 Found 76 hits with Last Name = 'shagufta' and Initial = 'na'
Found 76 hits with Last Name = 'shagufta' and Initial = 'na' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50233798
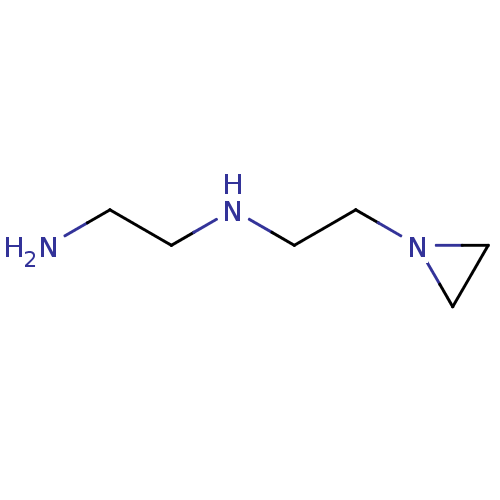
(CHEMBL398940 | N-(2-aminoethyl)-1-aziridine-ethana...)Show InChI InChI=1S/C6H15N3/c7-1-2-8-3-4-9-5-6-9/h8H,1-7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 4.59E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Functional Histamine H1 receptor antagonistic activity in vitro assay on guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123025
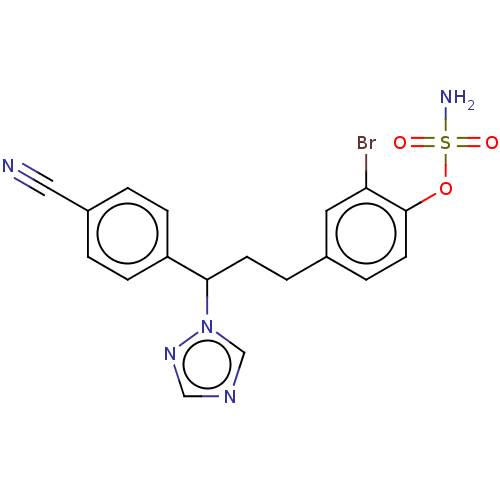
(CHEMBL3623231)Show SMILES NS(=O)(=O)Oc1ccc(CCC(c2ccc(cc2)C#N)n2cncn2)cc1Br Show InChI InChI=1S/C18H16BrN5O3S/c19-16-9-13(4-8-18(16)27-28(21,25)26)3-7-17(24-12-22-11-23-24)15-5-1-14(10-20)2-6-15/h1-2,4-6,8-9,11-12,17H,3,7H2,(H2,21,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human JEG-3 cells using [1beta-3H]androstenedione as substrate after 1 hr by scintillation spectrometry |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123026
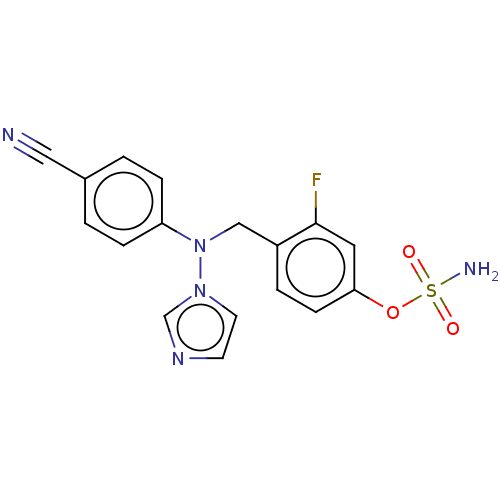
(CHEMBL3623232)Show SMILES NS(=O)(=O)Oc1ccc(CN(c2ccc(cc2)C#N)n2ccnc2)c(F)c1 Show InChI InChI=1S/C17H14FN5O3S/c18-17-9-16(26-27(20,24)25)6-3-14(17)11-23(22-8-7-21-12-22)15-4-1-13(10-19)2-5-15/h1-9,12H,11H2,(H2,20,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human JEG-3 cells using [1beta-3H]androstenedione as substrate after 1 hr by scintillation spectrometry |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50073850
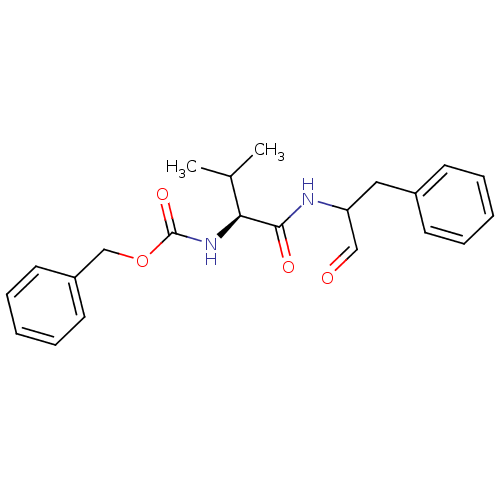
((S)-2-((S)-2-Benzyloxycarbonylamino-3-methyl-butyr...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccccc1)C=O |r| Show InChI InChI=1S/C22H26N2O4/c1-16(2)20(24-22(27)28-15-18-11-7-4-8-12-18)21(26)23-19(14-25)13-17-9-5-3-6-10-17/h3-12,14,16,19-20H,13,15H2,1-2H3,(H,23,26)(H,24,27)/t19?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
pA2 for NK2 receptor of human bladder IM9 cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50123026

