

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50052204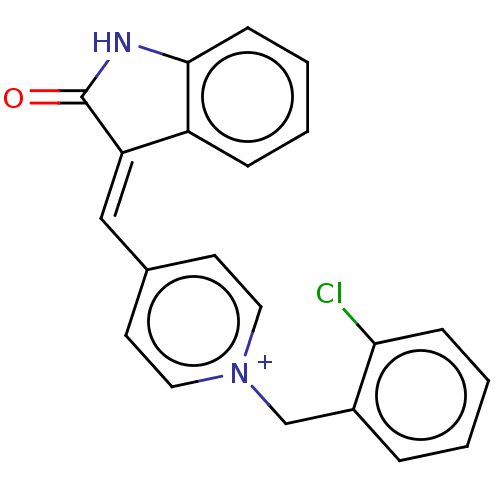 (CHEMBL3318392) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yazd University Curated by ChEMBL | Assay Description Mixed type inhibition of electric eel AChE using acetylthiocholine iodide substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 84: 375-81 (2014) Article DOI: 10.1016/j.ejmech.2014.01.017 BindingDB Entry DOI: 10.7270/Q2H996T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medicinal Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE pre-incubated for 3 mins before acetylthiocholine substrate addition by Lineweaver-Burk plot | Eur J Med Chem 97: 181-9 (2015) Article DOI: 10.1016/j.ejmech.2015.04.055 BindingDB Entry DOI: 10.7270/Q2P84DM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50440441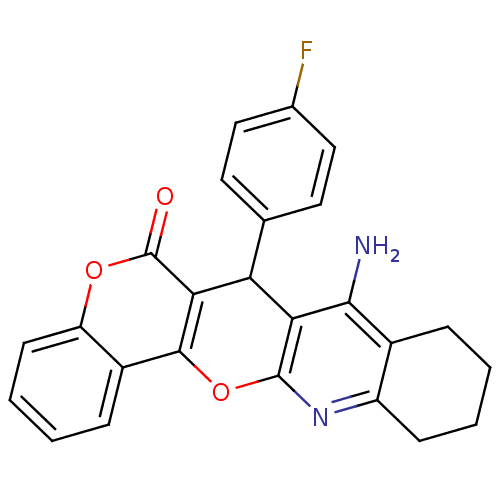 (CHEMBL2425846) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 68: 291-300 (2013) Article DOI: 10.1016/j.ejmech.2013.07.045 BindingDB Entry DOI: 10.7270/Q2794637 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50080392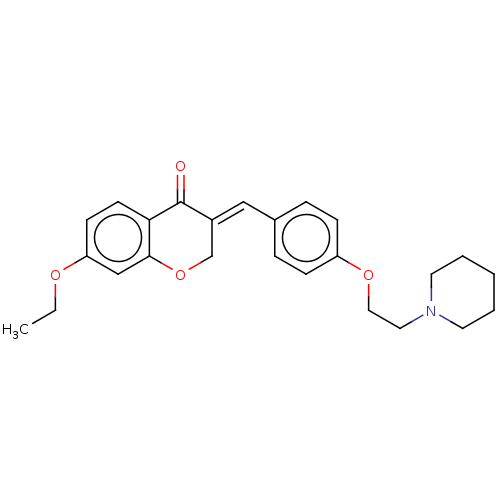 (CHEMBL3416513) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medicinal Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE pre-incubated for 3 mins before acetylthiocholine substrate addition by Lineweaver-Burk plot | Eur J Med Chem 97: 181-9 (2015) Article DOI: 10.1016/j.ejmech.2015.04.055 BindingDB Entry DOI: 10.7270/Q2P84DM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50038390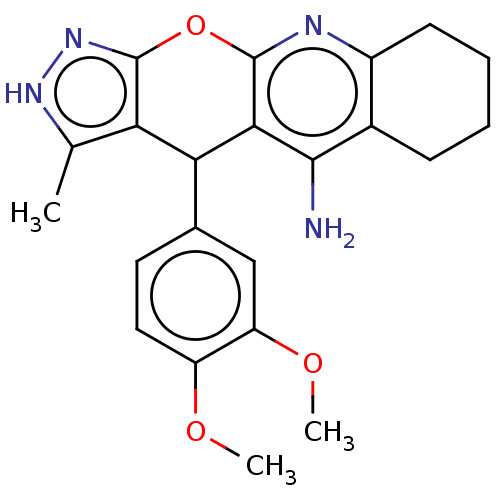 (CHEMBL3352904) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Lineweaver-Burk plot | Eur J Med Chem 89: 296-303 (2014) Article DOI: 10.1016/j.ejmech.2014.10.049 BindingDB Entry DOI: 10.7270/Q2T72K1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50204859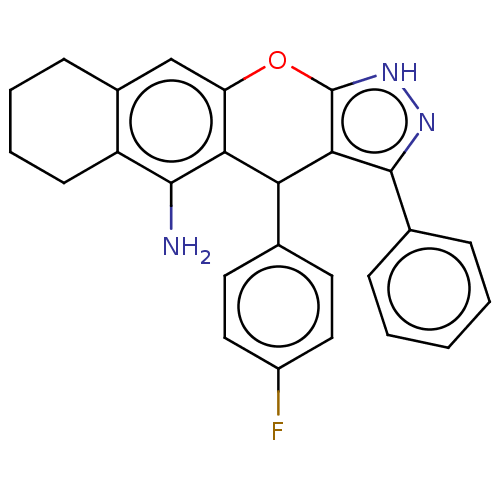 (CHEMBL3939483) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Mixed type inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured f... | Eur