 Found 57 hits with Last Name = 'shalev' and Initial = 'de'
Found 57 hits with Last Name = 'shalev' and Initial = 'de' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50374671

(CHEMBL272660)Show SMILES NC(=O)CN1CCCNC(=O)CCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:27.28,wD:16.16,49.51,38.40,(28.48,-20.35,;27.16,-19.57,;27.17,-18.03,;25.81,-20.32,;25.8,-21.86,;23.64,-21.92,;23.15,-20.32,;24.48,-19.55,;24.48,-18.01,;23.15,-17.24,;23.15,-15.7,;21.81,-18.01,;21.81,-19.55,;20.48,-20.32,;19.15,-19.55,;20.48,-21.86,;19.15,-22.63,;17.81,-21.86,;16.48,-22.63,;15.15,-21.85,;13.82,-22.62,;13.81,-24.16,;15.16,-24.93,;16.48,-24.16,;19.15,-24.17,;17.81,-24.94,;20.48,-24.94,;20.48,-26.48,;19.15,-27.25,;19.15,-28.79,;17.81,-29.56,;17.8,-31.09,;19.14,-31.87,;20.48,-31.09,;20.47,-29.55,;21.81,-27.25,;21.81,-28.79,;23.15,-26.48,;24.48,-27.25,;24.48,-28.79,;25.81,-29.56,;25.81,-31.1,;27.15,-31.87,;27.15,-33.41,;28.48,-34.18,;25.81,-34.18,;25.81,-26.48,;27.15,-27.25,;25.81,-24.94,;27.15,-24.17,;28.48,-24.94,;29.82,-24.17,;29.98,-22.63,;31.49,-22.31,;32.24,-23.66,;33.75,-23.99,;34.21,-25.46,;33.18,-26.59,;31.67,-26.26,;31.21,-24.8,;27.15,-22.63,;28.48,-21.86,)| Show InChI InChI=1S/C44H55N11O7/c45-37(56)27-55-22-10-21-48-38(57)18-19-39(58)51-34(23-28-11-3-1-4-12-28)41(60)53-35(24-29-13-5-2-6-14-29)42(61)52-33(17-9-20-49-44(46)47)40(59)54-36(43(55)62)25-30-26-50-32-16-8-7-15-31(30)32/h1-8,11-16,26,33-36,50H,9-10,17-25,27H2,(H2,45,56)(H,48,57)(H,51,58)(H,52,61)(H,53,60)(H,54,59)(H4,46,47,49)/t33-,34-,35+,36-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDP-alpha-MSH from MC4R |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50374672

(CHEMBL271586)Show SMILES NC(=O)CN1CCCNC(=O)CCCCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:29.30,wD:51.53,18.18,40.42,(30.64,-.68,;29.3,.09,;29.31,1.63,;27.97,-.68,;26.64,.1,;26.64,1.64,;27.98,2.4,;27.98,3.94,;25.3,3.95,;23.96,4.72,;23.96,6.26,;22.63,3.95,;21.3,4.72,;19.96,3.95,;19.96,2.41,;18.63,1.64,;17.29,2.41,;18.63,.1,;17.29,-.67,;15.96,.1,;14.63,-.67,;13.3,.11,;11.96,-.66,;11.96,-2.2,;13.31,-2.97,;14.63,-2.2,;17.29,-2.21,;15.96,-2.98,;18.63,-2.98,;18.63,-4.52,;17.29,-5.29,;17.29,-6.83,;15.95,-7.59,;15.95,-9.13,;17.29,-9.9,;18.63,-9.12,;18.62,-7.59,;19.96,-5.29,;19.96,-6.83,;21.3,-4.52,;22.63,-5.29,;22.63,-6.83,;23.96,-7.6,;23.96,-9.14,;25.3,-9.91,;25.3,-11.45,;26.63,-12.22,;23.96,-12.22,;23.96,-4.52,;25.3,-5.29,;23.96,-2.98,;25.3,-2.21,;26.63,-2.98,;28.17,-2.98,;29.08,-1.72,;30.54,-2.19,;30.53,-3.74,;31.67,-4.77,;31.35,-6.28,;29.88,-6.75,;28.74,-5.71,;29.07,-4.21,;25.3,-.67,;23.97,.1,)| Show InChI InChI=1S/C46H59N11O7/c47-39(58)29-57-24-12-23-50-40(59)20-9-10-21-41(60)53-36(25-30-13-3-1-4-14-30)43(62)55-37(26-31-15-5-2-6-16-31)44(63)54-35(19-11-22-51-46(48)49)42(61)56-38(45(57)64)27-32-28-52-34-18-8-7-17-33(32)34/h1-8,13-18,28,35-38,52H,9-12,19-27,29H2,(H2,47,58)(H,50,59)(H,53,60)(H,54,63)(H,55,62)(H,56,61)(H4,48,49,51)/t35-,36-,37+,38-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDP-alpha-MSH from MC4R |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50374673