(CHEMBL3623232)Show SMILES NS(=O)(=O)Oc1ccc(CN(c2ccc(cc2)C#N)n2ccnc2)c(F)c1 Show InChI InChI=1S/C17H14FN5O3S/c18-17-9-16(26-27(20,24)25)6-3-14(17)11-23(22-8-7-21-12-22)15-4-1-13(10-19)2-5-15/h1-9,12H,11H2,(H2,20,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human JEG-3 cells using [6,7-3H]E1S as substrate after 1 hr by scintillation spectrometry |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM24306
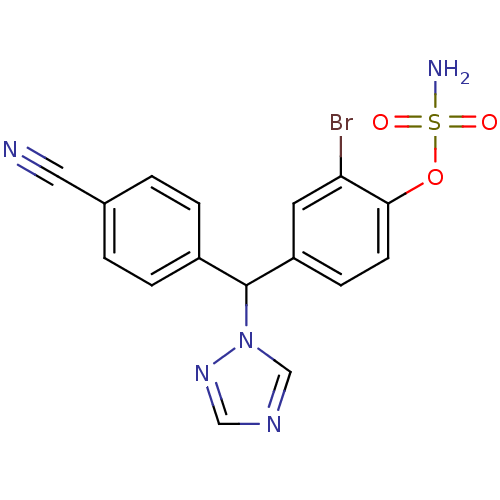
(Letrozole derivative, 40 | {2-bromo-4-[(4-cyanophe...)Show SMILES NS(=O)(=O)Oc1ccc(cc1Br)C(c1ccc(cc1)C#N)n1cncn1 Show InChI InChI=1S/C16H12BrN5O3S/c17-14-7-13(5-6-15(14)25-26(19,23)24)16(22-10-20-9-21-22)12-3-1-11(8-18)2-4-12/h1-7,9-10,16H,(H2,19,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase in human JEG-3 cells using [1beta-3H]androstenedione as substrate after 1 hr by scintillation spectrometry |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50398452
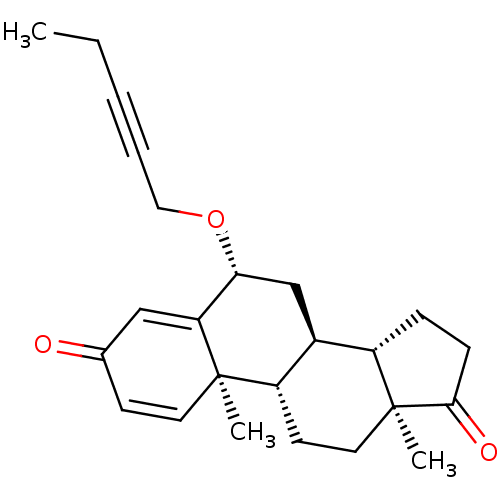
(CHEMBL2179110)Show SMILES CCC#CCO[C@@H]1C[C@H]2[C@@H]3CCC(=O)[C@@]3(C)CC[C@@H]2[C@@]2(C)C=CC(=O)C=C12 |r,c:23,t:27| Show InChI InChI=1S/C24H30O3/c1-4-5-6-13-27-21-15-17-18-7-8-22(26)24(18,3)12-10-19(17)23(2)11-9-16(25)14-20(21)23/h9,11,14,17-19,21H,4,7-8,10,12-13,15H2,1-3H3/t17-,18-,19-,21+,23+,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50324804
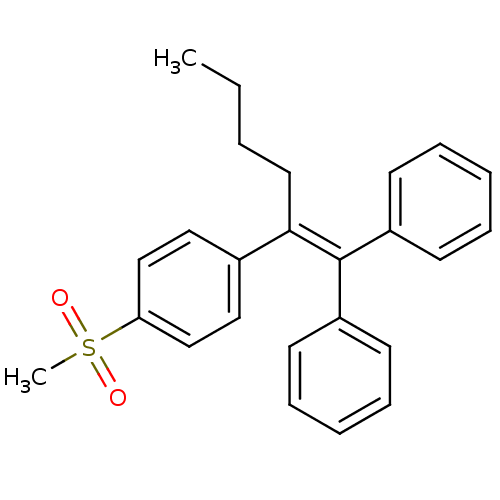
((2-(4-(methylsulfonyl)phenyl)hex-1-ene-1,1-diyl)di...)Show SMILES [#6]-[#6]-[#6]-[#6]\[#6](=[#6](/c1ccccc1)-c1ccccc1)-c1ccc(cc1)S([#6])(=O)=O Show InChI InChI=1S/C25H26O2S/c1-3-4-15-24(20-16-18-23(19-17-20)28(2,26)27)25(21-11-7-5-8-12-21)22-13-9-6-10-14-22/h5-14,16-19H,3-4,15H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 using arachidonic acid as substrate assessed as reduction in PGF2alpha production preincubated for 5 mins followed by substr... |
Eur J Med Chem 143: 515-531 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.056
BindingDB Entry DOI: 10.7270/Q21V5HMQ |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50464567

(CHEMBL4278683)Show SMILES CCC(Cc1ccccc1)=C(c1ccc(O)cc1)c1ccc(OCCN2CCCC2)cc1 Show InChI InChI=1S/C29H33NO2/c1-2-24(22-23-8-4-3-5-9-23)29(25-10-14-27(31)15-11-25)26-12-16-28(17-13-26)32-21-20-30-18-6-7-19-30/h3-5,8-17,31H,2,6-7,18-22H2,1H3/b29-24- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Binding affinity to estrogen receptor (unknown origin) |
Eur J Med Chem 143: 515-531 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.056
BindingDB Entry DOI: 10.7270/Q21V5HMQ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50035204

(4-(6-Methoxy-1a,2,3,7b-tetrahydro-1H-cyclopropa[a]...)Show InChI InChI=1S/C17H17NO/c1-19-13-4-2-11-3-5-14-16(17(14)15(11)10-13)12-6-8-18-9-7-12/h2,4,6-10,14,16-17H,3,5H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of human placental aromatase |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50035204