J Med Chem 123: 298-308 (2016) Article DOI: 10.1016/j.ejmech.2016.07.043 BindingDB Entry DOI: 10.7270/Q2V40X55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50197934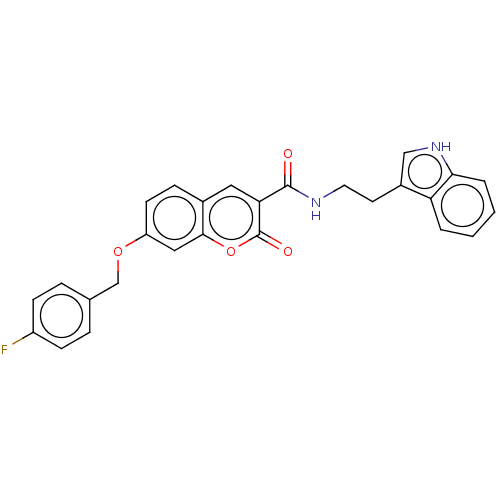 (CHEMBL3889532) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Islamic Azad University Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured for 2 mins by Lineweaver-Burk plot analysis | Eur J Med Chem 121: 40-46 (2016) Article DOI: 10.1016/j.ejmech.2016.05.014 BindingDB Entry DOI: 10.7270/Q2XS5XBG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020730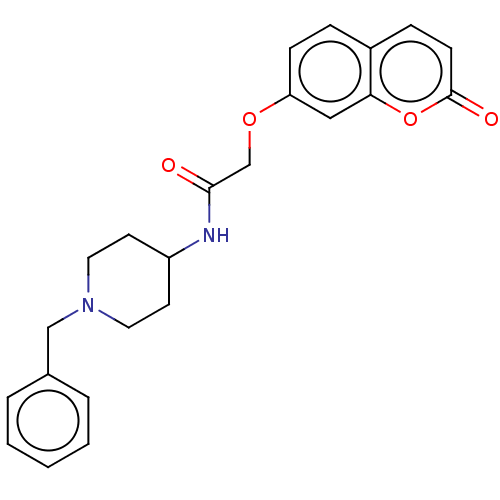 (CHEMBL1469070) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine as substrate by Lineweavere-Burk plot | Eur J Med Chem 82: 536-44 (2014) Article DOI: 10.1016/j.ejmech.2014.05.056 BindingDB Entry DOI: 10.7270/Q27D2WQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50235322 (CHEMBL4066593) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of rat cortex AchE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 128: 332-345 (2017) Article DOI: 10.1016/j.ejmech.2016.10.060 BindingDB Entry DOI: 10.7270/Q2HM5BQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50402577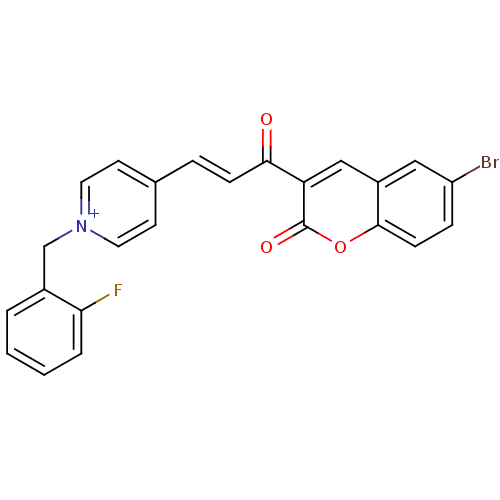 (CHEMBL2206129) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50402583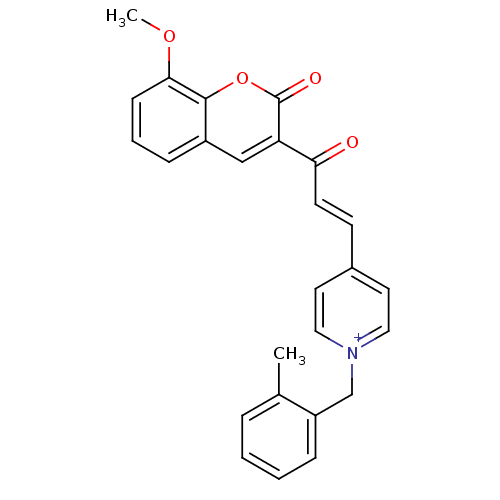 (CHEMBL2206123) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9009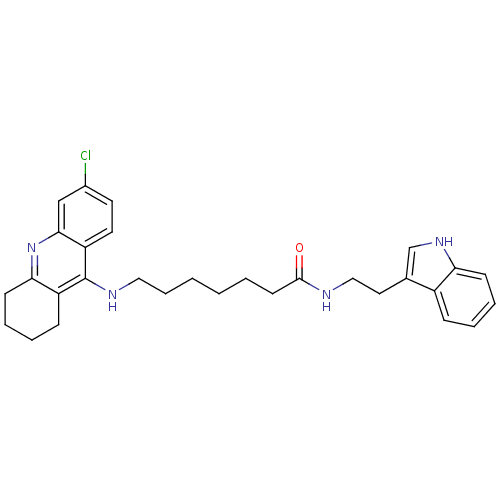 (7-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibitory activity was determined against bovine liver beta galactosidase | Eur J Med Chem 128: 332-345 (2017) Article DOI: 10.1016/j.ejmech.2016.10.060 BindingDB Entry DOI: 10.7270/Q2HM5BQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50235323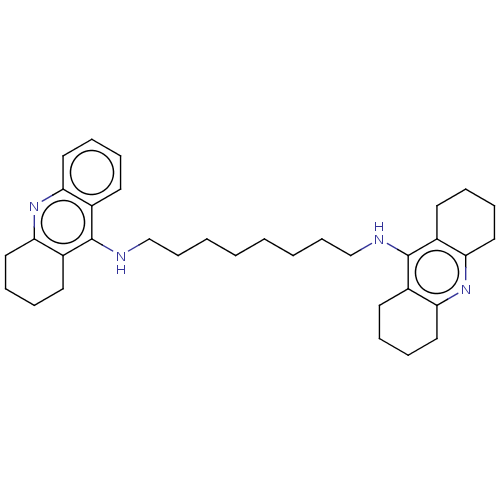 (CHEMBL4080023) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of rat cortex AchE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 128: 332-345 (2017) Article DOI: 10.1016/j.ejmech.2016.10.060 BindingDB Entry DOI: 