(CHEMBL408398)Show SMILES NC(=O)CN1CCNC(=O)CCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:26.27,wD:15.15,48.50,37.39,(3.62,2.67,;2.29,1.89,;.96,2.65,;2.3,.35,;.97,-.43,;.99,1.1,;-.35,1.87,;-.35,3.41,;-1.68,4.18,;-1.68,5.72,;-3.01,3.41,;-3.01,1.87,;-4.35,1.1,;-5.68,1.87,;-4.35,-.44,;-5.68,-1.21,;-7.01,-.44,;-8.35,-1.21,;-9.68,-.43,;-11.01,-1.19,;-11.01,-2.74,;-9.67,-3.51,;-8.34,-2.73,;-5.68,-2.75,;-7.01,-3.52,;-4.35,-3.52,;-4.35,-5.06,;-5.68,-5.83,;-5.68,-7.37,;-7.02,-8.13,;-7.02,-9.67,;-5.69,-10.44,;-4.35,-9.66,;-4.36,-8.12,;-3.01,-5.83,;-3.01,-7.37,;-1.68,-5.06,;-.35,-5.83,;-.35,-7.37,;.99,-8.14,;.99,-9.68,;2.32,-10.45,;2.32,-11.99,;3.65,-12.76,;.99,-12.76,;.99,-5.06,;2.32,-5.83,;.99,-3.52,;2.32,-2.75,;3.65,-3.52,;4.99,-2.75,;5.16,-1.2,;6.66,-.89,;7.42,-2.23,;8.92,-2.56,;9.39,-4.03,;8.35,-5.17,;6.85,-4.84,;6.38,-3.37,;2.32,-1.21,;3.65,-.44,)| Show InChI InChI=1S/C43H53N11O7/c44-36(55)26-54-21-20-47-37(56)17-18-38(57)50-33(22-27-10-3-1-4-11-27)40(59)52-34(23-28-12-5-2-6-13-28)41(60)51-32(16-9-19-48-43(45)46)39(58)53-35(42(54)61)24-29-25-49-31-15-8-7-14-30(29)31/h1-8,10-15,25,32-35,49H,9,16-24,26H2,(H2,44,55)(H,47,56)(H,50,57)(H,51,60)(H,52,59)(H,53,58)(H4,45,46,48)/t32-,33-,34+,35-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDP-alpha-MSH from MC4R |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50374670

(CHEMBL270923)Show SMILES NC(=O)CN1CCNC(=O)c2ccccc2C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:30.32,wD:52.55,19.20,41.44,(6.67,-17.07,;5.13,-17.07,;4.36,-15.74,;4.37,-18.41,;2.83,-18.41,;2.05,-17.08,;.51,-17.09,;-.25,-18.42,;-1.79,-18.43,;-2.56,-19.76,;-2.57,-17.1,;-1.81,-15.76,;-2.59,-14.43,;-4.14,-14.45,;-4.89,-15.78,;-4.11,-17.1,;-4.87,-18.43,;-6.41,-18.44,;-4.1,-19.76,;-5.43,-20.54,;-6.76,-19.76,;-8.09,-20.54,;-9.42,-19.75,;-10.76,-20.53,;-10.76,-22.07,;-9.41,-22.83,;-8.09,-22.06,;-5.43,-22.08,;-6.76,-22.84,;-4.1,-22.84,;-4.1,-24.38,;-5.43,-25.16,;-5.43,-26.7,;-6.77,-27.46,;-6.77,-28.99,;-5.43,-29.77,;-4.1,-28.99,;-4.1,-27.45,;-2.76,-25.16,;-2.76,-26.7,;-1.42,-24.38,;-.09,-25.16,;-.09,-26.7,;1.24,-27.46,;1.24,-29,;2.58,-29.78,;2.58,-31.32,;3.91,-32.08,;1.24,-32.08,;1.24,-24.38,;2.58,-25.16,;1.24,-22.84,;2.58,-22.08,;3.91,-22.84,;5.24,-22.08,;5.41,-20.53,;6.91,-20.22,;7.67,-21.56,;9.18,-21.89,;9.64,-23.36,;8.6,-24.49,;7.1,-24.16,;6.63,-22.7,;2.06,-19.75,;.52,-19.75,)| Show InChI InChI=1S/C47H53N11O7/c48-40(59)28-58-23-22-51-41(60)33-17-7-8-18-34(33)42(61)55-37(24-29-12-3-1-4-13-29)45(64)56-38(25-30-14-5-2-6-15-30)44(63)54-36(20-11-21-52-47(49)50)43(62)57-39(46(58)65)26-31-27-53-35-19-10-9-16-32(31)35/h1-10,12-19,27,36-39,53H,11,20-26,28H2,(H2,48,59)(H,51,60)(H,54,63)(H,55,61)(H,56,64)(H,57,62)(H4,49,50,52)/t36-,37-,38+,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDP-alpha-MSH from MC4R |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50347967