(4-(6-Methoxy-1a,2,3,7b-tetrahydro-1H-cyclopropa[a]...)Show InChI InChI=1S/C17H17NO/c1-19-13-4-2-11-3-5-14-16(17(14)15(11)10-13)12-6-8-18-9-7-12/h2,4,6-10,14,16-17H,3,5H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of human placental aromatase |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50075009
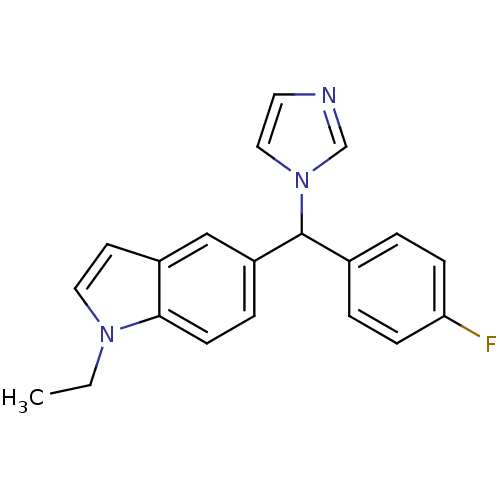
(1-Ethyl-5-[(4-fluoro-phenyl)-imidazol-1-yl-methyl]...)Show InChI InChI=1S/C20H18FN3/c1-2-23-11-9-16-13-17(5-8-19(16)23)20(24-12-10-22-14-24)15-3-6-18(21)7-4-15/h3-14,20H,2H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM420297

(acs.jmedchem.1c00409_ST.30 | med.21724, Compound 6...)Show SMILES Fc1cccc(C[C@H](NC(=O)c2cc3ccccc3[nH]2)C(=O)N[C@@H](C[C@@H]2CCNC2=O)C=O)c1 Show InChI InChI=1S/C25H25FN4O4/c26-18-6-3-4-15(10-18)11-21(24(33)28-19(14-31)12-17-8-9-27-23(17)32)30-25(34)22-13-16-5-1-2-7-20(16)29-22/h1-7,10,13-14,17,19,21,29H,8-9,11-12H2,(H,27,32)(H,28,33)(H,30,34)/t17-,19-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Functional H2 receptor antagonistic activity in vitro assay on the isolated spontaneously beating guinea-pig right atrium |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50097366

(4-((1H-imidazol-1-yl)methyl)-1-nitro-4aH-xanthen-9...)Show SMILES [O-][N+](=O)c1ccc(Cn2ccnc2)c2oc3ccccc3c(=O)c12 Show InChI InChI=1S/C17H11N3O4/c21-16-12-3-1-2-4-14(12)24-17-11(9-19-8-7-18-10-19)5-6-13(15(16)17)20(22)23/h1-8,10H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of human placental aromatase in presence of [1beta,2beta-3H] testosterone by Thompson and Siiteri method |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123152
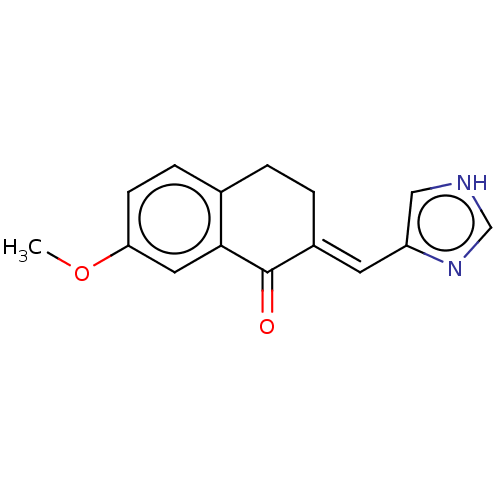
(CHEMBL3219600)Show InChI InChI=1S/C15H14N2O2/c1-19-13-5-4-10-2-3-11(15(18)14(10)7-13)6-12-8-16-9-17-12/h4-9H,2-3H2,1H3,(H,16,17)/b11-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123024
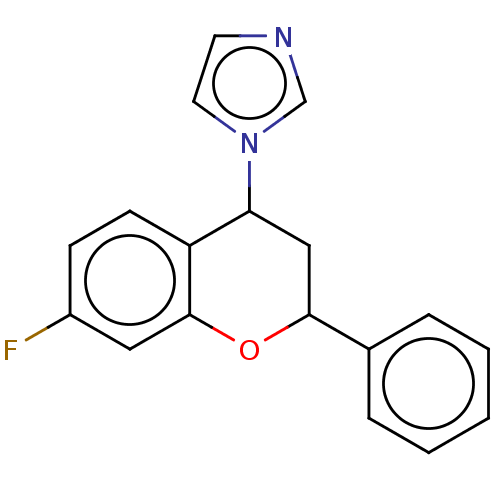
(CHEMBL3623227)Show InChI InChI=1S/C18H15FN2O/c19-14-6-7-15-16(21-9-8-20-12-21)11-17(22-18(15)10-14)13-4-2-1-3-5-13/h1-10,12,16-17H,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of human placental aromatase using [1,2,6,7-3H] androstenedione as substrate |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50097373
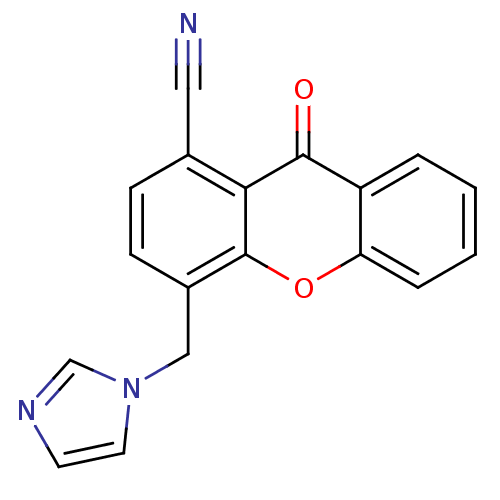
(4-((1H-imidazol-1-yl)methyl)-9-oxo-9H-xanthene-1-c...)Show InChI InChI=1S/C18H11N3O2/c19-9-12-5-6-13(10-21-8-7-20-11-21)18-16(12)17(22)14-3-1-2-4-15(14)23-18/h1-8,11H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of human placental aromatase in presence of [1beta,2beta-3H] testosterone by Thompson and Siiteri method |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9475
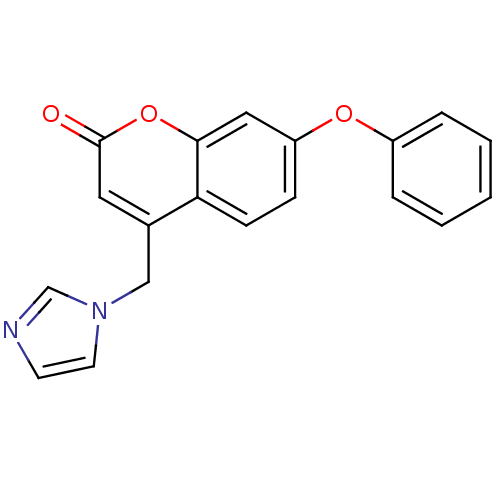
(4-(1H-Imidazol-1-ylmethyl)-7-phenoxy-2H-chromen-2-...)Show InChI InChI=1S/C19H14N2O3/c22-19-10-14(12-21-9-8-20-13-21)17-7-6-16(11-18(17)24-19)23-15-4-2-1-3-5-15/h1-11,13H,12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of human placental aromatase in presence of [1beta,2beta-3H] testosterone by Thompson and Siiteri method |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM420296
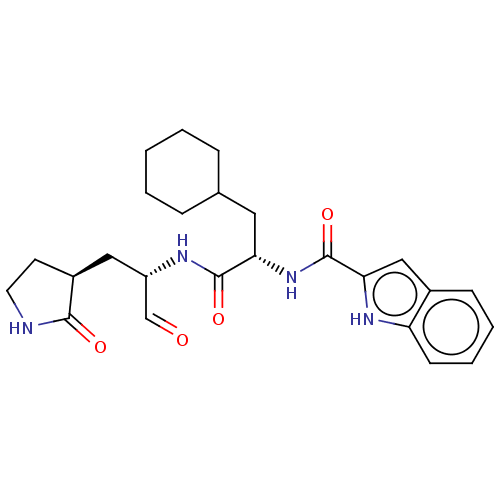
(Advanced SARS-CoV-2 Inhibitor 11a | MPI10 | acs.jm...)Show SMILES O=C[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@H](CC1CCCCC1)NC(=O)c1cc2ccccc2[nH]1 Show InChI InChI=1S/C25H32N4O4/c30-15-19(13-18-10-11-26-23(18)31)27-24(32)21(12-16-6-2-1-3-7-16)29-25(33)22-14-17-8-4-5-9-20(17)28-22/h4-5,8-9,14-16,18-19,21,28H,1-3,6-7,10-13H2,(H,26,31)(H,27,32)(H,29,33)/t18-,19-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Functional Histamine H1 receptor antagonistic activity in vitro assay on guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Mus musculus (Mouse)) | BDBM50407514