10.7270/Q2HM5BQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50443517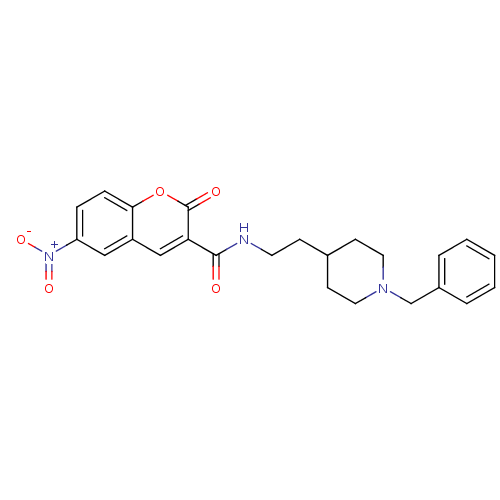 (CHEMBL3087899) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Kerman University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine as substrate preincubated for 15 mins prior to substrate addition by Ellman's method | Eur J Med Chem 70: 623-30 (2013) Article DOI: 10.1016/j.ejmech.2013.10.024 BindingDB Entry DOI: 10.7270/Q2GQ7070 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50235324 (CHEMBL4104258) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of rat cortex AchE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 128: 332-345 (2017) Article DOI: 10.1016/j.ejmech.2016.10.060 BindingDB Entry DOI: 10.7270/Q2HM5BQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50052204 (CHEMBL3318392) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Yazd University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman method based spectrophotometry | Eur J Med Chem 84: 375-81 (2014) Article DOI: 10.1016/j.ejmech.2014.01.017 BindingDB Entry DOI: 10.7270/Q2H996T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50402576 (CHEMBL2206130) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50402587 (CHEMBL2206119) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50402590 (CHEMBL2205576) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Science Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Bioorg Med Chem 20: 7214-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.052 BindingDB Entry DOI: 10.7270/Q2SQ91J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9006 (6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AchE using acetylthiocholine as substrate by Ellman's method | Eur J Med Chem 128: 332-345 (2017) Article DOI: 10.1016/j.ejmech.2016.10.060 BindingDB Entry DOI: 10.7270/Q2HM5BQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50052206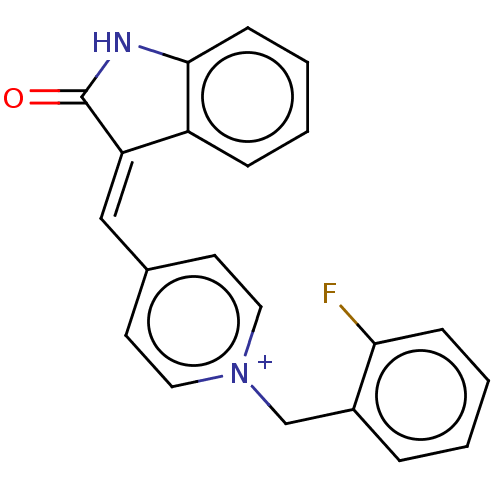 (CHEMBL3318391) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Yazd