 Found 74 hits with Last Name = 'shahabi' and Initial = 'd'
Found 74 hits with Last Name = 'shahabi' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50613803
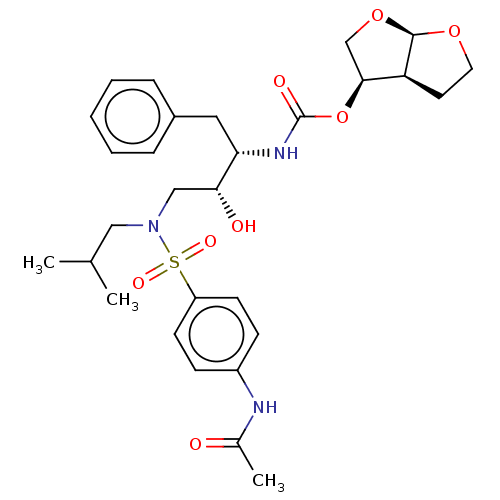
(CHEMBL5291125)Show SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(NC(C)=O)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50613809
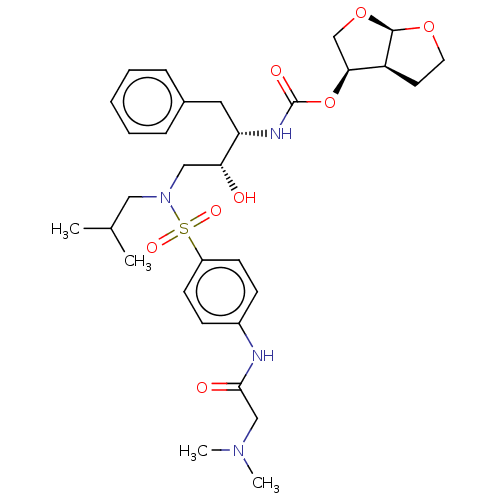
(CHEMBL5266228)Show SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(NC(=O)CN(C)C)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50613802
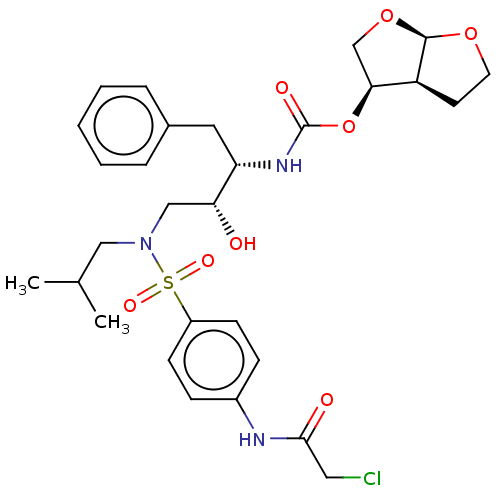
(CHEMBL5287201)Show SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(NC(=O)CCl)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50613808
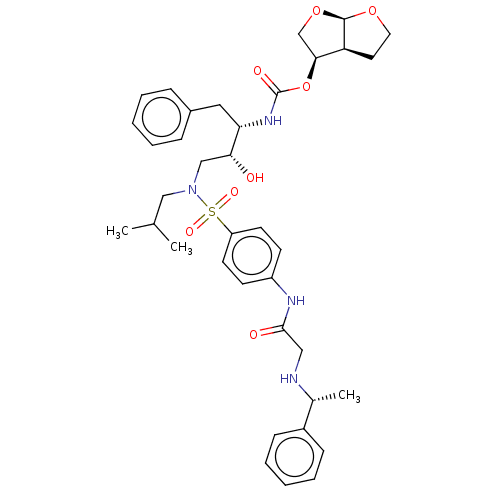
(CHEMBL5267260)Show SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(NC(=O)CN[C@H](C)c2ccccc2)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50613429
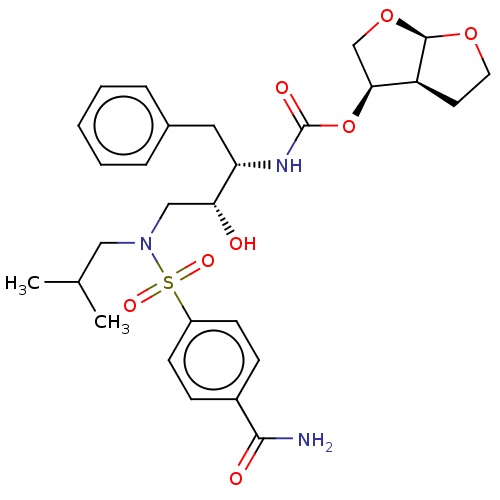
(CHEMBL5271223)Show SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(cc1)C(N)=O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50613806
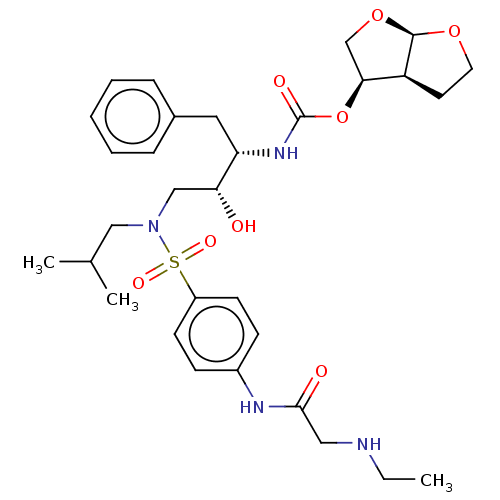
(CHEMBL5280093)Show SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(NC(=O)CNCC)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50608624
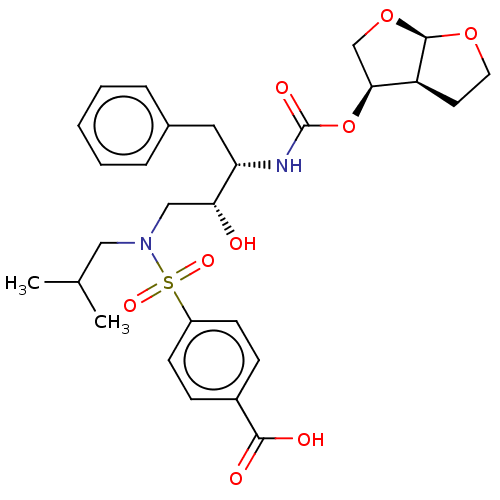
(CHEMBL5274743)Show SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(cc1)C(O)=O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496891
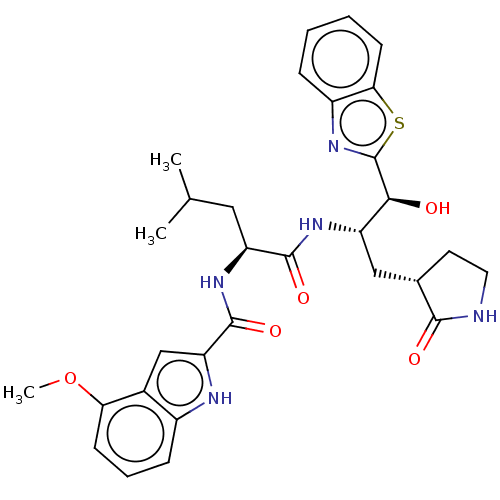
(GRL-2420)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)[C@H](O)c1nc2ccccc2s1 Show InChI InChI=1S/C30H35N5O5S/c1-16(2)13-22(34-29(39)23-15-18-19(32-23)8-6-9-24(18)40-3)28(38)33-21(14-17-11-12-31-27(17)37)26(36)30-35-20-7-4-5-10-25(20)41-30/h4-10,15-17,21-22,26,32,36H,11-14H2,1-3H3,(H,31,37)(H,33,38)(H,34,39)/t17-,21-,22-,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... |
J Med Chem 64: 14702-14714 (2021)
