroxy pregnenolone [21-3H] as the s... | US Patent USRE45173 (2014) BindingDB Entry DOI: 10.7270/Q2MW2FT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM158428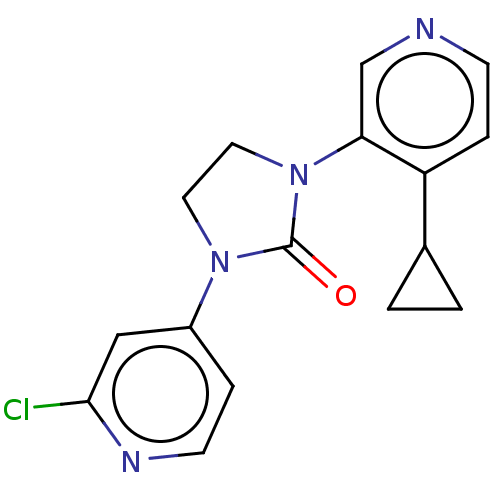 (US9029399, 1A | US9339501, 1A) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG US Patent | Assay Description Assay method was adopted from a published protocol with some modifications to suit our requirements (Dmitry N Grigoryev et al, Analytical Biochemistr... | US Patent US9029399 (2015) BindingDB Entry DOI: 10.7270/Q28G8JFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM132216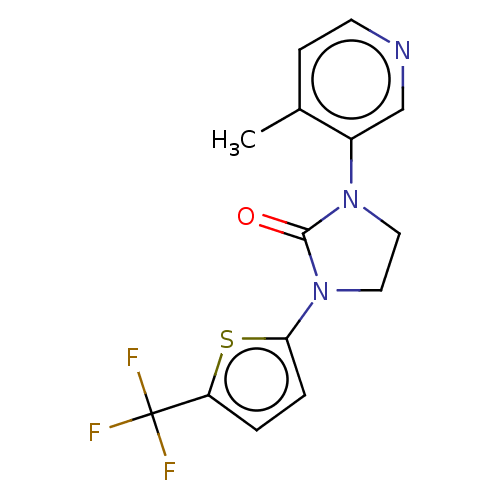 (USRE45173, 155A | USRE45173, 192A) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Cytochrome P450, 17-20 lyase (CYP17-lyase) assay development using recombinant human CYP17 enzyme and 17-alpha -hydroxy pregnenolone [21-3H] as the s... | US Patent USRE45173 (2014) BindingDB Entry DOI: 10.7270/Q2MW2FT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM132216 (USRE45173, 155A | USRE45173, 192A) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Cytochrome P450, 17-20 lyase (CYP17-lyase) assay development using recombinant human CYP17 enzyme and 17-alpha -hydroxy pregnenolone [21-3H] as the s... | US Patent USRE45173 (2014) BindingDB Entry DOI: 10.7270/Q2MW2FT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM158445 (US9029399, 19A | US9339501, 19A) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description Assay method was adopted from a published protocol with some modifications to suit our requirements (Dmitry N Grigoryev et al, Analytical Biochemistr... | US Patent US9339501 (2016) BindingDB Entry DOI: 10.7270/Q26T0KG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM158445 (US9029399, 19A | US9339501, 19A) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG US Patent | Assay Description Assay method was adopted from a published protocol with some modifications to suit our requirements (Dmitry N Grigoryev et al, Analytical Biochemistr... | US Patent US9029399 (2015) BindingDB Entry DOI: 10.7270/Q28G8JFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM158428 (US9029399, 1A | US9339501, 1A) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description Assay method was adopted from a published protocol with some modifications to suit our requirements (Dmitry N Grigoryev et al, Analytical Biochemistr... | US Patent US9339501 (2016) BindingDB Entry DOI: 10.7270/Q26T0KG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50413752 (CHEMBL2012519 | L-783277) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of human VEGFR2 using poly[Glu:Tyr] (4:1) substrate and [gamma-33P]-ATP by radiometric biochemical kinase assay | J Med Chem 62: 9141-9160 (2019) Article DOI: 10.1021/acs.jmedchem.9b01025 BindingDB Entry DOI: 10.7270/Q2H41VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM158460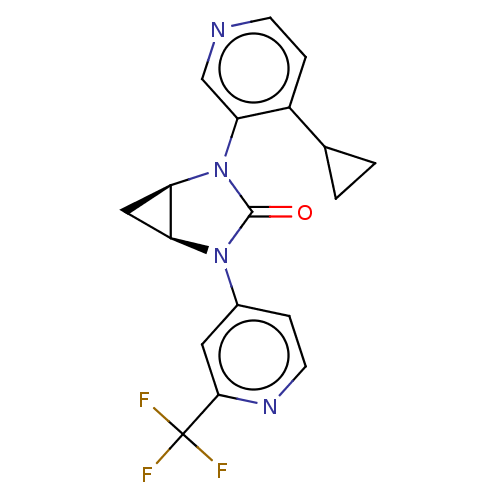 (US9029399, 32A-II | US9339501, 32A-II) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description Assay method was adopted from a published protocol with some modifications to suit our requirements (Dmitry N Grigoryev et al, Analytical Biochemistr... | US Patent US9339501 (2016) BindingDB Entry DOI: 10.7270/Q26T0KG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM158460 (US9029399, 32A-II | US9339501, 