University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman method based spectrophotometry | Eur J Med Chem 84: 375-81 (2014) Article DOI: 10.1016/j.ejmech.2014.01.017 BindingDB Entry DOI: 10.7270/Q2H996T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50052208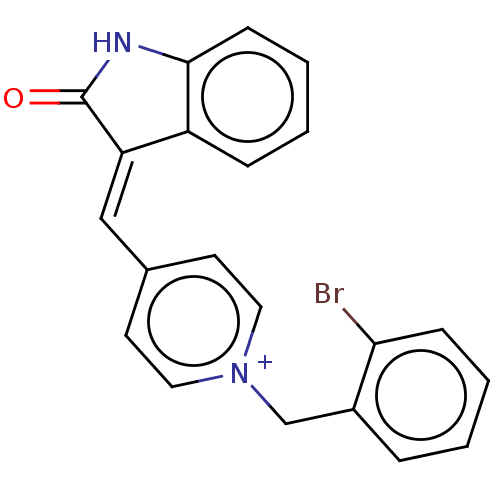 (CHEMBL3318394) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yazd University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman method based spectrophotometry | Eur J Med Chem 84: 375-81 (2014) Article DOI: 10.1016/j.ejmech.2014.01.017 BindingDB Entry DOI: 10.7270/Q2H996T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50189988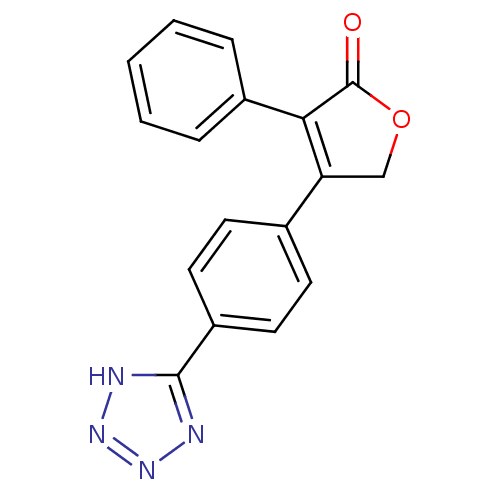 (CHEMBL214101 | Rofecoxib analogue) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of COX2 | Bioorg Med Chem Lett 16: 4483-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.032 BindingDB Entry DOI: 10.7270/Q2W095JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50189989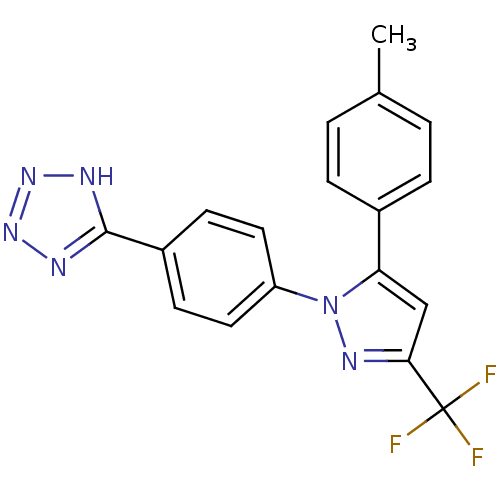 (4-(4-methylphenyl)-1-[4-(5-tetrazolyl)phenyl]-3-(t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of COX2 | Bioorg Med Chem Lett 16: 4483-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.032 BindingDB Entry DOI: 10.7270/Q2W095JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50443513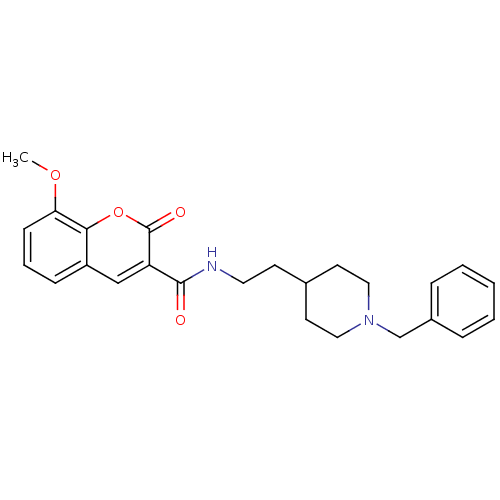 (CHEMBL3087903) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Kerman University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine as substrate preincubated for 15 mins prior to substrate addition by Ellman's method | Eur J Med Chem 70: 623-30 (2013) Article DOI: 10.1016/j.ejmech.2013.10.024 BindingDB Entry DOI: 10.7270/Q2GQ7070 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50443518 (CHEMBL3087898) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Kerman University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine as substrate preincubated for 15 mins prior to substrate addition by Ellman's method | Eur J Med Chem 70: 623-30 (2013) Article DOI: 