Article DOI: 10.1021/acs.jmedchem.1c01214
BindingDB Entry DOI: 10.7270/Q2CC13T6 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50613804
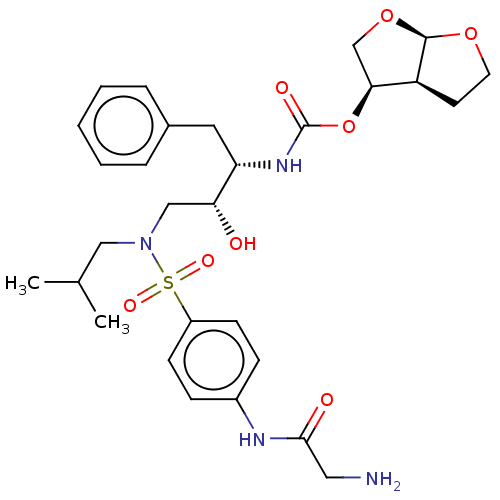
(CHEMBL5287711)Show SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(NC(=O)CN)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50613810
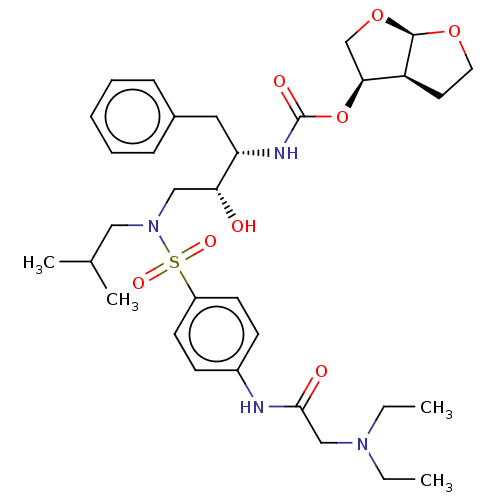
(CHEMBL5271083)Show SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(NC(=O)CN(CC)CC)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50613807
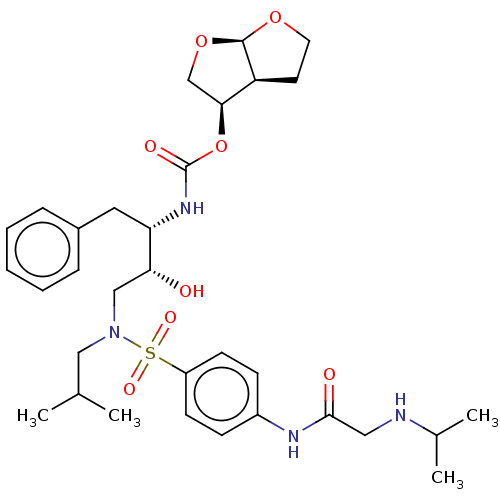
(CHEMBL5288746)Show SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(NC(=O)CNC(C)C)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 388 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50613813
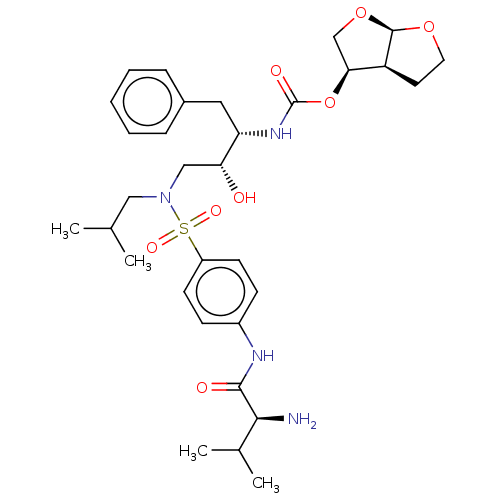
(CHEMBL5284706)Show SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(NC(=O)[C@@H](N)C(C)C)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 445 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50613812
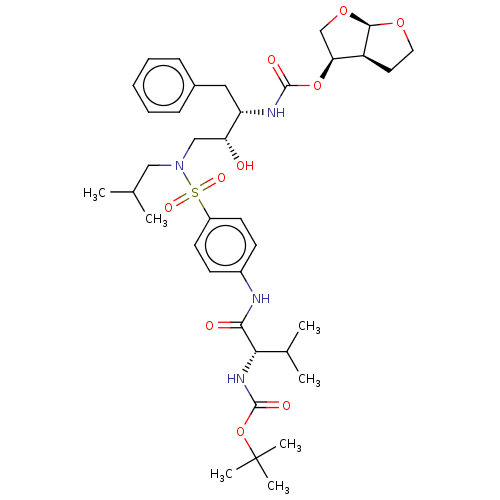
(CHEMBL5268648)Show SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(NC(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)C)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.78E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50613805
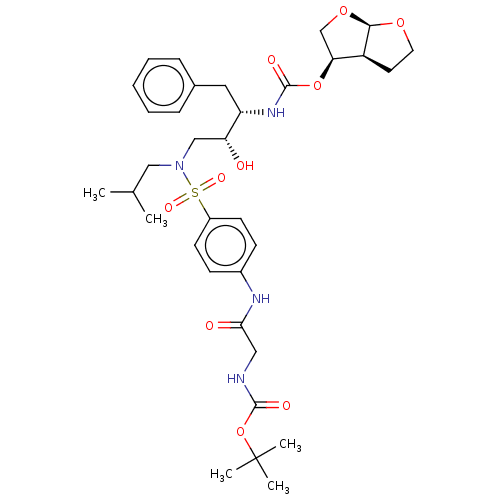
(CHEMBL5281851)Show SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(NC(=O)CNC(=O)OC(C)(C)C)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50613811
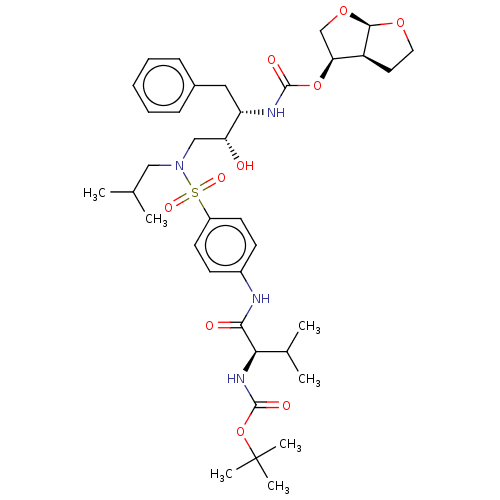
(CHEMBL5278603)Show SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(NC(=O)[C@H](NC(=O)OC(C)(C)C)C(C)C)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.37E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496875
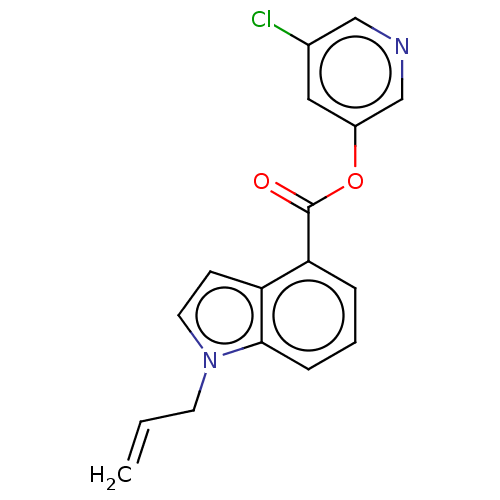
(indole chloropyridinyl-ester derived, 7d)Show InChI InChI=1S/C17H13ClN2O2/c1-2-7-20-8-6-14-15(4-3-5-16(14)20)17(21)22-13-9-12(18)10-19-11-13/h2-6,8-11H,1,7H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... |