32A-II) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG US Patent | Assay Description Assay method was adopted from a published protocol with some modifications to suit our requirements (Dmitry N Grigoryev et al, Analytical Biochemistr... | US Patent US9029399 (2015) BindingDB Entry DOI: 10.7270/Q28G8JFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM148406 (US8962637, 132) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Debiopharm S.A.; Aurigene Discovery Technologies Ltd. US Patent | Assay Description Compounds were screened in the TR-FRET assay for JAK2 and c-Src kinase inhibition. Ultra light poly GT (Perkin Elmer) was used as the substrate for J... | US Patent US8962637 (2015) BindingDB Entry DOI: 10.7270/Q2MS3RGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM148297 (US8962637, 17) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Debiopharm S.A.; Aurigene Discovery Technologies Ltd. US Patent | Assay Description Compounds were screened in the TR-FRET assay for JAK2 and c-Src kinase inhibition. Ultra light poly GT (Perkin Elmer) was used as the substrate for J... | US Patent US8962637 (2015) BindingDB Entry DOI: 10.7270/Q2MS3RGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM148398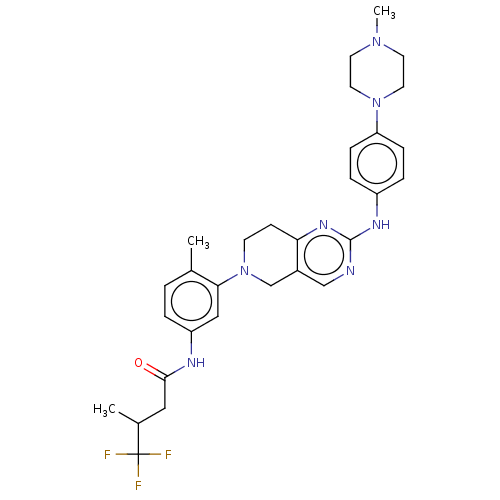 (US8962637, 123) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Debiopharm S.A.; Aurigene Discovery Technologies Ltd. US Patent | Assay Description Compounds were screened in the TR-FRET assay for JAK2 and c-Src kinase inhibition. Ultra light poly GT (Perkin Elmer) was used as the substrate for J... | US Patent US8962637 (2015) BindingDB Entry DOI: 10.7270/Q2MS3RGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM132118 (USRE45173, 29A) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Cytochrome P450, 17-20 lyase (CYP17-lyase) assay development using recombinant human CYP17 enzyme and 17-alpha -hydroxy pregnenolone [21-3H] as the s... | US Patent USRE45173 (2014) BindingDB Entry DOI: 10.7270/Q2MW2FT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM132121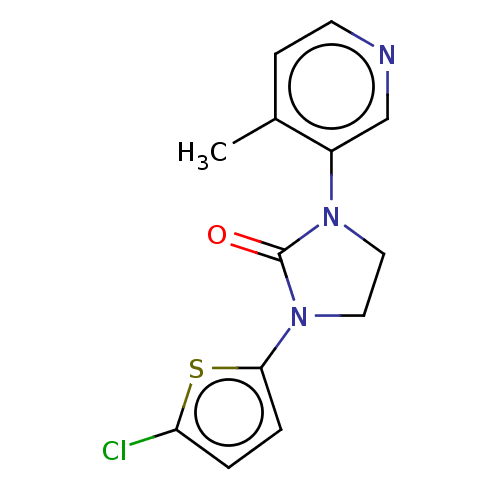 (USRE45173, 41A) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Cytochrome P450, 17-20 lyase (CYP17-lyase) assay development using recombinant human CYP17 enzyme and 17-alpha -hydroxy pregnenolone [21-3H] as the s... | US Patent USRE45173 (2014) BindingDB Entry DOI: 10.7270/Q2MW2FT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50262079 (4-(6-(4-(piperazin-1-yl)phenyl)pyrazolo[1,5-a]pyri...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of 10 uM ATP by radiometric kinase assay | J Med Chem 60: 1495-1508 (2017) Article DOI: 10.1021/acs.jmedchem.6b01679 BindingDB Entry DOI: 10.7270/Q2TQ63S4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM132220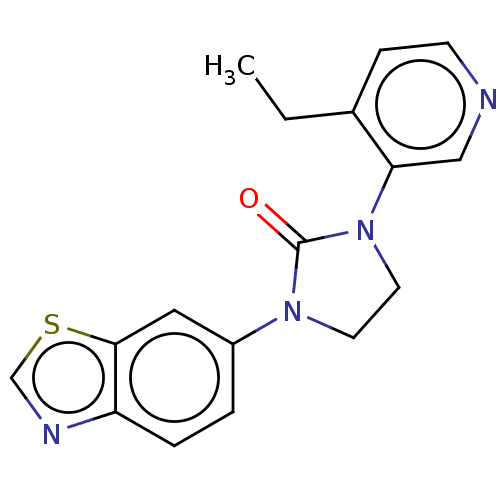 (USRE45173, 159A | USRE45173, 196A) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Cytochrome P450, 17-20 lyase (CYP17-lyase) assay development using recombinant human CYP17 enzyme and 17-alpha -hydroxy pregnenolone [21-3H] as the s... | US Patent USRE45173 (2014) BindingDB Entry DOI: 10.7270/Q2MW2FT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM132220 (USRE45173, 159A | USRE45173, 196A) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Cytochrome P450, 17-20 lyase (CYP17-lyase) assay development using