10.1016/j.ejmech.2013.10.024 BindingDB Entry DOI: 10.7270/Q2GQ7070 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of COX2 | Bioorg Med Chem Lett 16: 4483-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.032 BindingDB Entry DOI: 10.7270/Q2W095JB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16047 ((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) expressed in baculovirus expression system using Rh-EVNLDAEFK-Quencher as substrate after 90 mins by FRET assay | Bioorg Med Chem 21: 2396-412 (2013) Article DOI: 10.1016/j.bmc.2013.01.064 BindingDB Entry DOI: 10.7270/Q2BV7J12 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of COX1 | Bioorg Med Chem Lett 16: 4483-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.032 BindingDB Entry DOI: 10.7270/Q2W095JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50189988 (CHEMBL214101 | Rofecoxib analogue) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of COX1 | Bioorg Med Chem Lett 16: 4483-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.032 BindingDB Entry DOI: 10.7270/Q2W095JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50073207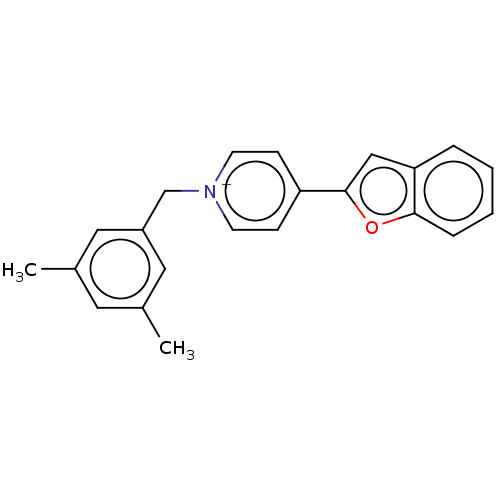 (CHEMBL3408398) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Islamic Azad University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine substrate by Ellman's reagent based method | Eur J Med Chem 93: 196-201 (2015) Article DOI: 10.1016/j.ejmech.2015.02.009 BindingDB Entry DOI: 10.7270/Q2CF9RTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50189989 (4-(4-methylphenyl)-1-[4-(5-tetrazolyl)phenyl]-3-(t...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of COX1 | Bioorg Med Chem Lett 16: 4483-7 (2006) Article DOI: 10.1016/j.bmcl.2006.06.032 BindingDB Entry DOI: 10.7270/Q2W095JB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50052210 (CHEMBL3318398) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Yazd University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman method based spectrophotometry | Eur J Med Chem 84: 375-81 (2014) Article DOI: 