J Med Chem 64: 14702-14714 (2021)
Article DOI: 10.1021/acs.jmedchem.1c01214
BindingDB Entry DOI: 10.7270/Q2CC13T6 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496873
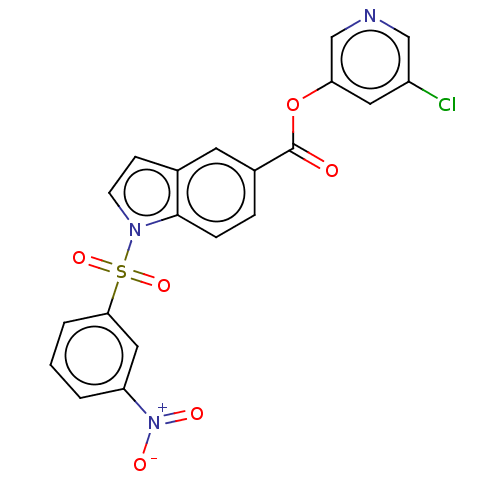
(indole chloropyridinyl-ester derived, 7b)Show SMILES [O-][N+](=O)c1cccc(c1)S(=O)(=O)n1ccc2cc(ccc12)C(=O)Oc1cncc(Cl)c1 Show InChI InChI=1S/C20H12ClN3O6S/c21-15-9-17(12-22-11-15)30-20(25)14-4-5-19-13(8-14)6-7-23(19)31(28,29)18-3-1-2-16(10-18)24(26)27/h1-12H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... |
J Med Chem 64: 14702-14714 (2021)
Article DOI: 10.1021/acs.jmedchem.1c01214
BindingDB Entry DOI: 10.7270/Q2CC13T6 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM429304
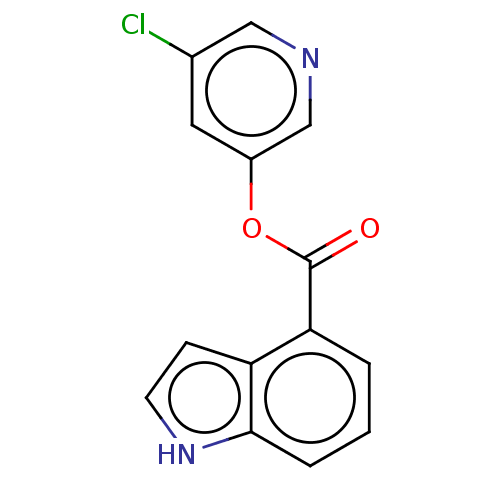
(CVD-0006354 | acs.jmedchem.1c00409_ST.426 | indole...)Show InChI InChI=1S/C14H9ClN2O2/c15-9-6-10(8-16-7-9)19-14(18)12-2-1-3-13-11(12)4-5-17-13/h1-8,17H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... |
J Med Chem 64: 14702-14714 (2021)
Article DOI: 10.1021/acs.jmedchem.1c01214
BindingDB Entry DOI: 10.7270/Q2CC13T6 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM429306
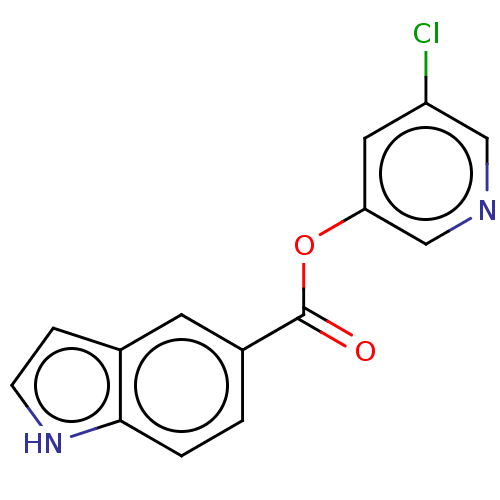
(acs.jmedchem.1c00409_ST.427 | indole chloropyridin...)Show InChI InChI=1S/C14H9ClN2O2/c15-11-6-12(8-16-7-11)19-14(18)10-1-2-13-9(5-10)3-4-17-13/h1-8,17H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... |
J Med Chem 64: 14702-14714 (2021)
Article DOI: 10.1021/acs.jmedchem.1c01214
BindingDB Entry DOI: 10.7270/Q2CC13T6 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496871
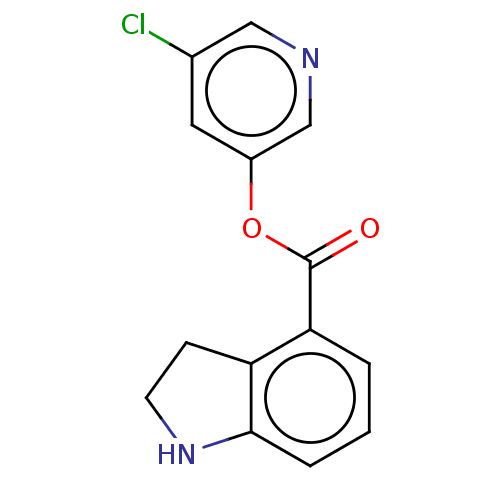
(indole chloropyridinyl-ester derived, 2)Show InChI InChI=1S/C14H11ClN2O2/c15-9-6-10(8-16-7-9)19-14(18)12-2-1-3-13-11(12)4-5-17-13/h1-3,6-8,17H,4-5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... |
J Med Chem 64: 14702-14714 (2021)
Article DOI: 10.1021/acs.jmedchem.1c01214