(CHEMBL1234732)Show InChI InChI=1S/C18H21N5S/c1-3-23(4-2)12-8-10-13(11-9-12)24-15-7-5-6-14-16(15)17(19)22-18(20)21-14/h5-11H,3-4H2,1-2H3,(H4,19,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Functional H2 receptor antagonistic activity in vitro assay on the isolated spontaneously beating guinea-pig right atrium |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM20607
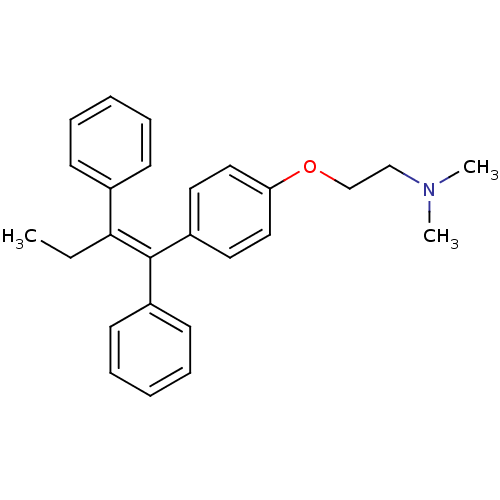
((2-{4-[(1Z)-1,2-diphenylbut-1-en-1-yl]phenoxy}ethy...)Show SMILES CC\C(=C(/c1ccccc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO/c1-4-25(21-11-7-5-8-12-21)26(22-13-9-6-10-14-22)23-15-17-24(18-16-23)28-20-19-27(2)3/h5-18H,4,19-20H2,1-3H3/b26-25- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Displacement of [3H]E2 from human recombinant ERalpha assessed as receptor binding after 45 mins by scintillation counting method |
Eur J Med Chem 143: 515-531 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.056
BindingDB Entry DOI: 10.7270/Q21V5HMQ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123153
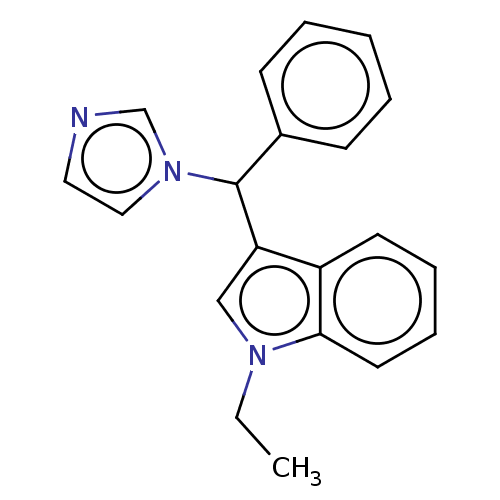
(CHEMBL3623225)Show InChI InChI=1S/C20H19N3/c1-2-22-14-18(17-10-6-7-11-19(17)22)20(23-13-12-21-15-23)16-8-4-3-5-9-16/h3-15,20H,2H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123016