10.1016/j.ejmech.2014.01.017 BindingDB Entry DOI: 10.7270/Q2H996T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50052212 (CHEMBL3318400) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Yazd University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman method based spectrophotometry | Eur J Med Chem 84: 375-81 (2014) Article DOI: 10.1016/j.ejmech.2014.01.017 BindingDB Entry DOI: 10.7270/Q2H996T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50073217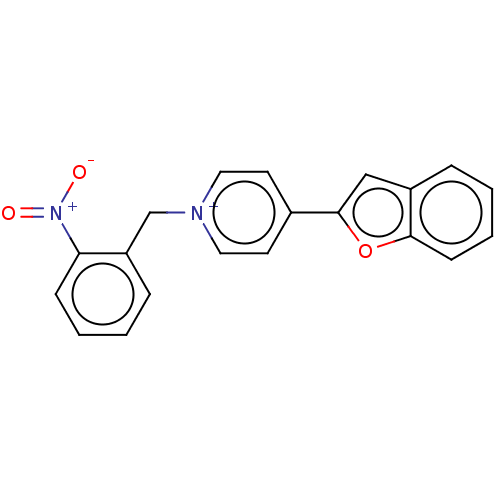 (CHEMBL3408242) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Islamic Azad University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine substrate by Ellman's reagent based method | Eur J Med Chem 93: 196-201 (2015) Article DOI: 10.1016/j.ejmech.2015.02.009 BindingDB Entry DOI: 10.7270/Q2CF9RTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50440441 (CHEMBL2425846) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins prior to substrate addition by Ellman's method | Eur J Med Chem 68: 291-300 (2013) Article DOI: 10.1016/j.ejmech.2013.07.045 BindingDB Entry DOI: 10.7270/Q2794637 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50443515 (CHEMBL3087901) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kerman University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylcholine as substrate preincubated for 15 mins prior to substrate addition by Ellman's method | Eur J Med Chem 70: 623-30 (2013) Article DOI: 10.1016/j.ejmech.2013.10.024 BindingDB Entry DOI: 10.7270/Q2GQ7070 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50204847 (CHEMBL3895802) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured for 1 ... | Eur J Med Chem 123: 298-308 (2016) Article DOI: 10.1016/j.ejmech.2016.07.043 BindingDB Entry DOI: 10.7270/Q2V40X55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50052214 (CHEMBL3318402) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Yazd University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman method based spectrophotometry | Eur J Med Chem 84: 