(CHEMBL1800386)Show SMILES [#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7](-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7](-[#6]-[#6]-[#6]-[#7])-[#6]-[#6](-[#7])=O |r| Show InChI InChI=1S/C49H90N18O9/c1-4-12-35(62-43(72)36(14-10-24-60-49(57)58)61-41(70)30-67(25-8-6-5-7-21-50)46(75)34(53)13-9-23-59-48(55)56)42(71)64-38(27-32-16-18-33(68)19-17-32)44(73)65-39(28-52)45(74)63-37(20-15-31(2)3)47(76)66(26-11-22-51)29-40(54)69/h16-19,31,34-39,68H,4-15,20-30,50-53H2,1-3H3,(H2,54,69)(H,61,70)(H,62,72)(H,63,74)(H,64,71)(H,65,73)(H4,55,56,59)(H4,57,58,60)/t34-,35-,36-,37-,38-,39-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of Akt using RPRTSSF peptide and [gamma32]ATP by cell-free radioactive assay |
J Med Chem 54: 5154-64 (2011)
Article DOI: 10.1021/jm2003969
BindingDB Entry DOI: 10.7270/Q26D5TBX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50347981

(CHEMBL1800388)Show SMILES [#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7](-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6]-[#6](-[#7])=O |r| Show InChI InChI=1S/C47H86N18O9/c1-4-10-33(60-41(70)34(12-8-22-58-47(55)56)59-39(68)28-65(24-9-20-49)44(73)32(51)11-7-21-57-46(53)54)40(69)62-36(25-30-14-16-31(66)17-15-30)42(71)63-37(26-50)43(72)61-35(18-13-29(2)3)45(74)64(27-38(52)67)23-6-5-19-48/h14-17,29,32-37,66H,4-13,18-28,48-51H2,1-3H3,(H2,52,67)(H,59,68)(H,60,70)(H,61,72)(H,62,69)(H,63,71)(H4,53,54,57)(H4,55,56,58)/t32-,33-,34-,35-,36-,37-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of Akt using RPRTSSF peptide and [gamma32]ATP by cell-free radioactive assay |
J Med Chem 54: 5154-64 (2011)
Article DOI: 10.1021/jm2003969
BindingDB Entry DOI: 10.7270/Q26D5TBX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50347969
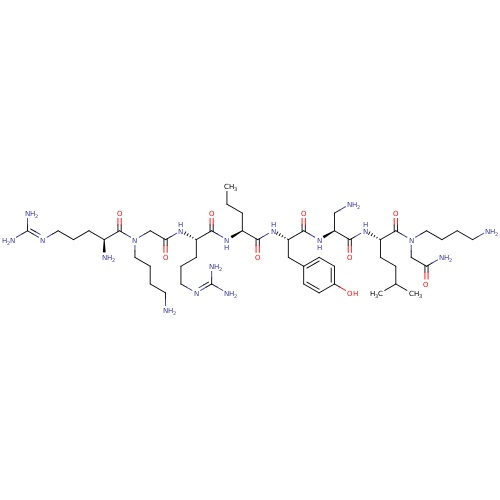
(CHEMBL1800389)Show SMILES [#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6]-[#6](-[#7])=O |r| Show InChI InChI=1S/C48H88N18O9/c1-4-11-34(61-42(71)35(13-10-23-59-48(56)57)60-40(69)29-66(25-8-6-21-50)45(74)33(52)12-9-22-58-47(54)55)41(70)63-37(26-31-15-17-32(67)18-16-31)43(72)64-38(27-51)44(73)62-36(19-14-30(2)3)46(75)65(28-39(53)68)24-7-5-20-49/h15-18,30,33-38,67H,4-14,19-29,49-52H2,1-3H3,(H2,53,68)(H,60,69)(H,61,71)(H,62,73)(H,63,70)(H,64,72)(H4,54,55,58)(H4,56,57,59)/t33-,34-,35-,36-,37-,38-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of Akt using RPRTSSF peptide and [gamma32]ATP by cell-free radioactive assay |
J Med Chem 54: 5154-64 (2011)
Article DOI: 10.1021/jm2003969
BindingDB Entry DOI: 10.7270/Q26D5TBX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50347965

(CHEMBL1800268)Show SMILES [#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7](-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7](-[#6]-[#6]-[#6]-[#7])-[#6]-[#6](-[#7])=O |r| Show InChI InChI=1S/C46H84N18O9/c1-4-9-32(59-40(69)33(11-6-21-57-46(54)55)58-38(67)27-64(23-8-19-48)43(72)31(50)10-5-20-56-45(52)53)39(68)61-35(24-29-13-15-30(65)16-14-29)41(70)62-36(25-49)42(71)60-34(17-12-28(2)3)44(73)63(22-7-18-47)26-37(51)66/h13-16,28,31-36,65H,4-12,17-27,47-50H2,1-3H3,(H2,51,66)(H,58,67)(H,59,69)(H,60,71)(H,61,68)(H,62,70)(H4,52,53,56)(H4,54,55,57)/t31-,32-,33-,34-,35-,36-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of Akt using RPRTSSF peptide and [gamma32]ATP by cell-free radioactive assay |
J Med Chem 54: 5154-64 (2011)
Article DOI: 10.1021/jm2003969
BindingDB Entry DOI: 10.7270/Q26D5TBX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50347966