BindingDB Entry DOI: 10.7270/Q2CC13T6 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496881
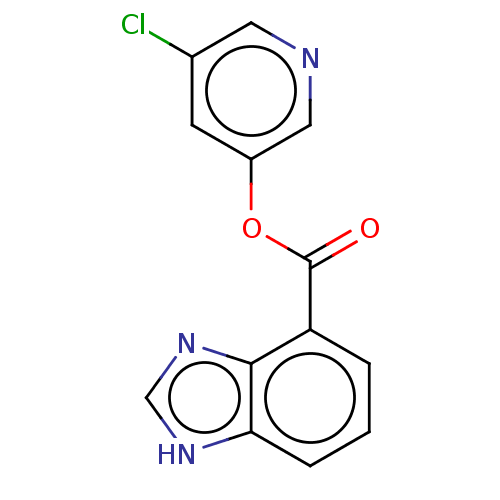
(indole chloropyridinyl-ester derived, 7j)Show InChI InChI=1S/C13H8ClN3O2/c14-8-4-9(6-15-5-8)19-13(18)10-2-1-3-11-12(10)17-7-16-11/h1-7H,(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... |
J Med Chem 64: 14702-14714 (2021)
Article DOI: 10.1021/acs.jmedchem.1c01214
BindingDB Entry DOI: 10.7270/Q2CC13T6 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496876
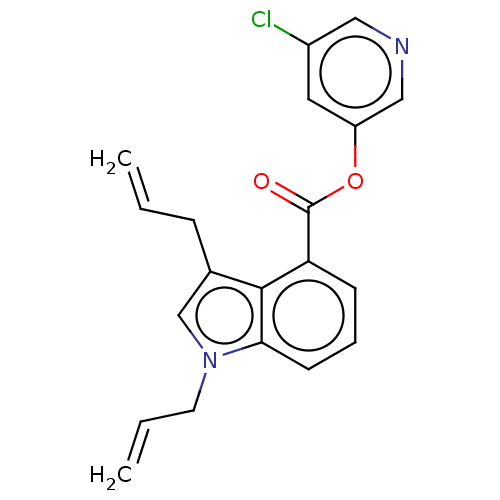
(indole chloropyridinyl-ester derived, 7e)Show SMILES Clc1cncc(OC(=O)c2cccc3n(CC=C)cc(CC=C)c23)c1 Show InChI InChI=1S/C20H17ClN2O2/c1-3-6-14-13-23(9-4-2)18-8-5-7-17(19(14)18)20(24)25-16-10-15(21)11-22-12-16/h3-5,7-8,10-13H,1-2,6,9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... |
J Med Chem 64: 14702-14714 (2021)
Article DOI: 10.1021/acs.jmedchem.1c01214
BindingDB Entry DOI: 10.7270/Q2CC13T6 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496884

(indole chloropyridinyl-ester derived, 7m)Show InChI InChI=1S/C14H11ClN2O3/c15-9-6-10(8-16-7-9)20-14(18)11-2-1-3-12-13(11)19-5-4-17-12/h1-3,6-8,17H,4-5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 419 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... |
J Med Chem 64: 14702-14714 (2021)
Article DOI: 10.1021/acs.jmedchem.1c01214
BindingDB Entry DOI: 10.7270/Q2CC13T6 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496877
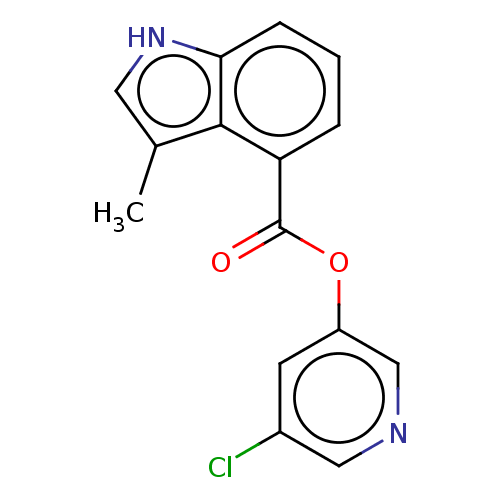
(indole chloropyridinyl-ester derived, 7f)Show InChI InChI=1S/C15H11ClN2O2/c1-9-6-18-13-4-2-3-12(14(9)13)15(19)20-11-5-10(16)7-17-8-11/h2-8,18H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... |
J Med Chem 64: 14702-14714 (2021)
Article DOI: 10.1021/acs.jmedchem.1c01214
BindingDB Entry DOI: 10.7270/Q2CC13T6 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496882
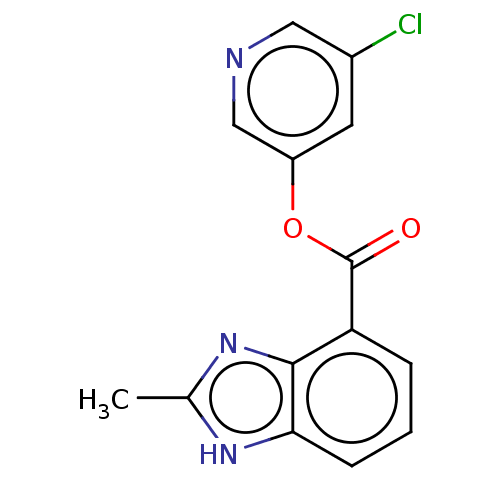