(CHEMBL3623214)Show SMILES [H][C@@]12C[C@@H](C(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)C=C[C@]12C)n1ccnc1 |r,c:22,t:18| Show InChI InChI=1S/C22H26N2O2/c1-21-7-5-15(25)11-14(21)3-4-16-17(21)6-8-22(2)18(16)12-19(20(22)26)24-10-9-23-13-24/h5,7,9-11,13,16-19H,3-4,6,8,12H2,1-2H3/t16-,17+,18+,19+,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Rattus norvegicus) | BDBM50049763
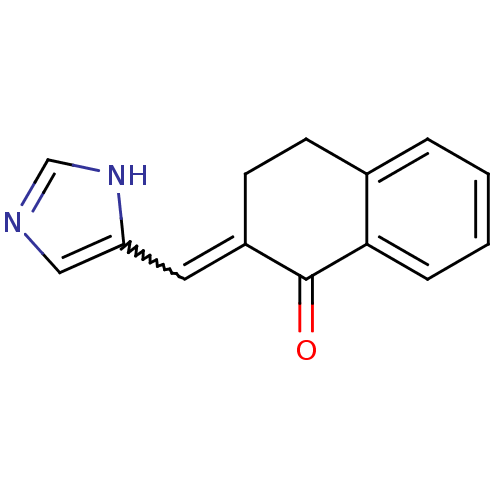
(2-[1-(3H-Imidazol-4-yl)-meth-(E)-ylidene]-3,4-dihy...)Show InChI InChI=1S/C14H12N2O/c17-14-11(7-12-8-15-9-16-12)6-5-10-3-1-2-4-13(10)14/h1-4,7-9H,5-6H2,(H,15,16) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of rat ovarian aromatase |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123012
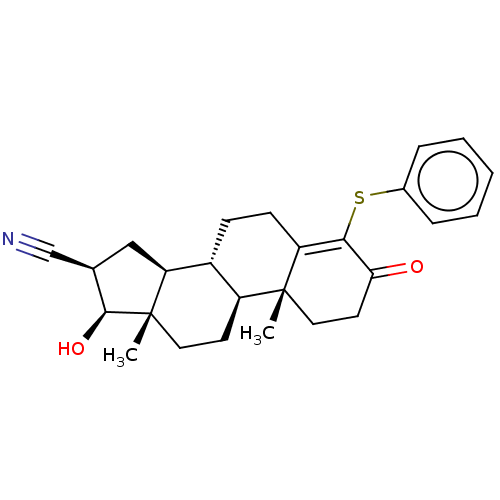
(CHEMBL3623217)Show SMILES [H][C@@]12C[C@H](C#N)[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=C(Sc3ccccc3)C(=O)CC[C@]12C |r,c:20| Show InChI InChI=1S/C26H31NO2S/c1-25-13-11-22(28)23(30-17-6-4-3-5-7-17)20(25)9-8-18-19(25)10-12-26(2)21(18)14-16(15-27)24(26)29/h3-7,16,18-19,21,24,29H,8-14H2,1-2H3/t16-,18-,19+,21+,24+,25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM420283
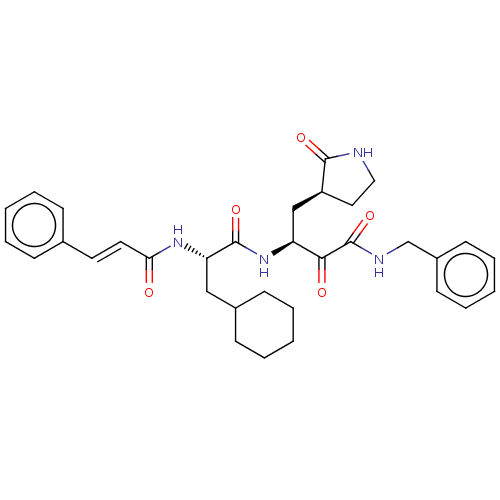
(alpha-ketoamide inhibitor 11r | med.21724, Compoun...)Show SMILES O=C(N[C@@H](CC1CCCCC1)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(=O)C(=O)NCc1ccccc1)\C=C\c1ccccc1 Show InChI InChI=1S/C33H40N4O5/c38-29(17-16-23-10-4-1-5-11-23)36-28(20-24-12-6-2-7-13-24)32(41)37-27(21-26-18-19-34-31(26)40)30(39)33(42)35-22-25-14-8-3-9-15-25/h1,3-5,8-11,14-17,24,26-28H,2,6-7,12-13,18-22H2,(H,34,40)(H,35,42)(H,36,38)(H,37,41)/b17-16+/t26-,27-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Functional Histamine H1 receptor antagonistic activity in vitro assay on guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123017

(CHEMBL3623213)Show SMILES [H][C@@]12C[C@@H](C(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C)n1ccnc1 |r,t:18| Show InChI InChI=1S/C22H28N2O2/c1-21-7-5-15(25)11-14(21)3-4-16-17(21)6-8-22(2)18(16)12-19(20(22)26)24-10-9-23-13-24/h9-11,13,16-19H,3-8,12H2,1-2H3/t16-,17+,18+,19+,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123023

(CHEMBL3623226)Show InChI InChI=1S/C17H12FN3O/c18-14-7-5-12(6-8-14)17(21-11-19-10-20-21)16-9-13-3-1-2-4-15(13)22-16/h1-11,17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of human placental aromatase |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Mus musculus (Mouse)) | BDBM50407515

(CHEMBL5285968)Show InChI InChI=1S/C17H18N4O3S/c1-22-11-7-9(8-12(23-2)15(11)24-3)25-13-6-4-5-10-14(13)16(18)21-17(19)20-10/h4-8H,1-3H3,(H4,18,19,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Functional H2 receptor antagonistic activity in vitro assay on the isolated spontaneously beating guinea-pig right atrium |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123019

(CHEMBL3621226)Show SMILES [H][C@@]12C=CC3=CC(=O)CC[C@]3(C)[C@@]1([H])CC[C@@](C)([C@H]2CC#N)C(C)=O |r,c:2,t:4| Show InChI InChI=1S/C20H25NO2/c1-13(22)19(2)10-7-17-16(18(19)8-11-21)5-4-14-12-15(23)6-9-20(14,17)3/h4-5,12,16-18H,6-10H2,1-3H3/t16-,17+,18+,19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM20607