(CHEMBL1800269)Show SMILES [#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7](-[#6]-[#6]-[#6]-[#7])-[#6]-[#6](-[#7])=O |r| Show InChI InChI=1S/C47H86N18O9/c1-4-10-33(60-41(70)34(12-8-22-58-47(55)56)59-39(68)28-65(23-6-5-19-48)44(73)32(51)11-7-21-57-46(53)54)40(69)62-36(25-30-14-16-31(66)17-15-30)42(71)63-37(26-50)43(72)61-35(18-13-29(2)3)45(74)64(24-9-20-49)27-38(52)67/h14-17,29,32-37,66H,4-13,18-28,48-51H2,1-3H3,(H2,52,67)(H,59,68)(H,60,70)(H,61,72)(H,62,69)(H,63,71)(H4,53,54,57)(H4,55,56,58)/t32-,33-,34-,35-,36-,37-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of Akt using RPRTSSF peptide and [gamma32]ATP by cell-free radioactive assay |
J Med Chem 54: 5154-64 (2011)
Article DOI: 10.1021/jm2003969
BindingDB Entry DOI: 10.7270/Q26D5TBX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50347970

(CHEMBL1800390)Show SMILES [#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7](-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6]-[#6](-[#7])=O |r| Show InChI InChI=1S/C50H92N18O9/c1-4-13-36(63-44(73)37(15-12-25-61-50(58)59)62-42(71)31-68(26-9-6-5-7-22-51)47(76)35(54)14-11-24-60-49(56)57)43(72)65-39(28-33-17-19-34(69)20-18-33)45(74)66-40(29-53)46(75)64-38(21-16-32(2)3)48(77)67(30-41(55)70)27-10-8-23-52/h17-20,32,35-40,69H,4-16,21-31,51-54H2,1-3H3,(H2,55,70)(H,62,71)(H,63,73)(H,64,75)(H,65,72)(H,66,74)(H4,56,57,60)(H4,58,59,61)/t35-,36-,37-,38-,39-,40-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of Akt using RPRTSSF peptide and [gamma32]ATP by cell-free radioactive assay |
J Med Chem 54: 5154-64 (2011)
Article DOI: 10.1021/jm2003969
BindingDB Entry DOI: 10.7270/Q26D5TBX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50347973