375-81 (2014) Article DOI: 10.1016/j.ejmech.2014.01.017 BindingDB Entry DOI: 10.7270/Q2H996T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50073208 (CHEMBL3408397) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Islamic Azad University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine substrate by Ellman's reagent based method | Eur J Med Chem 93: 196-201 (2015) Article DOI: 10.1016/j.ejmech.2015.02.009 BindingDB Entry DOI: 10.7270/Q2CF9RTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50204842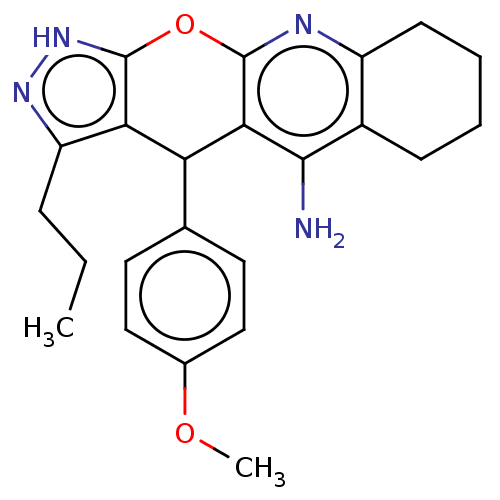 (CHEMBL3927698) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured for 1 ... | Eur J Med Chem 123: 298-308 (2016) Article DOI: 10.1016/j.ejmech.2016.07.043 BindingDB Entry DOI: 10.7270/Q2V40X55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50052215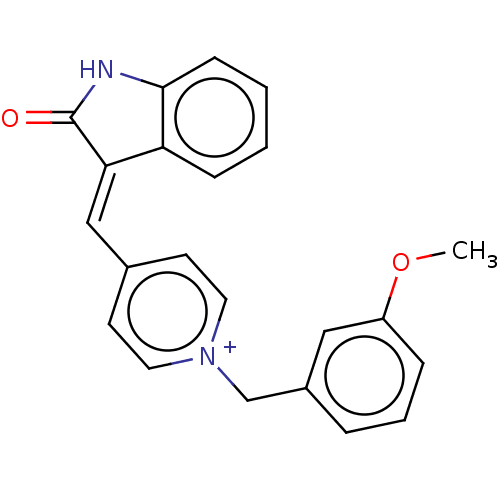 (CHEMBL3318403) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Yazd University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide substrate by Ellman method based spectrophotometry | Eur J Med Chem 84: 375-81 (2014) Article DOI: 10.1016/j.ejmech.2014.01.017 BindingDB Entry DOI: 10.7270/Q2H996T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50073220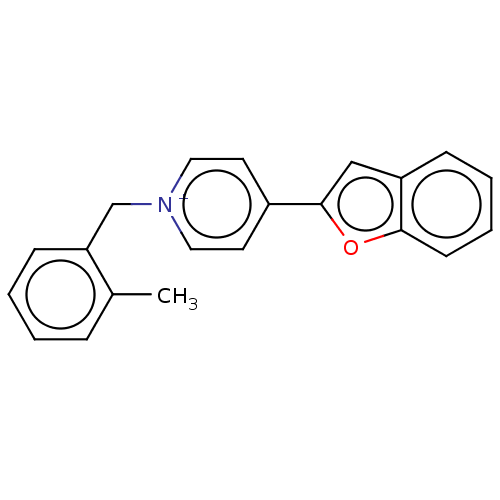 (CHEMBL3408241) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Islamic Azad University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine substrate by Ellman's reagent based method | Eur J Med Chem 93: 196-201 (2015) Article DOI: 10.1016/j.ejmech.2015.02.009 BindingDB Entry DOI: 10.7270/Q2CF9RTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50204851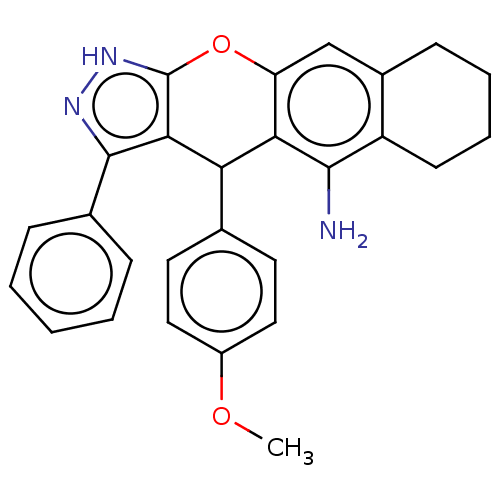 (CHEMBL3947445) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured for 1 ... | Eur J Med Chem 123: 298-308 (2016) Article DOI: 10.1016/j.ejmech.2016.07.043 BindingDB Entry DOI: 10.7270/Q2V40X55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50204859 (CHEMBL3939483) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured for 1 ... | Eur J Med Chem 123: 