 Found 5 hits Enz. Inhib. hit(s) with Target = 'Angiotensin-converting enzyme 2' and Ligand = 'BDBM21489'
Found 5 hits Enz. Inhib. hit(s) with Target = 'Angiotensin-converting enzyme 2' and Ligand = 'BDBM21489' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21489
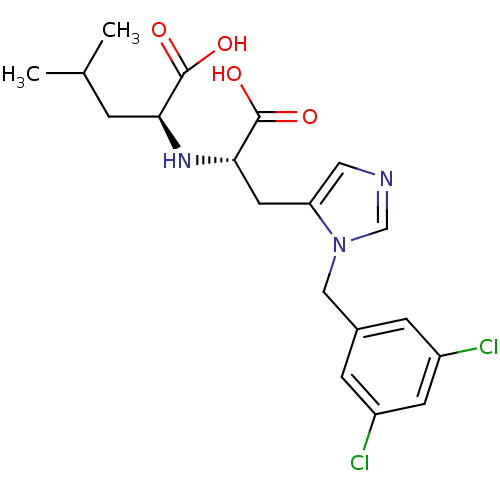
((2S)-2-{[(1S)-1-carboxy-2-{1-[(3,5-dichlorophenyl)...)Show SMILES CC(C)C[C@H](N[C@@H](Cc1cncn1Cc1cc(Cl)cc(Cl)c1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H23Cl2N3O4/c1-11(2)3-16(18(25)26)23-17(19(27)28)7-15-8-22-10-24(15)9-12-4-13(20)6-14(21)5-12/h4-6,8,10-11,16-17,23H,3,7,9H2,1-2H3,(H,25,26)(H,27,28)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Millennium Pharmaceuticals
| Assay Description
Enzyme assay was conducted in 384-well microplates format. Activity was monitored by measuring increase in fluorescence (excitation @320 nm, emission... |
J Am Chem Soc 124: 11852-3 (2002)
Article DOI: 10.1021/ja0277226
BindingDB Entry DOI: 10.7270/Q2KP80FN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21489

((2S)-2-{[(1S)-1-carboxy-2-{1-[(3,5-dichlorophenyl)...)Show SMILES CC(C)C[C@H](N[C@@H](Cc1cncn1Cc1cc(Cl)cc(Cl)c1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H23Cl2N3O4/c1-11(2)3-16(18(25)26)23-17(19(27)28)7-15-8-22-10-24(15)9-12-4-13(20)6-14(21)5-12/h4-6,8,10-11,16-17,23H,3,7,9H2,1-2H3,(H,25,26)(H,27,28)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ACE2 |
Bioorg Med Chem Lett 18: 1681-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.046
BindingDB Entry DOI: 10.7270/Q29K49Z2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21489

((2S)-2-{[(1S)-1-carboxy-2-{1-[(3,5-dichlorophenyl)...)Show SMILES CC(C)C[C@H](N[C@@H](Cc1cncn1Cc1cc(Cl)cc(Cl)c1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H23Cl2N3O4/c1-11(2)3-16(18(25)26)23-17(19(27)28)7-15-8-22-10-24(15)9-12-4-13(20)6-14(21)5-12/h4-6,8,10-11,16-17,23H,3,7,9H2,1-2H3,(H,25,26)(H,27,28)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL)
Curated by ChEMBL
| Assay Description
Inhibition of ACE2 (unknown origin) |
J Med Chem 63: 1978-1995 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01780
BindingDB Entry DOI: 10.7270/Q20868NG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21489

((2S)-2-{[(1S)-1-carboxy-2-{1-[(3,5-dichlorophenyl)...)Show SMILES CC(C)C[C@H](N[C@@H](Cc1cncn1Cc1cc(Cl)cc(Cl)c1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H23Cl2N3O4/c1-11(2)3-16(18(25)26)23-17(19(27)28)7-15-8-22-10-24(15)9-12-4-13(20)6-14(21)5-12/h4-6,8,10-11,16-17,23H,3,7,9H2,1-2H3,(H,25,26)(H,27,28)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116389
BindingDB Entry DOI: 10.7270/Q2GF0ZM3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21489

((2S)-2-{[(1S)-1-carboxy-2-{1-[(3,5-dichlorophenyl)...)Show SMILES CC(C)C[C@H](N[C@@H](Cc1cncn1Cc1cc(Cl)cc(Cl)c1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H23Cl2N3O4/c1-11(2)3-16(18(25)26)23-17(19(27)28)7-15-8-22-10-24(15)9-12-4-13(20)6-14(21)5-12/h4-6,8,10-11,16-17,23H,3,7,9H2,1-2H3,(H,25,26)(H,27,28)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Functional H2 receptor antagonistic activity in vitro assay on the isolated spontaneously beating guinea-pig right atrium |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data