(CHEMBL1800402)Show SMILES [#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7](-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#7](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C50H88N18O10/c1-4-12-35-42(72)65-38(27-32-16-18-33(69)19-17-32)44(74)66-39(28-51)45(75)64-37(20-15-31(2)3)47(77)67(29-40(53)70)26-11-24-61-50(78)60-21-7-5-6-8-25-68(46(76)34(52)13-9-22-58-48(54)55)30-41(71)62-36(43(73)63-35)14-10-23-59-49(56)57/h16-19,31,34-39,69H,4-15,20-30,51-52H2,1-3H3,(H2,53,70)(H,62,71)(H,63,73)(H,64,75)(H,65,72)(H,66,74)(H4,54,55,58)(H4,56,57,59)(H2,60,61,78)/t34-,35-,36-,37-,38-,39-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of Akt using RPRTSSF peptide and [gamma32]ATP by cell-free radioactive assay |
J Med Chem 54: 5154-64 (2011)
Article DOI: 10.1021/jm2003969
BindingDB Entry DOI: 10.7270/Q26D5TBX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50347975
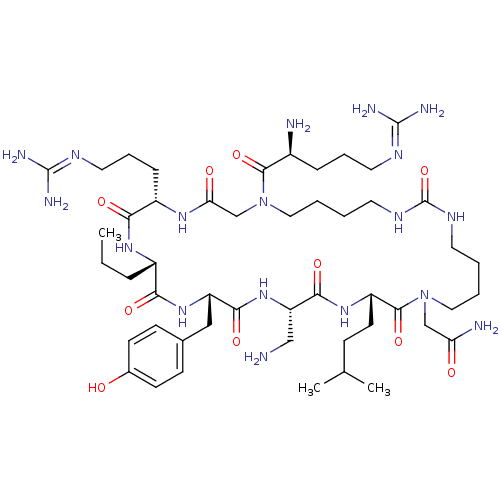
(CHEMBL1800566)Show SMILES [#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7](-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#7](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C49H86N18O10/c1-4-11-34-41(71)64-37(26-31-15-17-32(68)18-16-31)43(73)65-38(27-50)44(74)63-36(19-14-30(2)3)46(76)66(28-39(52)69)24-7-5-20-59-49(77)60-21-6-8-25-67(45(75)33(51)12-9-22-57-47(53)54)29-40(70)61-35(42(72)62-34)13-10-23-58-48(55)56/h15-18,30,33-38,68H,4-14,19-29,50-51H2,1-3H3,(H2,52,69)(H,61,70)(H,62,72)(H,63,74)(H,64,71)(H,65,73)(H4,53,54,57)(H4,55,56,58)(H2,59,60,77)/t33-,34-,35-,36-,37-,38-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of Akt using RPRTSSF peptide and [gamma32]ATP by cell-free radioactive assay |
J Med Chem 54: 5154-64 (2011)
Article DOI: 10.1021/jm2003969
BindingDB Entry DOI: 10.7270/Q26D5TBX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50347968
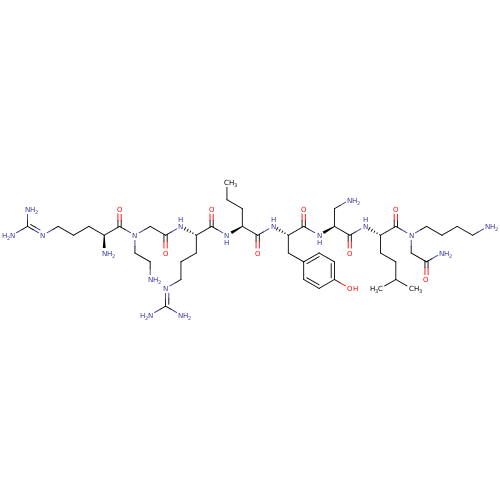
(CHEMBL1800387)Show SMILES [#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7](-[#6]-[#6]-[#7])-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6]-[#6](-[#7])=O |r| Show InChI