((2-{4-[(1Z)-1,2-diphenylbut-1-en-1-yl]phenoxy}ethy...)Show SMILES CC\C(=C(/c1ccccc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO/c1-4-25(21-11-7-5-8-12-21)26(22-13-9-6-10-14-22)23-15-17-24(18-16-23)28-20-19-27(2)3/h5-18H,4,19-20H2,1-3H3/b26-25- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 222 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor (unknown origin) |
Eur J Med Chem 143: 515-531 (2018)
Article DOI: 10.1016/j.ejmech.2017.11.056
BindingDB Entry DOI: 10.7270/Q21V5HMQ |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123020
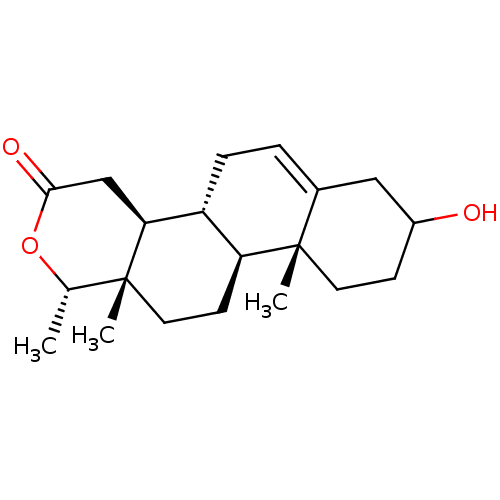
(CHEMBL3623209)Show SMILES [H][C@@]12CC=C3CC(O)CC[C@]3(C)[C@@]1([H])CC[C@]1(C)[C@H](C)OC(=O)C[C@@]21[H] |r,t:3| Show InChI InChI=1S/C20H30O3/c1-12-19(2)9-7-16-15(17(19)11-18(22)23-12)5-4-13-10-14(21)6-8-20(13,16)3/h4,12,14-17,21H,5-11H2,1-3H3/t12-,14?,15+,16-,17-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Rattus norvegicus) | BDBM50123022

(CHEMBL169251)Show InChI InChI=1S/C14H12N2O/c17-14-11(7-12-8-15-9-16-12)6-5-10-3-1-2-4-13(10)14/h1-4,7-9H,5-6H2,(H,15,16)/b11-7- | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of rat ovarian aromatase |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123021

(CHEMBL3623208)Show SMILES [H][C@@]12CCC3=CC(=O)CC[C@]3(C)[C@@]1([H])CC[C@@](C)([C@H]2CC#N)C(C)=O |r,t:4| Show InChI InChI=1S/C20H27NO2/c1-13(22)19(2)10-7-17-16(18(19)8-11-21)5-4-14-12-15(23)6-9-20(14,17)3/h12,16-18H,4-10H2,1-3H3/t16-,17+,18+,19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21489
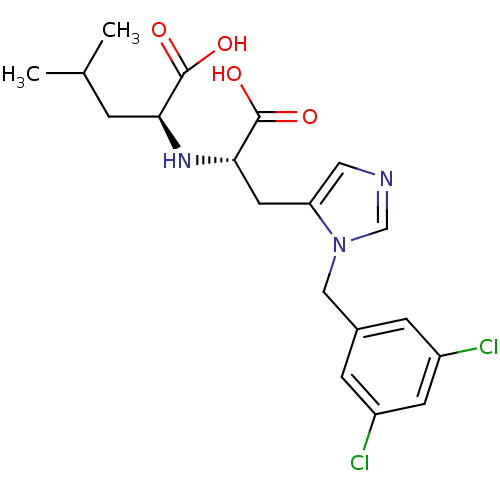
((2S)-2-{[(1S)-1-carboxy-2-{1-[(3,5-dichlorophenyl)...)Show SMILES CC(C)C[C@H](N[C@@H](Cc1cncn1Cc1cc(Cl)cc(Cl)c1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H23Cl2N3O4/c1-11(2)3-16(18(25)26)23-17(19(27)28)7-15-8-22-10-24(15)9-12-4-13(20)6-14(21)5-12/h4-6,8,10-11,16-17,23H,3,7,9H2,1-2H3,(H,25,26)(H,27,28)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Functional H2 receptor antagonistic activity in vitro assay on the isolated spontaneously beating guinea-pig right atrium |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aromatase
(Homo sapiens (Human)) | BDBM50123151
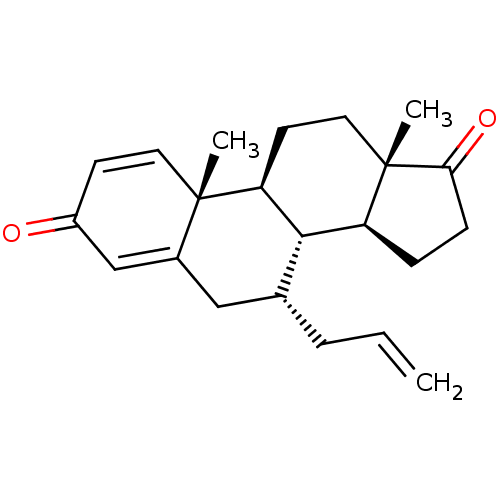
(CHEMBL3623223)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])[C@H](CC=C)CC2=CC(=O)C=C[C@]12C |r,c:25,t:21| Show InChI InChI=1S/C22H28O2/c1-4-5-14-12-15-13-16(23)8-10-21(15,2)18-9-11-22(3)17(20(14)18)6-7-19(22)24/h4,8,10,13-14,17-18,20H,1,5-7,9,11-12H2,2-3H3/t14-,17+,18+,20+,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123030
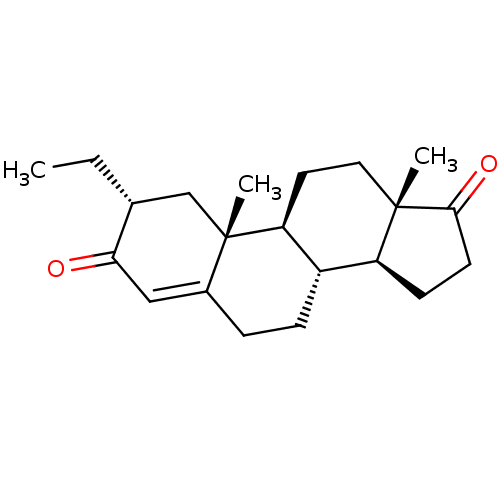
(CHEMBL3623219)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)[C@H](CC)C[C@]12C |r,t:18| Show InChI InChI=1S/C21H30O2/c1-4-13-12-21(3)14(11-18(13)22)5-6-15-16-7-8-19(23)20(16,2)10-9-17(15)21/h11,13,15-17H,4-10,12H2,1-3H3/t13-,15+,16+,17+,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM7461
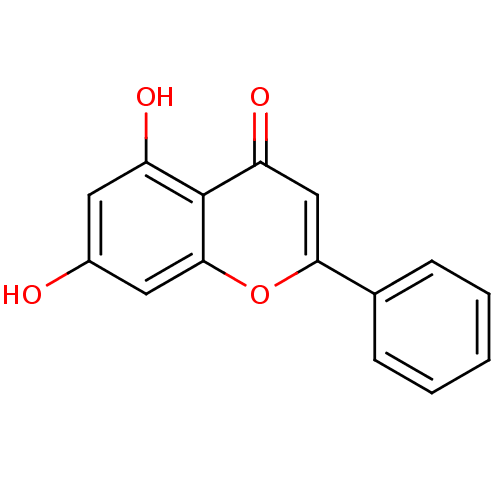
(5,7-dihydroxy-2-phenyl-4H-chromen-4-one | 5,7-dihy...)Show InChI InChI=1S/C15H10O4/c16-10-6-11(17)15-12(18)8-13(19-14(15)7-10)9-4-2-1-3-5-9/h1-8,16-17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10027
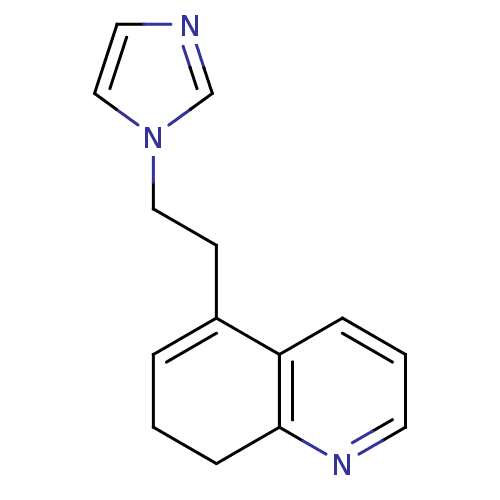
(5-[2-(1H-imidazol-1-yl)ethyl]-7,8-dihydroquinoline...)Show InChI InChI=1S/C14H15N3/c1-3-12(6-9-17-10-8-15-11-17)13-4-2-7-16-14(13)5-1/h2-4,7-8,10-11H,1,5-6,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123014