298-308 (2016) Article DOI: 10.1016/j.ejmech.2016.07.043 BindingDB Entry DOI: 10.7270/Q2V40X55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50204906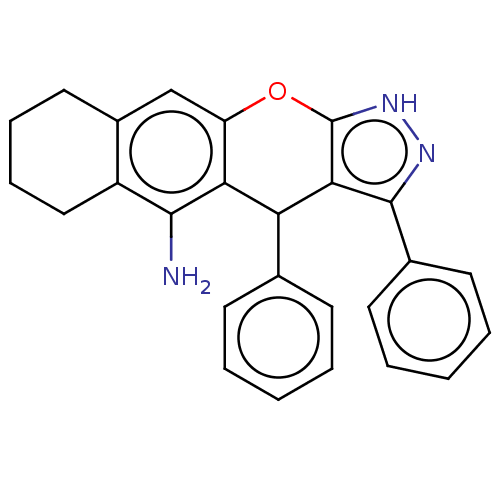 (CHEMBL3911514) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured for 1 ... | Eur J Med Chem 123: 298-308 (2016) Article DOI: 10.1016/j.ejmech.2016.07.043 BindingDB Entry DOI: 10.7270/Q2V40X55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50204852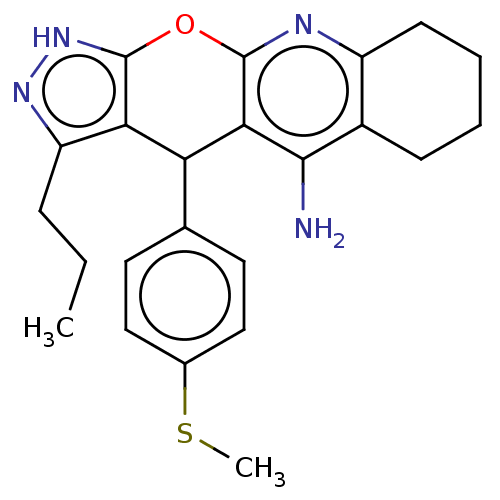 (CHEMBL3982934) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured for 1 ... | Eur J Med Chem 123: 298-308 (2016) Article DOI: 10.1016/j.ejmech.2016.07.043 BindingDB Entry DOI: 10.7270/Q2V40X55 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50073202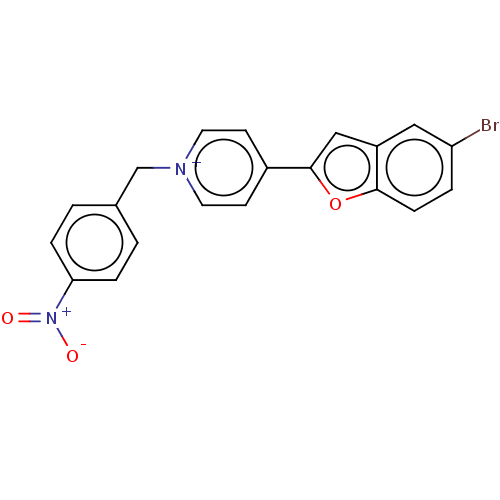 (CHEMBL3408403) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Islamic Azad University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine substrate by Ellman's reagent based method | Eur J Med Chem 93: 196-201 (2015) Article DOI: 10.1016/j.ejmech.2015.02.009 BindingDB Entry DOI: 10.7270/Q2CF9RTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50073204 (CHEMBL3408401) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Islamic Azad University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine substrate by Ellman's reagent based method | Eur J Med Chem 93: 196-201 (2015) Article DOI: 10.1016/j.ejmech.2015.02.009 BindingDB Entry DOI: 10.7270/Q2CF9RTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM13065 (5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-(trifluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences Curated by ChEMBL | Assay Description Inhibition of COX1 in human blood assessed as PGE2 level incubated for 15 mins prior to LPS-challenge measured after 24 hrs by enzyme immunoassay | Bioorg Med Chem 21: 2355-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.058 BindingDB Entry DOI: 10.7270/Q2VT1TFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 717 total ) | Next | Last >> |