InChI=1S/C46H84N18O9/c1-4-9-32(59-40(69)33(11-8-21-57-46(54)55)58-38(67)27-64(23-19-48)43(72)31(50)10-7-20-56-45(52)53)39(68)61-35(24-29-13-15-30(65)16-14-29)41(70)62-36(25-49)42(71)60-34(17-12-28(2)3)44(73)63(26-37(51)66)22-6-5-18-47/h13-16,28,31-36,65H,4-12,17-27,47-50H2,1-3H3,(H2,51,66)(H,58,67)(H,59,69)(H,60,71)(H,61,68)(H,62,70)(H4,52,53,56)(H4,54,55,57)/t31-,32-,33-,34-,35-,36-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 176 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of Akt using RPRTSSF peptide and [gamma32]ATP by cell-free radioactive assay |
J Med Chem 54: 5154-64 (2011)
Article DOI: 10.1021/jm2003969
BindingDB Entry DOI: 10.7270/Q26D5TBX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50347962
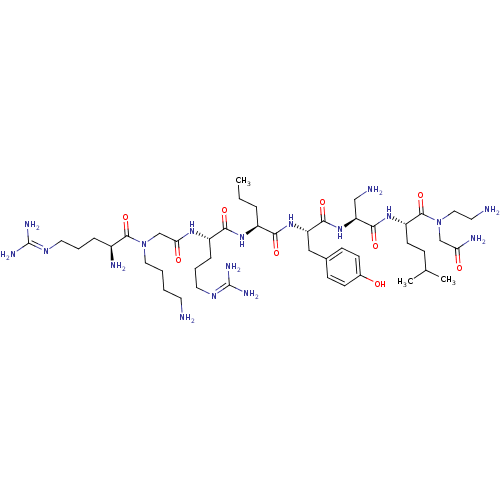
(CHEMBL1800266)Show SMILES [#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7](-[#6]-[#6]-[#7])-[#6]-[#6](-[#7])=O |r| Show InChI InChI=1S/C46H84N18O9/c1-4-9-32(59-40(69)33(11-8-21-57-46(54)55)58-38(67)27-63(22-6-5-18-47)43(72)31(50)10-7-20-56-45(52)53)39(68)61-35(24-29-13-15-30(65)16-14-29)41(70)62-36(25-49)42(71)60-34(17-12-28(2)3)44(73)64(23-19-48)26-37(51)66/h13-16,28,31-36,65H,4-12,17-27,47-50H2,1-3H3,(H2,51,66)(H,58,67)(H,59,69)(H,60,71)(H,61,68)(H,62,70)(H4,52,53,56)(H4,54,55,57)/t31-,32-,33-,34-,35-,36-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of Akt using RPRTSSF peptide and [gamma32]ATP by cell-free radioactive assay |
J Med Chem 54: 5154-64 (2011)
Article DOI: 10.1021/jm2003969
BindingDB Entry DOI: 10.7270/Q26D5TBX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50347980
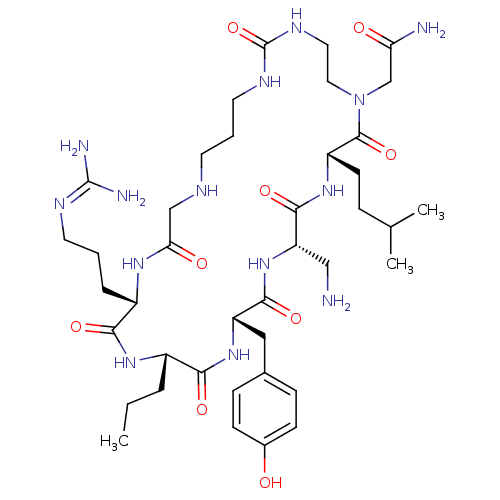
(CHEMBL1800699)Show SMILES [#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#7]-[#6]-[#6]-[#7](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O |r| Show InChI InChI=1S/C40H68N14O9/c1-4-7-27-34(58)52-30(20-25-10-12-26(55)13-11-25)36(60)53-31(21-41)37(61)51-29(14-9-24(2)3)38(62)54(23-32(42)56)19-18-48-40(63)47-17-6-15-45-22-33(57)49-28(35(59)50-27)8-5-16-46-39(43)44/h10-13,24,27-31,45,55H,4-9,14-23,41H2,1-3H3,(H2,42,56)(H,49,57)(H,50,59)(H,51,61)(H,52,58)(H,53,60)(H4,43,44,46)(H2,47,48,63)/t27-,28-,29-,30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of Akt using RPRTSSF peptide and [gamma32]ATP by cell-free radioactive assay |
J Med Chem 54: 5154-64 (2011)
Article DOI: 10.1021/jm2003969
BindingDB Entry DOI: 10.7270/Q26D5TBX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50347964