(CHEMBL3623215)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])C(=O)c3[nH]ncc3C[C@]12C |r| Show InChI InChI=1S/C20H28N2O2/c1-19-8-7-14-12(13(19)5-6-16(19)23)3-4-15-18(24)17-11(10-21-22-17)9-20(14,15)2/h10,12-16,23H,3-9H2,1-2H3,(H,21,22)/t12-,13-,14-,15-,16-,19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50407519
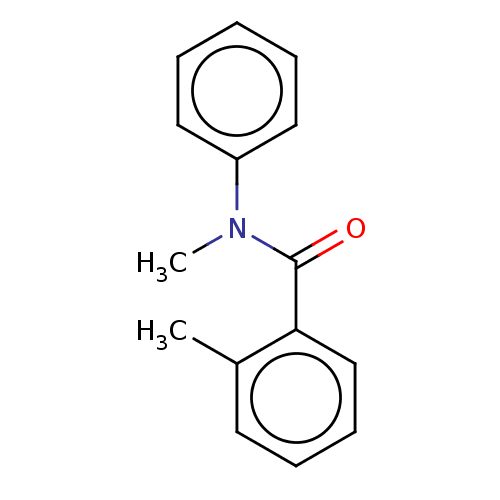
(CHEMBL5268979)Show InChI InChI=1S/C4H7N3/c5-1-4-2-6-3-7-4/h2-3H,1,5H2,(H,6,7) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Functional H2 receptor antagonistic activity in vitro assay on the isolated spontaneously beating guinea-pig right atrium |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM420295
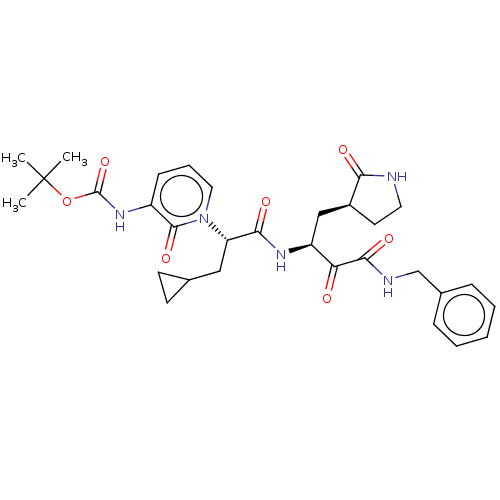
(alpha-ketoamide inhibitor 13b | med.21724, Compoun...)Show SMILES CC(C)(C)OC(=O)Nc1cccn([C@@H](CC2CC2)C(=O)N[C@@H](C[C@@H]2CCNC2=O)C(=O)C(=O)NCc2ccccc2)c1=O Show InChI InChI=1S/C31H39N5O7/c1-31(2,3)43-30(42)35-22-10-7-15-36(29(22)41)24(16-19-11-12-19)27(39)34-23(17-21-13-14-32-26(21)38)25(37)28(40)33-18-20-8-5-4-6-9-20/h4-10,15,19,21,23-24H,11-14,16-18H2,1-3H3,(H,32,38)(H,33,40)(H,34,39)(H,35,42)/t21-,23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
UniChem
| | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
pA2 value against histamine h4 receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123011