(indole chloropyridinyl-ester derived, 7k)Show InChI InChI=1S/C14H10ClN3O2/c1-8-17-12-4-2-3-11(13(12)18-8)14(19)20-10-5-9(15)6-16-7-10/h2-7H,1H3,(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 489 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... |
J Med Chem 64: 14702-14714 (2021)
Article DOI: 10.1021/acs.jmedchem.1c01214
BindingDB Entry DOI: 10.7270/Q2CC13T6 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496879
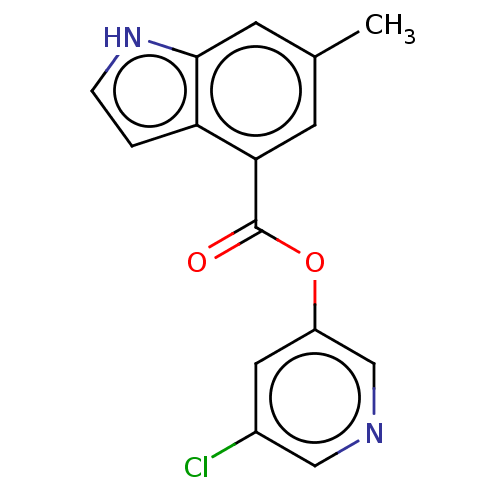
(indole chloropyridinyl-ester derived, 7h)Show InChI InChI=1S/C15H11ClN2O2/c1-9-4-13(12-2-3-18-14(12)5-9)15(19)20-11-6-10(16)7-17-8-11/h2-8,18H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... |
J Med Chem 64: 14702-14714 (2021)
Article DOI: 10.1021/acs.jmedchem.1c01214
BindingDB Entry DOI: 10.7270/Q2CC13T6 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496880
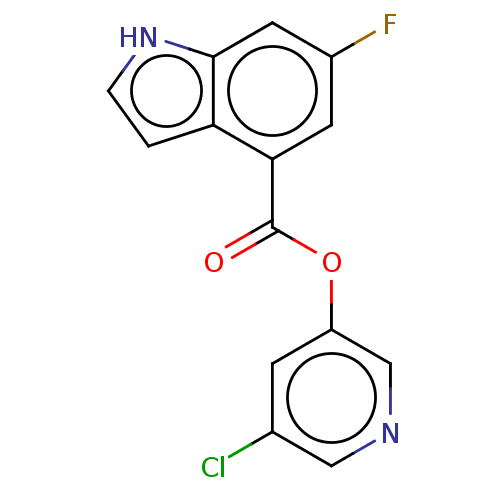
(indole chloropyridinyl-ester derived, 7i)Show InChI InChI=1S/C14H8ClFN2O2/c15-8-3-10(7-17-6-8)20-14(19)12-4-9(16)5-13-11(12)1-2-18-13/h1-7,18H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... |
J Med Chem 64: 14702-14714 (2021)
Article DOI: 10.1021/acs.jmedchem.1c01214
BindingDB Entry DOI: 10.7270/Q2CC13T6 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496874
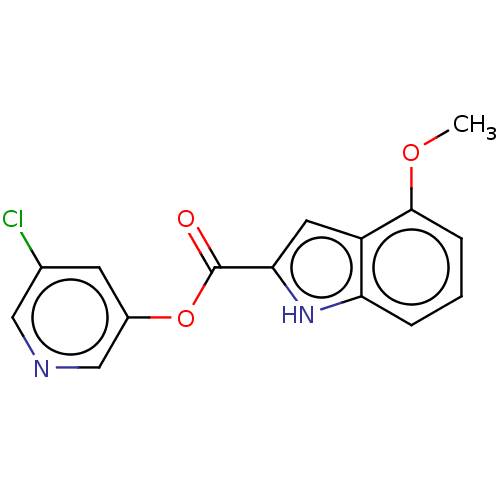
(indole chloropyridinyl-ester derived, 7c)Show InChI InChI=1S/C15H11ClN2O3/c1-20-14-4-2-3-12-11(14)6-13(18-12)15(19)21-10-5-9(16)7-17-8-10/h2-8,18H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... |
J Med Chem 64: 14702-14714 (2021)
Article DOI: 10.1021/acs.jmedchem.1c01214
BindingDB Entry DOI: 10.7270/Q2CC13T6 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496883
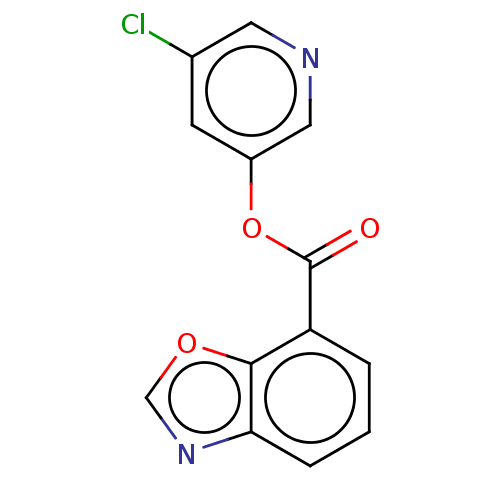
(indole chloropyridinyl-ester derived, 7l)Show InChI InChI=1S/C13H7ClN2O3/c14-8-4-9(6-15-5-8)19-13(17)10-2-1-3-11-12(10)18-7-16-11/h1-7H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... |
J Med Chem 64: 14702-14714 (2021)
Article DOI: 10.1021/acs.jmedchem.1c01214