recombinant human CYP17 enzyme and 17-alpha -hydroxy pregnenolone [21-3H] as the s... | US Patent USRE45173 (2014) BindingDB Entry DOI: 10.7270/Q2MW2FT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50509140 (CHEMBL4453304) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of human VEGFR2 using poly[Glu:Tyr] (4:1) substrate and [gamma-33P]-ATP by radiometric biochemical kinase assay | J Med Chem 62: 9141-9160 (2019) Article DOI: 10.1021/acs.jmedchem.9b01025 BindingDB Entry DOI: 10.7270/Q2H41VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM148422 (US8962637, 153) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Debiopharm S.A.; Aurigene Discovery Technologies Ltd. US Patent | Assay Description Compounds were screened in the TR-FRET assay for JAK2 and c-Src kinase inhibition. Ultra light poly GT (Perkin Elmer) was used as the substrate for J... | US Patent US8962637 (2015) BindingDB Entry DOI: 10.7270/Q2MS3RGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM132117 (USRE45173, 28A) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Cytochrome P450, 17-20 lyase (CYP17-lyase) assay development using recombinant human CYP17 enzyme and 17-alpha -hydroxy pregnenolone [21-3H] as the s... | US Patent USRE45173 (2014) BindingDB Entry DOI: 10.7270/Q2MW2FT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM148358 (US8962637, 83) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Debiopharm S.A.; Aurigene Discovery Technologies Ltd. US Patent | Assay Description Compounds were screened in the TR-FRET assay for JAK2 and c-Src kinase inhibition. Ultra light poly GT (Perkin Elmer) was used as the substrate for J... | US Patent US8962637 (2015) BindingDB Entry DOI: 10.7270/Q2MS3RGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM132152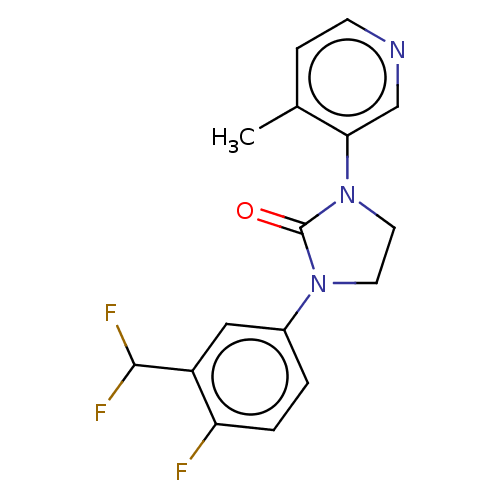 (USRE45173, 91A) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Cytochrome P450, 17-20 lyase (CYP17-lyase) assay development using recombinant human CYP17 enzyme and 17-alpha -hydroxy pregnenolone [21-3H] as the s... | US Patent USRE45173 (2014) BindingDB Entry DOI: 10.7270/Q2MW2FT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50509140 (CHEMBL4453304) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of human FLT3 using EAIYAAPFAKKK substrate and [gamma-33P]-ATP by radiometric biochemical kinase assay | J Med Chem 62: 9141-9160 (2019) Article DOI: 10.1021/acs.jmedchem.9b01025 BindingDB Entry DOI: 10.7270/Q2H41VR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM158436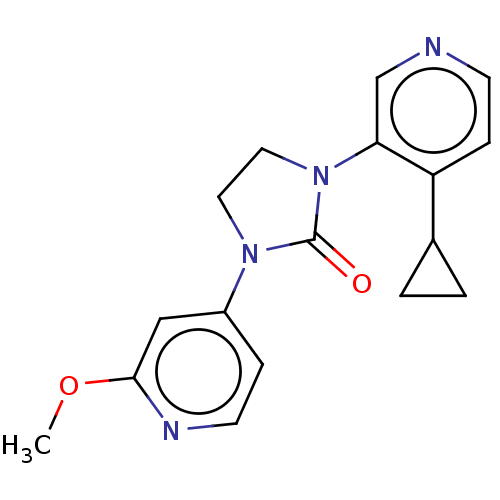 (US9029399, 9A | US9339501, 9A) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description Assay method was adopted from a published protocol with some modifications to suit our requirements (Dmitry N Grigoryev et al, Analytical Biochemistr... | US Patent US9339501 (2016) BindingDB Entry DOI: 10.7270/Q26T0KG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM158436 (US9029399, 9A | US9339501, 9A) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG US Patent | Assay Description Assay method was adopted from a published protocol with some modifications to suit our requirements (Dmitry N Grigoryev et al, Analytical Biochemistr... | US Patent US9029399 (2015) BindingDB Entry DOI: 10.7270/Q28G8JFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 482 total ) | Next | Last >> |