(CHEMBL1800259)Show SMILES [#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7](-[#6]-[#6]-[#7])-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7](-[#6]-[#6]-[#6]-[#7])-[#6]-[#6](-[#7])=O |r| Show InChI InChI=1S/C45H82N18O9/c1-4-8-31(58-39(68)32(10-6-20-56-45(53)54)57-37(66)26-63(22-18-47)42(71)30(49)9-5-19-55-44(51)52)38(67)60-34(23-28-12-14-29(64)15-13-28)40(69)61-35(24-48)41(70)59-33(16-11-27(2)3)43(72)62(21-7-17-46)25-36(50)65/h12-15,27,30-35,64H,4-11,16-26,46-49H2,1-3H3,(H2,50,65)(H,57,66)(H,58,68)(H,59,70)(H,60,67)(H,61,69)(H4,51,52,55)(H4,53,54,56)/t30-,31-,32-,33-,34-,35-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of Akt using RPRTSSF peptide and [gamma32]ATP by cell-free radioactive assay |
J Med Chem 54: 5154-64 (2011)
Article DOI: 10.1021/jm2003969
BindingDB Entry DOI: 10.7270/Q26D5TBX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50347976

(CHEMBL1800567)Show SMILES [#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7](-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#7](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C51H90N18O10/c1-4-13-36-43(73)66-39(28-33-17-19-34(70)20-18-33)45(75)67-40(29-52)46(76)65-38(21-16-32(2)3)48(78)68(30-41(54)71)27-10-8-23-62-51(79)61-22-7-5-6-9-26-69(47(77)35(53)14-11-24-59-49(55)56)31-42(72)63-37(44(74)64-36)15-12-25-60-50(57)58/h17-20,32,35-40,70H,4-16,21-31,52-53H2,1-3H3,(H2,54,71)(H,63,72)(H,64,74)(H,65,76)(H,66,73)(H,67,75)(H4,55,56,59)(H4,57,58,60)(H2,61,62,79)/t35-,36-,37-,38-,39-,40-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of Akt using RPRTSSF peptide and [gamma32]ATP by cell-free radioactive assay |
J Med Chem 54: 5154-64 (2011)
Article DOI: 10.1021/jm2003969
BindingDB Entry DOI: 10.7270/Q26D5TBX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50347974
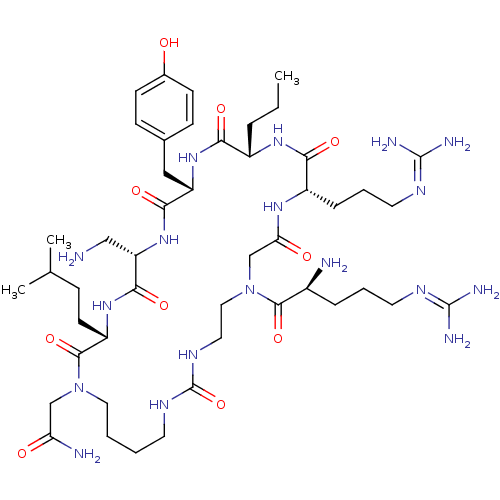
(CHEMBL1800403)Show SMILES [#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7](-[#6]-[#6]-[#7]-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#7](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C47H82N18O10/c1-4-9-32-39(69)62-35(24-29-13-15-30(66)16-14-29)41(71)63-36(25-48)42(72)61-34(17-12-28(2)3)44(74)64(26-37(50)67)22-6-5-18-57-47(75)58-21-23-65(43(73)31(49)10-7-19-55-45(51)52)27-38(68)59-33(40(70)60-32)11-8-20-56-46(53)54/h13-16,28,31-36,66H,4-12,17-27,48-49H2,1-3H3,(H2,50,67)(H,59,68)(H,60,70)(H,61,72)(H,62,69)(H,63,71)(H4,51,52,55)(H4,53,54,56)(H2,57,58,75)/t31-,32-,33-,34-,35-,36-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 282 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of Akt using RPRTSSF peptide and [gamma32]ATP by cell-free radioactive assay |
J Med Chem 54: 5154-64 (2011)
Article DOI: 10.1021/jm2003969
BindingDB Entry DOI: 10.7270/Q26D5TBX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50347977
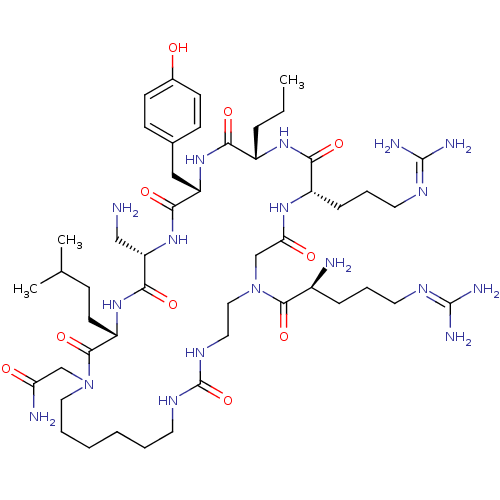
(CHEMBL1800568)Show SMILES [#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7](-[#6]-[#6]-[#7]-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#7](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C49H86N18O10/c1-4-11-34-41(71)64-37(26-31-15-17-32(68)18-16-31)43(73)65-38(27-50)44(74)63-36(19-14-30(2)3)46(76)66(28-39(52)69)24-8-6-5-7-20-59-49(77)60-23-25-67(45(75)33(51)12-9-21-57-47(53)54)29-40(70)61-35(42(72)62-34)13-10-22-58-48(55)56/h15-18,30,33-38,68H,4-14,19-29,50-51H2,1-3H3,(H2,52,69)(H,61,70)(H,62,72)(H,63,74)(H,64,71)(H,65,73)(H4,53,54,57)(H4,55,56,58)(H2,59,60,77)/t33-,34-,35-,36-,37-,38-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of Akt using RPRTSSF peptide and [gamma32]ATP by cell-free radioactive assay |
J Med Chem 54: 5154-64 (2011)
Article DOI: 10.1021/jm2003969
BindingDB Entry DOI: 10.7270/Q26D5TBX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50347978
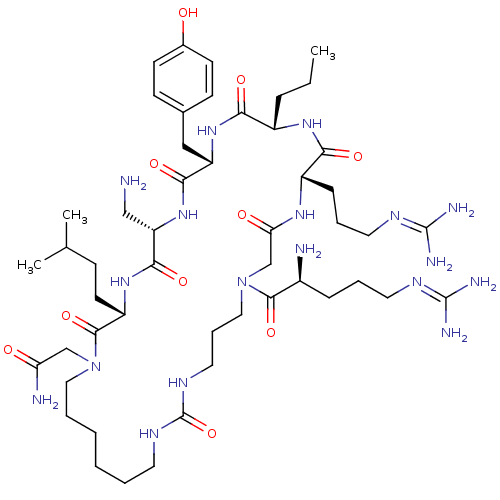
(CHEMBL1800569)Show SMILES [#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7](-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#7](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C50H88N18O10/c1-4-12-35-42(72)65-38(27-32-16-18-33(69)19-17-32)44(74)66-39(28-51)45(75)64-37(20-15-31(2)3)47(77)67(29-40(53)70)25-8-6-5-7-21-60-50(78)61-24-11-26-68(46(76)34(52)13-9-22-58-48(54)55)30-41(71)62-36(43(73)63-35)14-10-23-59-49(56)57/h16-19,31,34-39,69H,4-15,20-30,51-52H2,1-3H3,(H2,53,70)(H,62,71)(H,63,73)(H,64,75)(H,65,72)(H,66,74)(H4,54,55,58)(H4,56,57,59)(H2,60,61,78)/t34-,35-,36-,37-,38-,39-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of Akt using RPRTSSF peptide and [gamma32]ATP by cell-free radioactive assay |
J Med Chem 54: 5154-64 (2011)
Article DOI: 10.1021/jm2003969
BindingDB Entry DOI: 10.7270/Q26D5TBX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50347961