BindingDB Entry DOI: 10.7270/Q2CC13T6 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496888
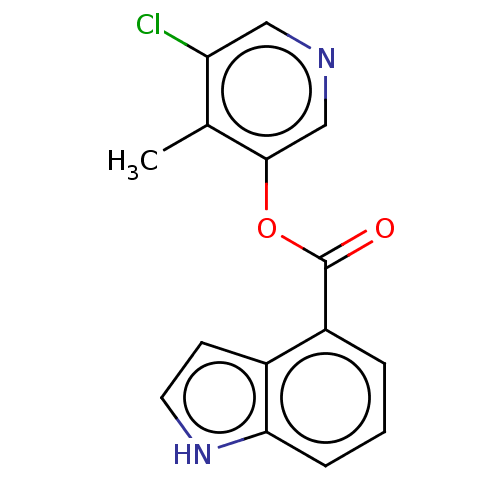
(indole chloropyridinyl-ester derived, 9d)Show InChI InChI=1S/C15H11ClN2O2/c1-9-12(16)7-17-8-14(9)20-15(19)11-3-2-4-13-10(11)5-6-18-13/h2-8,18H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... |
J Med Chem 64: 14702-14714 (2021)
Article DOI: 10.1021/acs.jmedchem.1c01214
BindingDB Entry DOI: 10.7270/Q2CC13T6 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
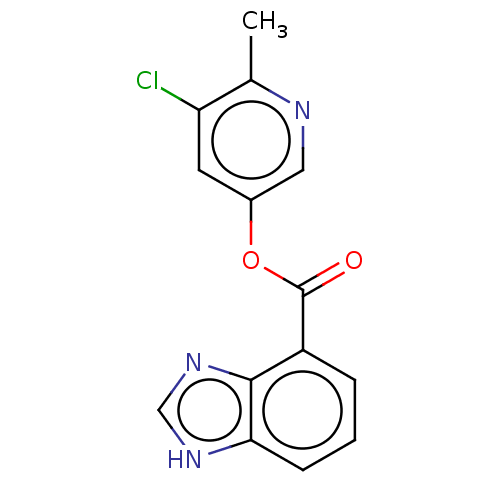
(2019-nCoV) | BDBM496890
(indole chloropyridinyl-ester derived, 9f)Show InChI InChI=1S/C14H10ClN3O2/c1-8-11(15)5-9(6-16-8)20-14(19)10-3-2-4-12-13(10)18-7-17-12/h2-7H,1H3,(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... |
J Med Chem 64: 14702-14714 (2021)
Article DOI: 10.1021/acs.jmedchem.1c01214
BindingDB Entry DOI: 10.7270/Q2CC13T6 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(2019-nCoV) | BDBM627570
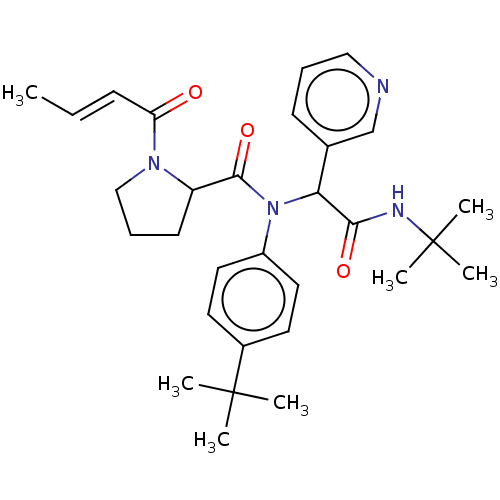
(US11795159, Compound 7 | US11795159, Compound 8)Show SMILES C\C=C\C(=O)N1CCCC1C(=O)N(C(C(=O)NC(C)(C)C)c1cccnc1)c1ccc(cc1)C(C)(C)C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496878
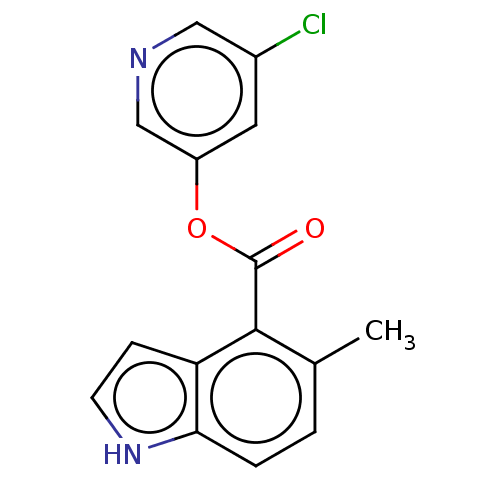
(indole chloropyridinyl-ester derived, 7g)Show InChI InChI=1S/C15H11ClN2O2/c1-9-2-3-13-12(4-5-18-13)14(9)15(19)20-11-6-10(16)7-17-8-11/h2-8,18H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... |
J Med Chem 64: 14702-14714 (2021)
Article DOI: 10.1021/acs.jmedchem.1c01214
BindingDB Entry DOI: 10.7270/Q2CC13T6 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496889
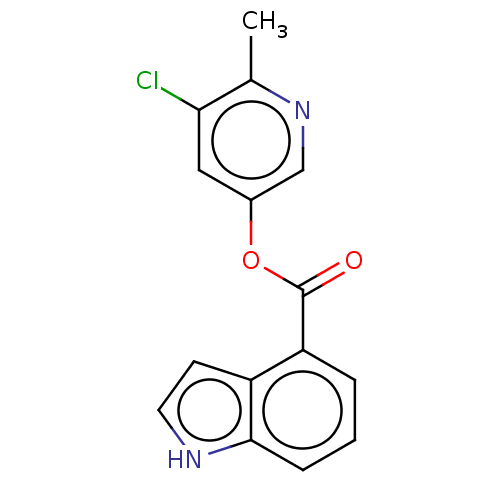
(indole chloropyridinyl-ester derived, 9e)Show InChI InChI=1S/C15H11ClN2O2/c1-9-13(16)7-10(8-18-9)20-15(19)12-3-2-4-14-11(12)5-6-17-14/h2-8,17H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... |
J Med Chem 64: 14702-14714 (2021)
Article DOI: 10.1021/acs.jmedchem.1c01214
BindingDB Entry DOI: 10.7270/Q2CC13T6 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(2019-nCoV) | BDBM627572
