

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50537918 (CHEMBL3951168) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50537922 (CHEMBL4645319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50537922 (CHEMBL4645319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50537919 (CHEMBL4633146) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50537918 (CHEMBL3951168) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50537919 (CHEMBL4633146) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM167444 (US9073906, 156) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50537921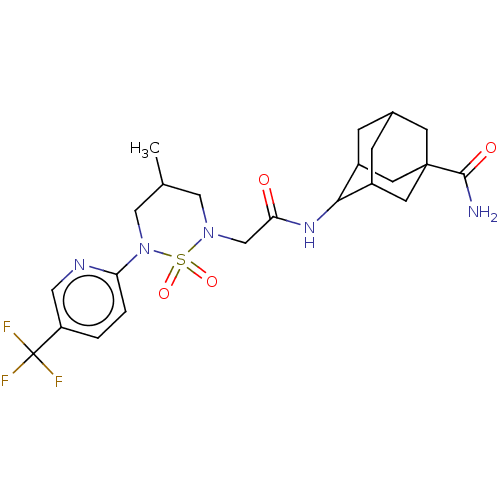 (CHEMBL4649416) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM167444 (US9073906, 156) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50537917 (CHEMBL4648389) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50444090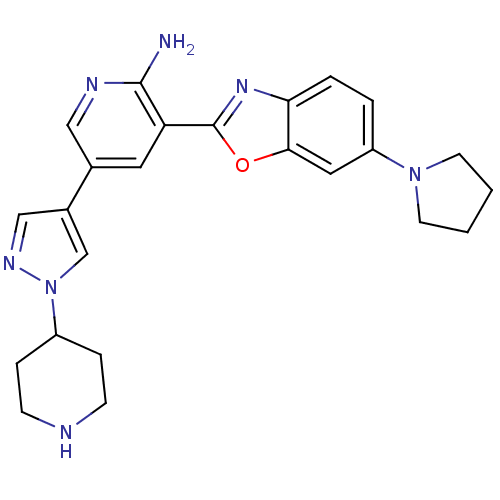 (CHEMBL3093151) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of c-Met (unknown origin) | Bioorg Med Chem Lett 23: 6711-6 (2013) Article DOI: 10.1016/j.bmcl.2013.10.036 BindingDB Entry DOI: 10.7270/Q2SX6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50537917 (CHEMBL4648389) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50537920 (CHEMBL4632806) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50537916 (CHEMBL4646414) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G protein-coupled receptor kinase 5 (Homo sapiens (Human)) | BDBM50444090 (CHEMBL3093151) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of GRK-5 (unknown origin) preincubated with enzyme for 10 mins before adding peptide substrate and ATP measured after 1 hr by LANCE-TR-FRE... | Bioorg Med Chem Lett 23: 6711-6 (2013) Article DOI: 10.1016/j.bmcl.2013.10.036 BindingDB Entry DOI: 10.7270/Q2SX6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50537916 (CHEMBL4646414) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G protein-coupled receptor kinase 5 (Homo sapiens (Human)) | BDBM50444135 (CHEMBL3093260) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of GRK-5 (unknown origin) preincubated with enzyme for 10 mins before adding peptide substrate and ATP measured after 1 hr by LANCE-TR-FRE... | Bioorg Med Chem Lett 23: 6711-6 (2013) Article DOI: 10.1016/j.bmcl.2013.10.036 BindingDB Entry DOI: 10.7270/Q2SX6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G protein-coupled receptor kinase 5 (Homo sapiens (Human)) | BDBM50444137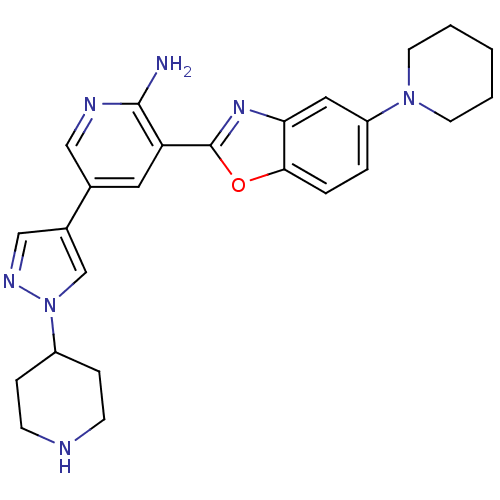 (CHEMBL3093258) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of GRK-5 (unknown origin) preincubated with enzyme for 10 mins before adding peptide substrate and ATP measured after 1 hr by LANCE-TR-FRE... | Bioorg Med Chem Lett 23: 6711-6 (2013) Article DOI: 10.1016/j.bmcl.2013.10.036 BindingDB Entry DOI: 10.7270/Q2SX6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 2 (Homo sapiens (Human)) | BDBM50057740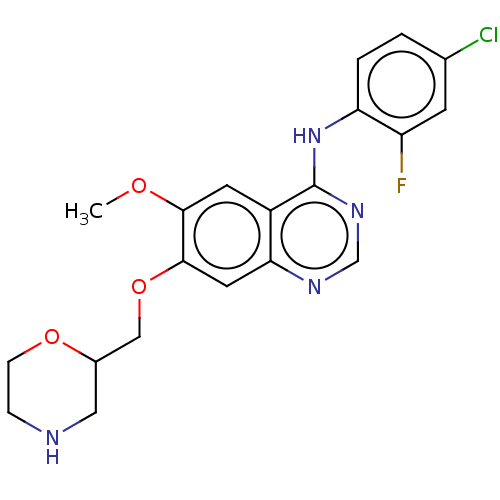 (CHEMBL3322987) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of EphA2 (unknown origin) pre-incubated with enzyme for 10 mins before adding peptide substrate and ATP using TK-substrate-biotin and SEB ... | Bioorg Med Chem Lett 24: 4080-3 (2014) Article DOI: 10.1016/j.bmcl.2014.07.081 BindingDB Entry DOI: 10.7270/Q2C53NHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 2 (Homo sapiens (Human)) | BDBM50057741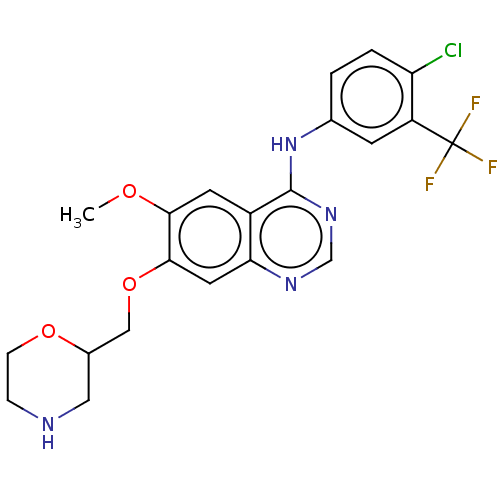 (CHEMBL3322988) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of EphA2 (unknown origin) pre-incubated with enzyme for 10 mins before adding peptide substrate and ATP using TK-substrate-biotin and SEB ... | Bioorg Med Chem Lett 24: 4080-3 (2014) Article DOI: 10.1016/j.bmcl.2014.07.081 BindingDB Entry DOI: 10.7270/Q2C53NHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50537915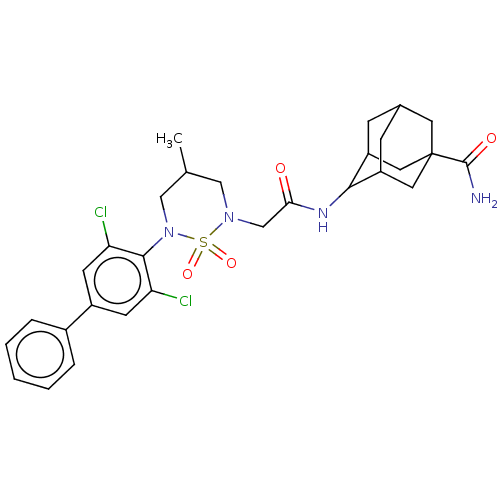 (CHEMBL4638272) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 174 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 2 (Homo sapiens (Human)) | BDBM50057737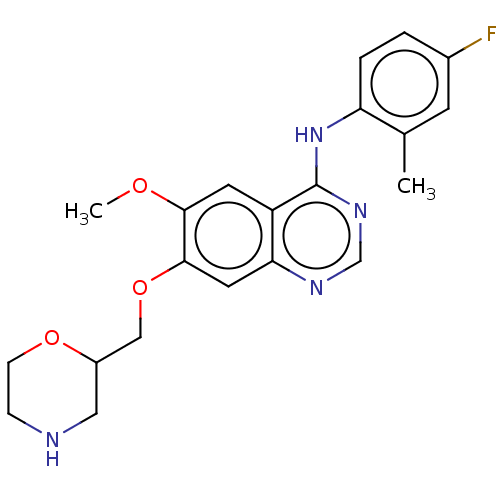 (CHEMBL3322986) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of EphA2 (unknown origin) pre-incubated with enzyme for 10 mins before adding peptide substrate and ATP using TK-substrate-biotin and SEB ... | Bioorg Med Chem Lett 24: 4080-3 (2014) Article DOI: 10.1016/j.bmcl.2014.07.081 BindingDB Entry DOI: 10.7270/Q2C53NHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G protein-coupled receptor kinase 5 (Homo sapiens (Human)) | BDBM50384021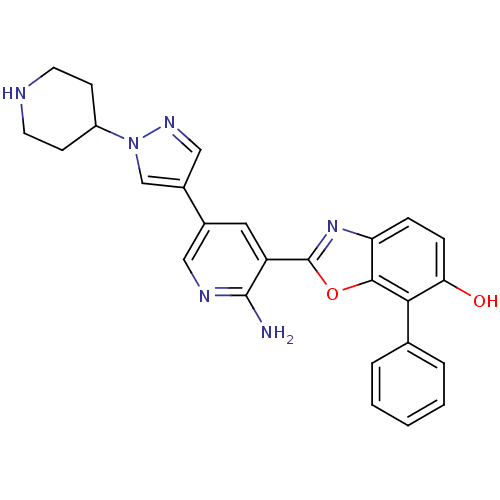 (CHEMBL2032283 | CHEMBL2079205) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of GRK-5 (unknown origin) preincubated with enzyme for 10 mins before adding peptide substrate and ATP measured after 1 hr by LANCE-TR-FRE... | Bioorg Med Chem Lett 23: 6711-6 (2013) Article DOI: 10.1016/j.bmcl.2013.10.036 BindingDB Entry DOI: 10.7270/Q2SX6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 2 (Homo sapiens (Human)) | BDBM50057747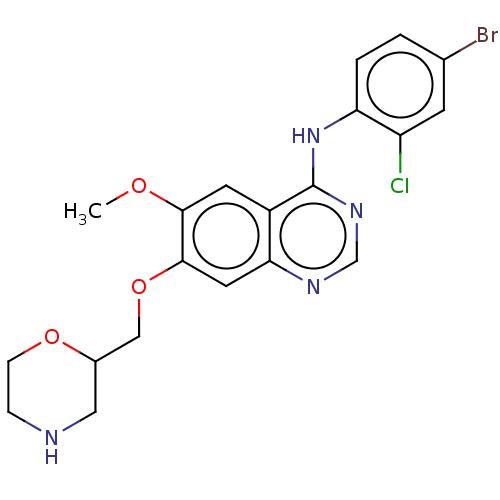 (CHEMBL3322990) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of EphA2 (unknown origin) pre-incubated with enzyme for 10 mins before adding peptide substrate and ATP using TK-substrate-biotin and SEB ... | Bioorg Med Chem Lett 24: 4080-3 (2014) Article DOI: 10.1016/j.bmcl.2014.07.081 BindingDB Entry DOI: 10.7270/Q2C53NHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50537915 (CHEMBL4638272) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 231 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50247012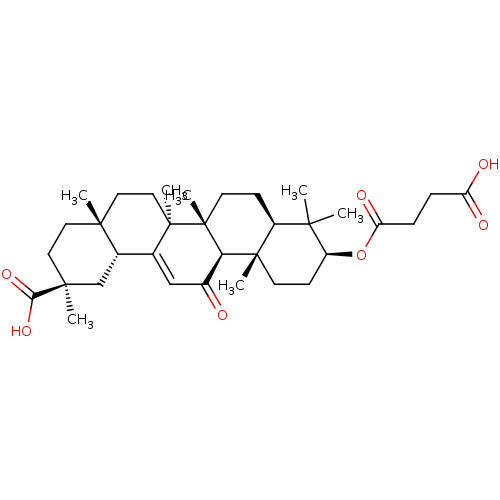 (3beta-O-Succinyl-18-beta-glycyrrhetinic acid | Car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 243 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 2 (Homo sapiens (Human)) | BDBM50057732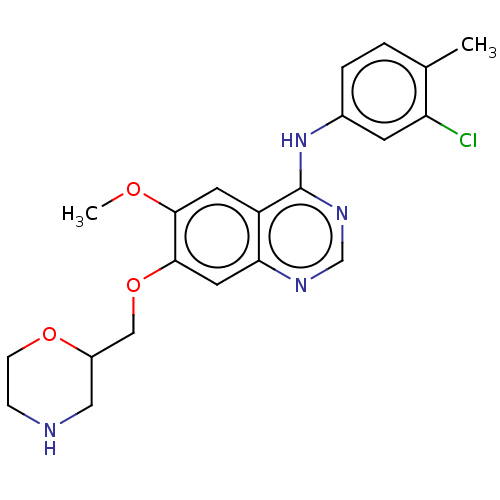 (CHEMBL3322983) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of EphA2 (unknown origin) pre-incubated with enzyme for 10 mins before adding peptide substrate and ATP using TK-substrate-biotin and SEB ... | Bioorg Med Chem Lett 24: 4080-3 (2014) Article DOI: 10.1016/j.bmcl.2014.07.081 BindingDB