(CHEMBL3623218)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)[C@H](F)C[C@]12C |r,t:18| Show InChI InChI=1S/C19H25FO2/c1-18-8-7-14-12(13(18)5-6-17(18)22)4-3-11-9-16(21)15(20)10-19(11,14)2/h9,12-15H,3-8,10H2,1-2H3/t12-,13-,14-,15+,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123013
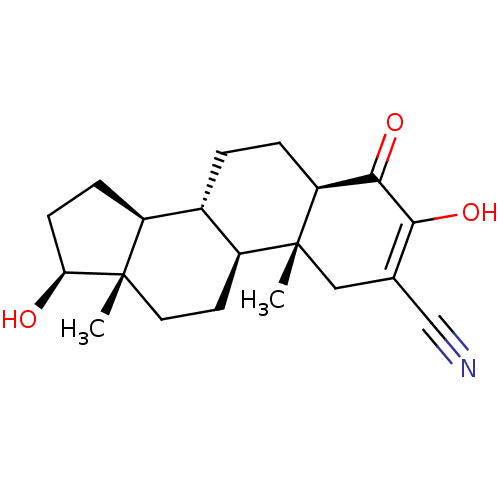
(CHEMBL3623216)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])C(=O)C(O)=C(C[C@]12C)C#N |r,c:23| Show InChI InChI=1S/C20H27NO3/c1-19-8-7-14-12(13(19)5-6-16(19)22)3-4-15-18(24)17(23)11(10-21)9-20(14,15)2/h12-16,22-23H,3-9H2,1-2H3/t12-,13-,14-,15-,16-,19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM7458

(5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...)Show InChI InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of aromatase (unknown origin) |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50407516

(CHEMBL5280512)Show InChI InChI=1S/C19H20N4O2S/c20-18-17-15(22-19(21)23-18)2-1-3-16(17)26-14-6-4-12(5-7-14)25-13-8-10-24-11-9-13/h1-7,13H,8-11H2,(H4,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Functional Histamine H1 receptor antagonistic activity in vitro assay on guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50431275
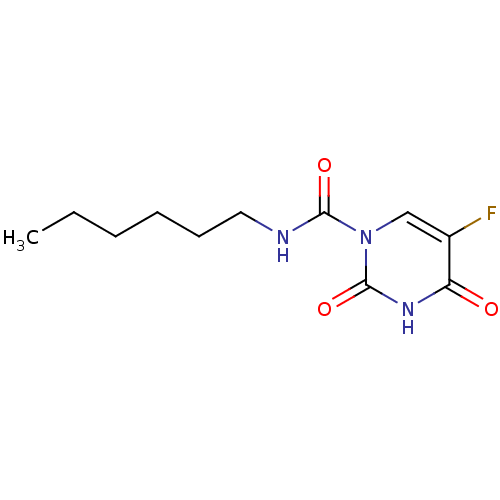
(CARMOFUR | Carm-ofur | Mifurol | med.21724, Compou...)Show InChI InChI=1S/C11H16FN3O3/c1-2-3-4-5-6-13-10(17)15-7-8(12)9(16)14-11(15)18/h7H,2-6H2,1H3,(H,13,17)(H,14,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Functional Histamine H1 receptor antagonistic activity in vitro assay on guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM420293
(alpha-ketoamide inhibitor 13a)Show SMILES CC(C)(C)OC(=O)Nc1cccn([C@@H](CC2CCCCC2)C(=O)N[C@@H](C[C@@H]2CCNC2=O)C(=O)C(=O)NC2CC2)c1=O Show InChI InChI=1S/C30H43N5O7/c1-30(2,3)42-29(41)34-21-10-7-15-35(28(21)40)23(16-18-8-5-4-6-9-18)26(38)33-22(17-19-13-14-31-25(19)37)24(36)27(39)32-20-11-12-20/h7,10,15,18-20,22-23H,4-6,8-9,11-14,16-17H2,1-3H3,(H,31,37)(H,32,39)(H,33,38)(H,34,41)/t19-,22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Functional Histamine H1 receptor antagonistic activity in vitro assay on guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50123027
(CHEMBL3623212)Show SMILES [H][C@@]12C\C(=C/c3ccc(OCCCn4ccnc4)c(OC)c3)[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@@H](O)CC[C@]12C |r,t:37| Show InChI InChI=1S/C33H44N2O4/c1-32-11-9-25(36)20-24(32)6-7-26-27(32)10-12-33(2)28(26)19-23(31(33)37)17-22-5-8-29(30(18-22)38-3)39-16-4-14-35-15-13-34-21-35/h5-6,8,13,15,17-18,21,25-28,31,36-37H,4,7,9-12,14,16,19-20H2,1-3H3/b23-17+/t25-,26+,27-,28-,31-,32-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American University of Ras Al Khaimah
Curated by ChEMBL
| Assay Description
Inhibition of human placental aromatase assessed as conversion of 3H2O from [1beta,2beta-3H] testosterone after 20 mins by scintillation spectrometer... |
Eur J Med Chem 102: 375-86 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.010
BindingDB Entry DOI: 10.7270/Q2ZC84Q6 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM7781
(4-Benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione ...)Show InChI InChI=1S/C10H10N2O2S/c1-11-9(13)12(10(14)15-11)7-8-5-3-2-4-6-8/h2-6H,7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Functional Histamine H3 receptor antagonistic activity in vitro assay on guinea pig ileum |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data