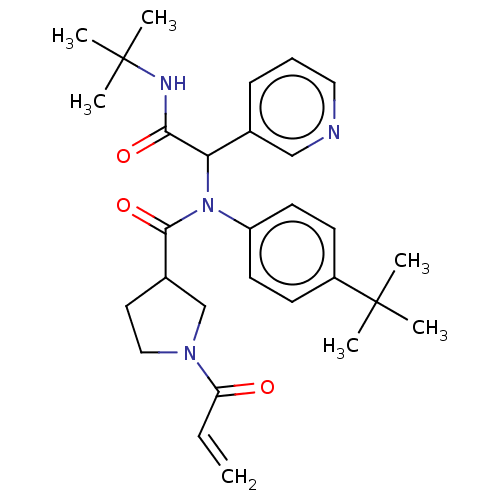
(US11795159, Compound 10 | US11795159, Compound 9)Show SMILES CC(C)(C)NC(=O)C(N(C(=O)C1CCN(C1)C(=O)C=C)c1ccc(cc1)C(C)(C)C)c1cccnc1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(2019-nCoV) | BDBM627566
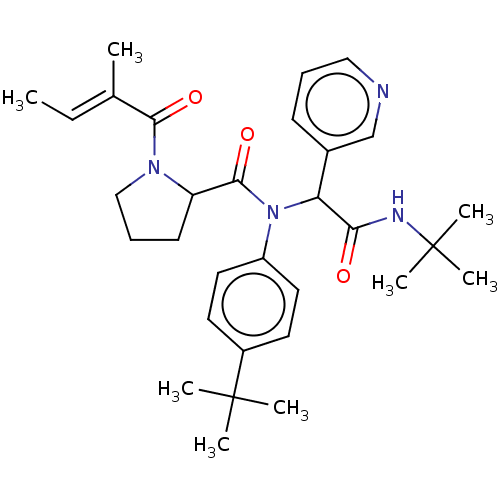
(US11795159, Compound 3 | US11795159, Compound 6)Show SMILES C\C=C(/C)C(=O)N1CCCC1C(=O)N(C(C(=O)NC(C)(C)C)c1cccnc1)c1ccc(cc1)C(C)(C)C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(2019-nCoV) | BDBM627572

(US11795159, Compound 10 | US11795159, Compound 9)Show SMILES CC(C)(C)NC(=O)C(N(C(=O)C1CCN(C1)C(=O)C=C)c1ccc(cc1)C(C)(C)C)c1cccnc1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(2019-nCoV) | BDBM627570

(US11795159, Compound 7 | US11795159, Compound 8)Show SMILES C\C=C\C(=O)N1CCCC1C(=O)N(C(C(=O)NC(C)(C)C)c1cccnc1)c1ccc(cc1)C(C)(C)C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496885
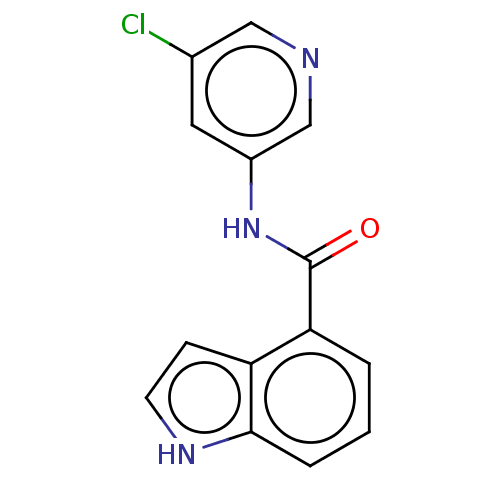
(indole chloropyridinyl-ester derived, 9a)Show InChI InChI=1S/C14H10ClN3O/c15-9-6-10(8-16-7-9)18-14(19)12-2-1-3-13-11(12)4-5-17-13/h1-8,17H,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... |
J Med Chem 64: 14702-14714 (2021)
Article DOI: 10.1021/acs.jmedchem.1c01214
BindingDB Entry DOI: 10.7270/Q2CC13T6 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(2019-nCoV) | BDBM627576
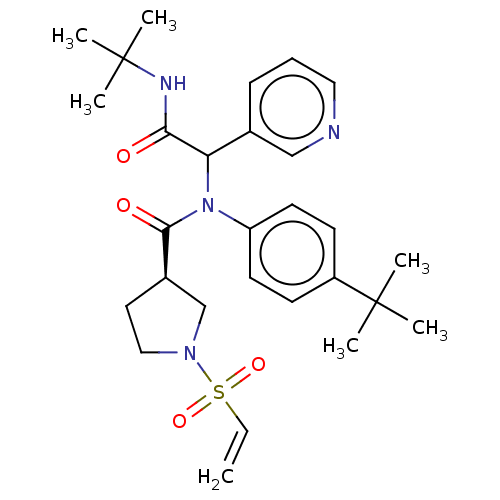
(US11795159, Compound 13 | US11795159, Compound 14)Show SMILES CC(C)(C)NC(=O)C(N(C(=O)[C@@H]1CCN(C1)S(=O)(=O)C=C)c1ccc(cc1)C(C)(C)C)c1cccnc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(2019-nCoV) | BDBM627576

(US11795159, Compound 13 | US11795159, Compound 14)Show SMILES CC(C)(C)NC(=O)C(N(C(=O)[C@@H]1CCN(C1)S(=O)(=O)C=C)c1ccc(cc1)C(C)(C)C)c1cccnc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(2019-nCoV) | BDBM627564
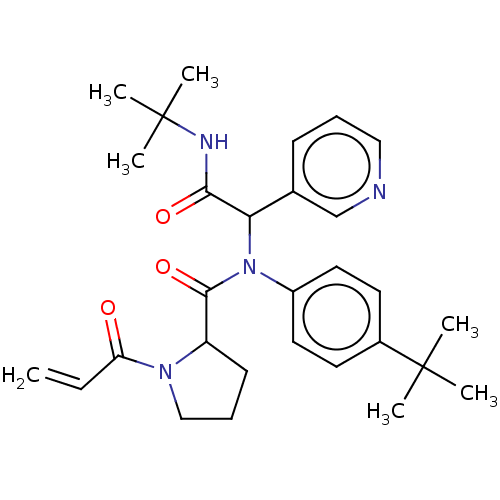