Entry DOI: 10.7270/Q2C53NHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 2 (Homo sapiens (Human)) | BDBM50057748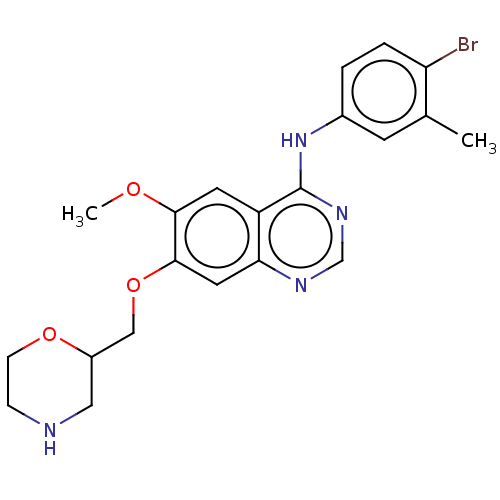 (CHEMBL3322991) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of EphA2 (unknown origin) pre-incubated with enzyme for 10 mins before adding peptide substrate and ATP using TK-substrate-biotin and SEB ... | Bioorg Med Chem Lett 24: 4080-3 (2014) Article DOI: 10.1016/j.bmcl.2014.07.081 BindingDB Entry DOI: 10.7270/Q2C53NHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50444090 (CHEMBL3093151) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of ALK (unknown origin) | Bioorg Med Chem Lett 23: 6711-6 (2013) Article DOI: 10.1016/j.bmcl.2013.10.036 BindingDB Entry DOI: 10.7270/Q2SX6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G protein-coupled receptor kinase 5 (Homo sapiens (Human)) | BDBM50444098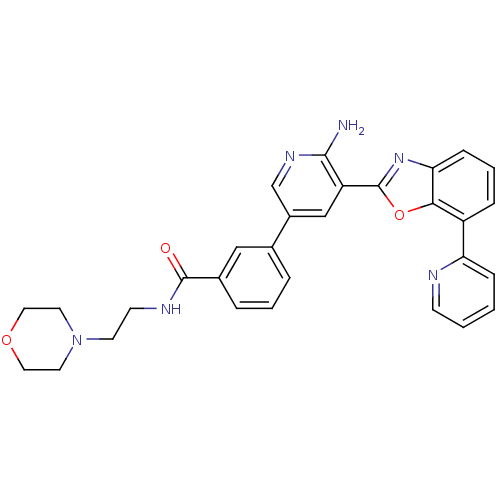 (CHEMBL3093253) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of GRK-5 (unknown origin) preincubated with enzyme for 10 mins before adding peptide substrate and ATP measured after 1 hr by LANCE-TR-FRE... | Bioorg Med Chem Lett 23: 6711-6 (2013) Article DOI: 10.1016/j.bmcl.2013.10.036 BindingDB Entry DOI: 10.7270/Q2SX6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 2 (Homo sapiens (Human)) | BDBM50057744 (CHEMBL3322989) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of EphA2 (unknown origin) pre-incubated with enzyme for 10 mins before adding peptide substrate and ATP using TK-substrate-biotin and SEB ... | Bioorg Med Chem Lett 24: 4080-3 (2014) Article DOI: 10.1016/j.bmcl.2014.07.081 BindingDB Entry DOI: 10.7270/Q2C53NHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G protein-coupled receptor kinase 5 (Homo sapiens (Human)) | BDBM50444095 (CHEMBL3093255) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of GRK-5 (unknown origin) preincubated with enzyme for 10 mins before adding peptide substrate and ATP measured after 1 hr by LANCE-TR-FRE... | Bioorg Med Chem Lett 23: 6711-6 (2013) Article DOI: 10.1016/j.bmcl.2013.10.036 BindingDB Entry DOI: 10.7270/Q2SX6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Homo sapiens (Human)) | BDBM50444090 (CHEMBL3093151) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of GRK-2 (unknown origin) preincubated with enzyme for 10 mins before adding peptide substrate and ATP measured after 1 hr by LANCE-TR-FRE... | Bioorg Med Chem Lett 23: 6711-6 (2013) Article DOI: 10.1016/j.bmcl.2013.10.036 BindingDB Entry DOI: 10.7270/Q2SX6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G protein-coupled receptor kinase 5 (Homo sapiens (Human)) | BDBM50444130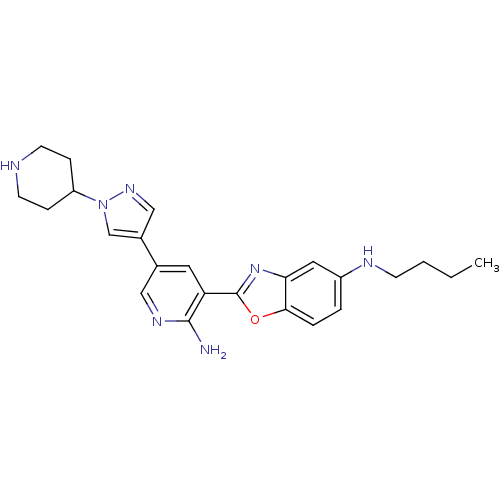 (CHEMBL3093145) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of GRK-5 (unknown origin) preincubated with enzyme for 10 mins before adding peptide substrate and ATP measured after 1 hr by LANCE-TR-FRE... | Bioorg Med Chem Lett 23: 6711-6 (2013) Article DOI: 10.1016/j.bmcl.2013.10.036 BindingDB Entry DOI: 10.7270/Q2SX6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G protein-coupled receptor kinase 5 (Homo sapiens (Human)) | BDBM50384020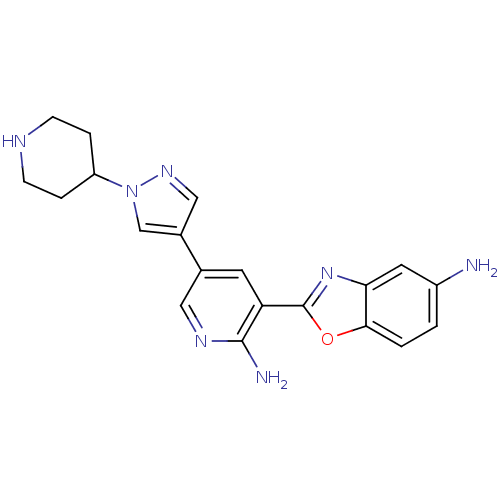 (CHEMBL2032284 | CHEMBL2079522) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of GRK-5 (unknown origin) preincubated with enzyme for 10 mins before adding peptide substrate and ATP measured after 1 hr by LANCE-TR-FRE... | Bioorg Med Chem Lett 23: 6711-6 (2013) Article DOI: 10.1016/j.bmcl.2013.10.036 BindingDB Entry DOI: 10.7270/Q2SX6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50537923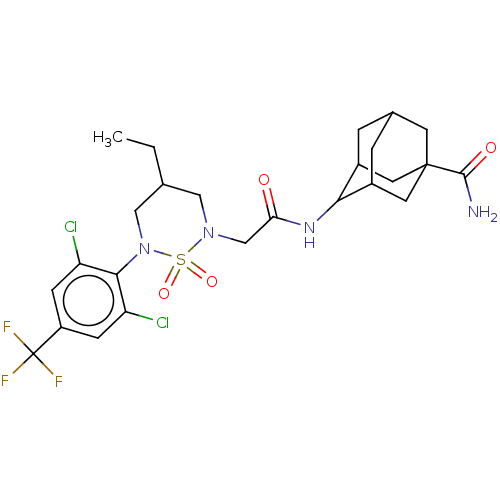 (CHEMBL4638268) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 529 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G protein-coupled receptor kinase 5 (Homo sapiens (Human)) | BDBM50444097 (CHEMBL3093254) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of GRK-5 (unknown origin) preincubated with enzyme for 10 mins before adding peptide substrate and ATP measured after 1 hr by LANCE-TR-FRE... | Bioorg Med Chem Lett 23: 6711-6 (2013) Article DOI: 10.1016/j.bmcl.2013.10.036 BindingDB Entry DOI: 10.7270/Q2SX6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G protein-coupled receptor kinase 5 (Homo sapiens (Human)) | BDBM50444108 (CHEMBL3093243) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of GRK-5 (unknown origin) preincubated with enzyme for 10 mins before adding peptide substrate and ATP measured after 1 hr by LANCE-TR-FRE... | Bioorg Med Chem Lett 23: 6711-6 (2013) Article DOI: 10.1016/j.bmcl.2013.10.036 BindingDB Entry DOI: 10.7270/Q2SX6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 2 (Homo sapiens (Human)) | BDBM50057717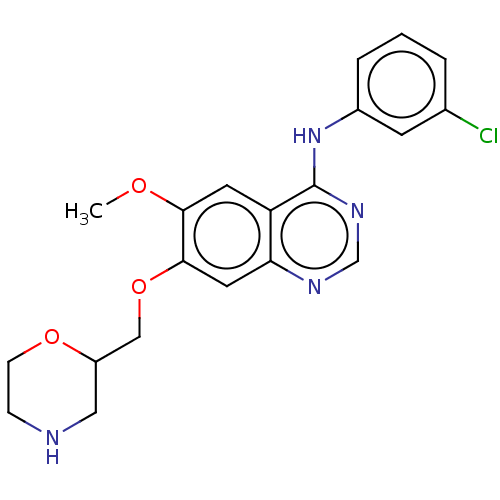 (CHEMBL3322978) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of EphA2 (unknown origin) pre-incubated with enzyme for 10 mins before adding peptide substrate and ATP using TK-substrate-biotin and SEB ... | Bioorg Med Chem Lett 24: 4080-3 (2014) Article DOI: 10.1016/j.bmcl.2014.07.081 BindingDB Entry DOI: 10.7270/Q2C53NHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 2 (Homo sapiens (Human)) | BDBM50057730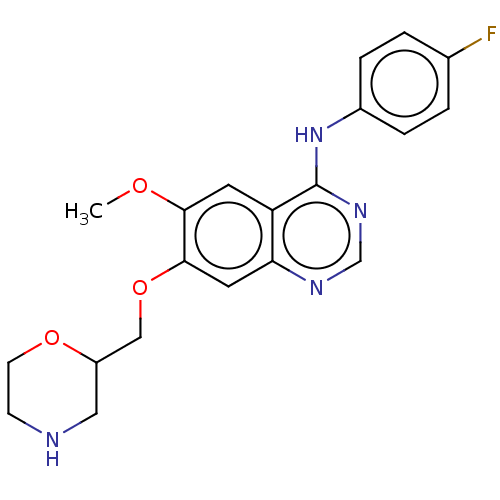 (CHEMBL3322981) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of EphA2 (unknown origin) pre-incubated with enzyme for 10 mins before adding peptide substrate and ATP using TK-substrate-biotin and SEB ... | Bioorg Med Chem Lett 24: 4080-3 (2014) Article DOI: 10.1016/j.bmcl.2014.07.081 BindingDB Entry DOI: 10.7270/Q2C53NHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G protein-coupled receptor kinase 5 (Homo sapiens (Human)) | BDBM50444104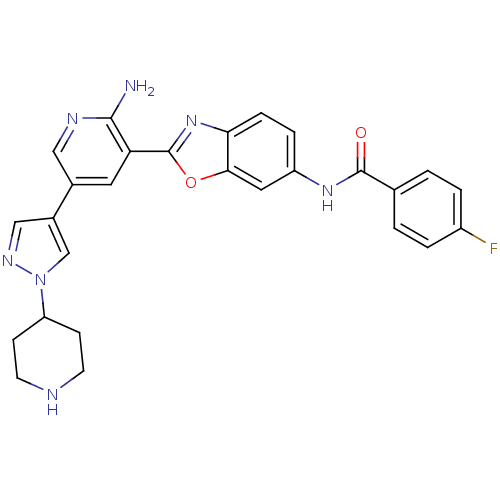 (CHEMBL3093247) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of GRK-5 (unknown origin) preincubated with enzyme for 10 mins before adding peptide substrate and ATP measured after 1 hr by LANCE-TR-FRE... | Bioorg Med Chem Lett 23: 6711-6 (2013) Article DOI: 10.1016/j.bmcl.2013.10.036 BindingDB Entry DOI: 10.7270/Q2SX6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 1 (Homo sapiens (Human)) | BDBM50444108 (CHEMBL3093243) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of GRK-2 (unknown origin) preincubated with enzyme for 10 mins before adding peptide substrate and ATP measured after 1 hr by LANCE-TR-FRE... | Bioorg Med Chem Lett 23: 6711-6 (2013) Article DOI: 10.1016/j.bmcl.2013.10.036 BindingDB Entry DOI: 10.7270/Q2SX6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G protein-coupled receptor kinase 5 (Homo sapiens (Human)) | BDBM50384035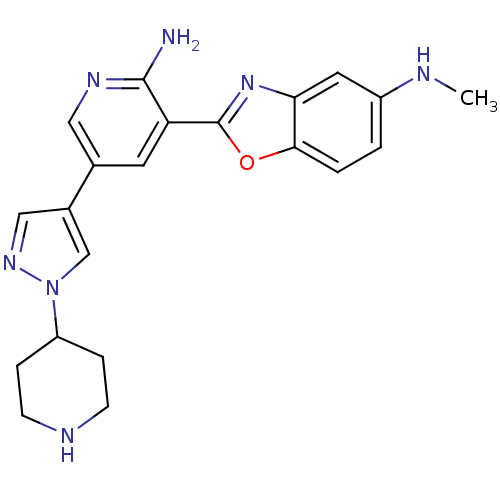 (CHEMBL2032158 | CHEMBL2078645) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of GRK-5 (unknown origin) preincubated with enzyme for 10 mins before adding peptide substrate and ATP measured after 1 hr by LANCE-TR-FRE... | Bioorg Med Chem Lett 23: 6711-6 (2013) Article DOI: 10.1016/j.bmcl.2013.10.036 BindingDB Entry DOI: 10.7270/Q2SX6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 2 (Homo sapiens (Human)) | BDBM50057729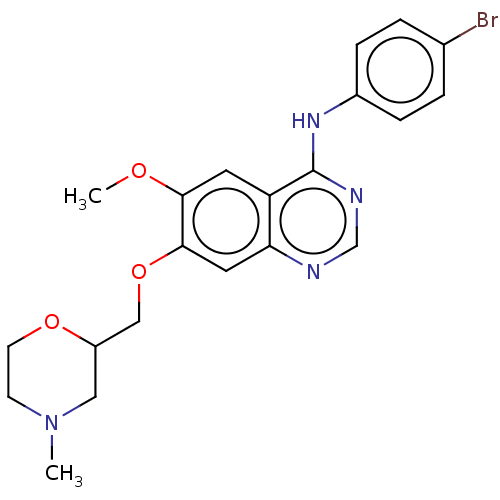 (CHEMBL3322977) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of EphA2 (unknown origin) pre-incubated with enzyme for 10 mins before adding peptide substrate and ATP using TK-substrate-biotin and SEB ... | Bioorg Med Chem Lett 24: 4080-3 (2014) Article DOI: 10.1016/j.bmcl.2014.07.081 BindingDB Entry DOI: 10.7270/Q2C53NHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50537923 (CHEMBL4638268) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 695 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 expressed in CHOK1 cells using XL665-labeled cortisol as substrate measured after 24 hrs by HTRF assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126787 BindingDB Entry DOI: 10.7270/Q2TX3JWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G protein-coupled receptor kinase 5 (Homo sapiens (Human)) | BDBM50444128 (CHEMBL3093147) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of GRK-5 (unknown origin) preincubated with enzyme for 10 mins before adding peptide substrate and ATP measured after 1 hr by LANCE-TR-FRE... | Bioorg Med Chem Lett 23: 6711-6 (2013) Article DOI: 10.1016/j.bmcl.2013.10.036 BindingDB Entry DOI: 10.7270/Q2SX6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 2 (Homo sapiens (Human)) | BDBM50057726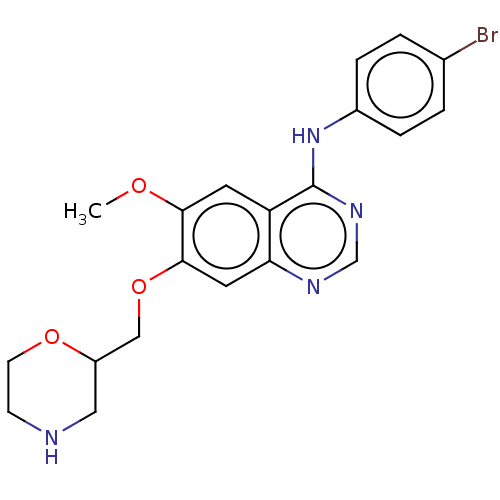 (CHEMBL3322980) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of EphA2 (unknown origin) pre-incubated with enzyme for 10 mins before adding peptide substrate and ATP using TK-substrate-biotin and SEB ... | Bioorg Med Chem Lett 24: 4080-3 (2014) Article DOI: 10.1016/j.bmcl.2014.07.081 BindingDB Entry DOI: 10.7270/Q2C53NHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 2 (Homo sapiens (Human)) | BDBM50057721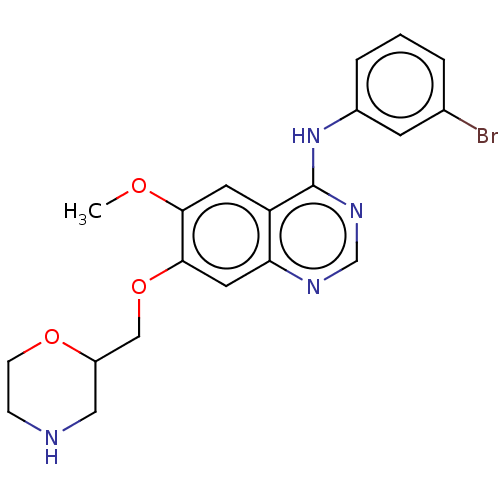 (CHEMBL3322979) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of EphA2 (unknown origin) pre-incubated with enzyme for 10 mins before adding peptide substrate and ATP using TK-substrate-biotin and SEB ... | Bioorg Med Chem Lett 24: 4080-3 (2014) Article DOI: 10.1016/j.bmcl.2014.07.081 BindingDB Entry DOI: 10.7270/Q2C53NHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 2 (Homo sapiens (Human)) | BDBM50057722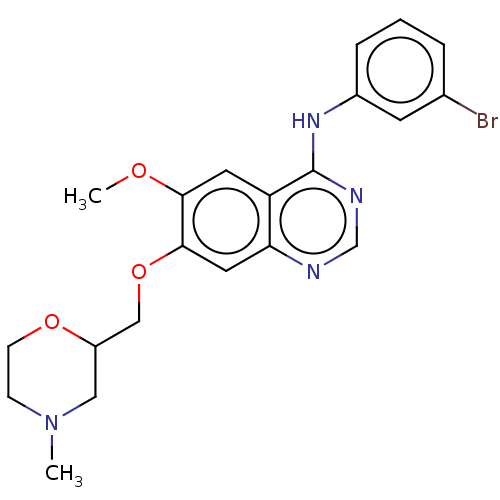 (CHEMBL3322975) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of EphA2 (unknown origin) pre-incubated with enzyme for 10 mins before adding peptide substrate and ATP using TK-substrate-biotin and SEB ... | Bioorg Med Chem Lett 24: 4080-3 (2014) Article DOI: 10.1016/j.bmcl.2014.07.081 BindingDB Entry DOI: 10.7270/Q2C53NHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G protein-coupled receptor kinase 5 (Homo sapiens (Human)) | BDBM50444125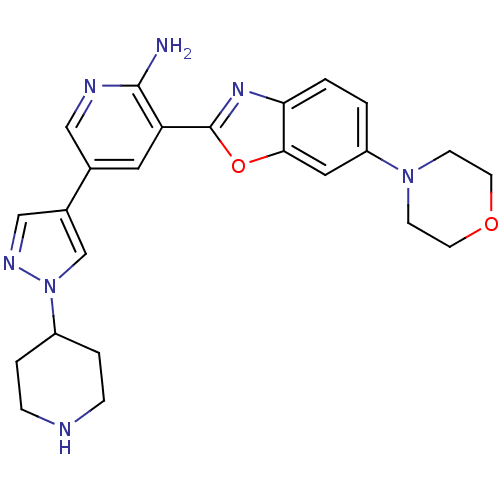 (CHEMBL3093150) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of GRK-5 (unknown origin) preincubated with enzyme for 10 mins before adding peptide substrate and ATP measured after 1 hr by LANCE-TR-FRE... | Bioorg Med Chem Lett 23: 6711-6 (2013) Article DOI: 10.1016/j.bmcl.2013.10.036 BindingDB Entry DOI: 10.7270/Q2SX6FPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 149 total ) | Next | Last >> |