(US11795159, Compound 1 | US11795159, Compound 12)Show SMILES CC(C)(C)NC(=O)C(N(C(=O)C1CCCN1C(=O)C=C)c1ccc(cc1)C(C)(C)C)c1cccnc1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(2019-nCoV) | BDBM627574

(US11795159, Compound 11)Show SMILES CC(C)(C)NC(=O)C(N(C(=O)C1CCCN1S(=O)(=O)C=C)c1ccc(cc1)C(C)(C)C)c1cccnc1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(2019-nCoV) | BDBM627568
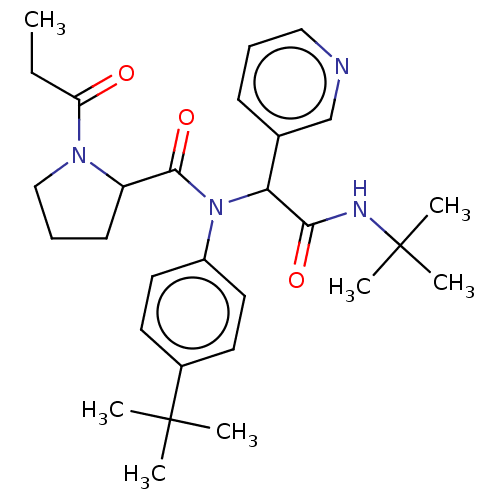
(US11795159, Compound 5)Show SMILES CCC(=O)N1CCCC1C(=O)N(C(C(=O)NC(C)(C)C)c1cccnc1)c1ccc(cc1)C(C)(C)C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(2019-nCoV) | BDBM627565
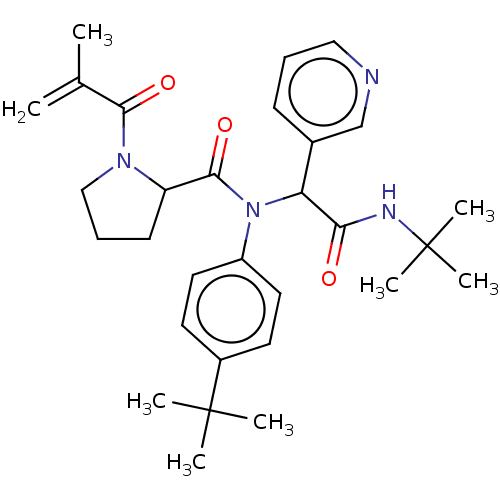
(US11795159, Compound 2 | US11795159, Compound 4)Show SMILES CC(=C)C(=O)N1CCCC1C(=O)N(C(C(=O)NC(C)(C)C)c1cccnc1)c1ccc(cc1)C(C)(C)C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(2019-nCoV) | BDBM627566

(US11795159, Compound 3 | US11795159, Compound 6)Show SMILES C\C=C(/C)C(=O)N1CCCC1C(=O)N(C(C(=O)NC(C)(C)C)c1cccnc1)c1ccc(cc1)C(C)(C)C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(2019-nCoV) | BDBM627565

(US11795159, Compound 2 | US11795159, Compound 4)Show SMILES CC(=C)C(=O)N1CCCC1C(=O)N(C(C(=O)NC(C)(C)C)c1cccnc1)c1ccc(cc1)C(C)(C)C | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(2019-nCoV) | BDBM627564

(US11795159, Compound 1 | US11795159, Compound 12)Show SMILES CC(C)(C)NC(=O)C(N(C(=O)C1CCCN1C(=O)C=C)c1ccc(cc1)C(C)(C)C)c1cccnc1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496887
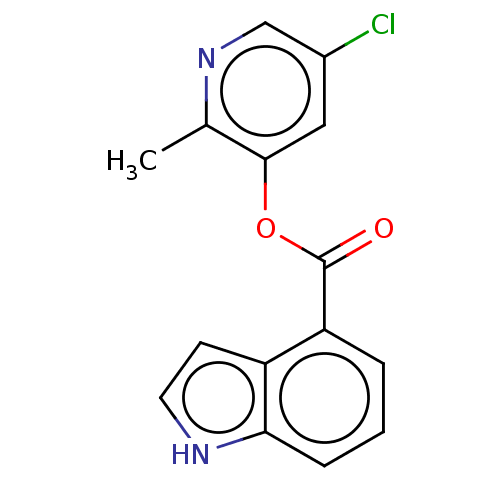
(indole chloropyridinyl-ester derived, 9c)Show InChI InChI=1S/C15H11ClN2O2/c1-9-14(7-10(16)8-18-9)20-15(19)12-3-2-4-13-11(12)5-6-17-13/h2-8,17H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
IC50 values were determined for compounds that covalently inhibit SARS-CoV-2 3CLpro using our recently described assay and data fitting methods that ... |
J Med Chem 64: 14702-14714 (2021)
Article DOI: 10.1021/acs.jmedchem.1c01214
BindingDB Entry DOI: 10.7270/Q2CC13T6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data