(CHEMBL1800265)Show SMILES [#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7](-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7](-[#6]-[#6]-[#7])-[#6]-[#6](-[#7])=O |r| Show InChI InChI=1S/C45H82N18O9/c1-4-8-31(58-39(68)32(10-6-20-56-45(53)54)57-37(66)26-62(21-7-17-46)42(71)30(49)9-5-19-55-44(51)52)38(67)60-34(23-28-12-14-29(64)15-13-28)40(69)61-35(24-48)41(70)59-33(16-11-27(2)3)43(72)63(22-18-47)25-36(50)65/h12-15,27,30-35,64H,4-11,16-26,46-49H2,1-3H3,(H2,50,65)(H,57,66)(H,58,68)(H,59,70)(H,60,67)(H,61,69)(H4,51,52,55)(H4,53,54,56)/t30-,31-,32-,33-,34-,35-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of Akt using RPRTSSF peptide and [gamma32]ATP by cell-free radioactive assay |
J Med Chem 54: 5154-64 (2011)
Article DOI: 10.1021/jm2003969
BindingDB Entry DOI: 10.7270/Q26D5TBX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50347963
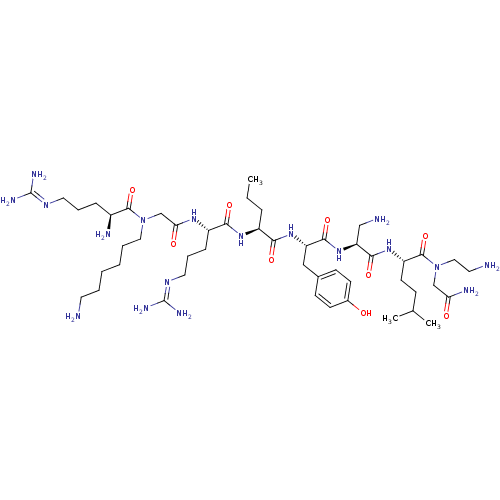
(CHEMBL1800267)Show SMILES [#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7](-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7](-[#6]-[#6]-[#7])-[#6]-[#6](-[#7])=O |r| Show InChI InChI=1S/C48H88N18O9/c1-4-11-34(61-42(71)35(13-10-23-59-48(56)57)60-40(69)29-65(24-8-6-5-7-20-49)45(74)33(52)12-9-22-58-47(54)55)41(70)63-37(26-31-15-17-32(67)18-16-31)43(72)64-38(27-51)44(73)62-36(19-14-30(2)3)46(75)66(25-21-50)28-39(53)68/h15-18,30,33-38,67H,4-14,19-29,49-52H2,1-3H3,(H2,53,68)(H,60,69)(H,61,71)(H,62,73)(H,63,70)(H,64,72)(H4,54,55,58)(H4,56,57,59)/t33-,34-,35-,36-,37-,38-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of Akt using RPRTSSF peptide and [gamma32]ATP by cell-free radioactive assay |
J Med Chem 54: 5154-64 (2011)
Article DOI: 10.1021/jm2003969
BindingDB Entry DOI: 10.7270/Q26D5TBX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50347979

(CHEMBL1800698)Show SMILES [#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7]-[#6]-[#6]-[#7]-[#6](=O)-[#7]-[#6]-[#6]-[#7](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O |r| Show InChI InChI=1S/C39H66N14O9/c1-4-6-26-33(57)51-29(19-24-9-11-25(54)12-10-24)35(59)52-30(20-40)36(60)50-28(13-8-23(2)3)37(61)53(22-31(41)55)18-17-47-39(62)46-16-15-44-21-32(56)48-27(34(58)49-26)7-5-14-45-38(42)43/h9-12,23,26-30,44,54H,4-8,13-22,40H2,1-3H3,(H2,41,55)(H,48,56)(H,49,58)(H,50,60)(H,51,57)(H,52,59)(H4,42,43,45)(H2,46,47,62)/t26-,27-,28-,29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of Akt using RPRTSSF peptide and [gamma32]ATP by cell-free radioactive assay |
J Med Chem 54: 5154-64 (2011)
Article DOI: 10.1021/jm2003969
BindingDB Entry DOI: 10.7270/Q26D5TBX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50347972

(CHEMBL1800400)Show SMILES [#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7](-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#7](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C47H82N18O10/c1-4-9-32-39(69)62-35(24-29-13-15-30(66)16-14-29)41(71)63-36(25-48)42(72)61-34(17-12-28(2)3)44(74)64(26-37(50)67)22-7-20-57-47(75)58-21-8-23-65(43(73)31(49)10-5-18-55-45(51)52)27-38(68)59-33(40(70)60-32)11-6-19-56-46(53)54/h13-16,28,31-36,66H,4-12,17-27,48-49H2,1-3H3,(H2,50,67)(H,59,68)(H,60,70)(H,61,72)(H,62,69)(H,63,71)(H4,51,52,55)(H4,53,54,56)(H2,57,58,75)/t31-,32-,33-,34-,35-,36-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of Akt using RPRTSSF peptide and [gamma32]ATP by cell-free radioactive assay |
J Med Chem 54: 5154-64 (2011)
Article DOI: 10.1021/jm2003969
BindingDB Entry DOI: 10.7270/Q26D5TBX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50347959
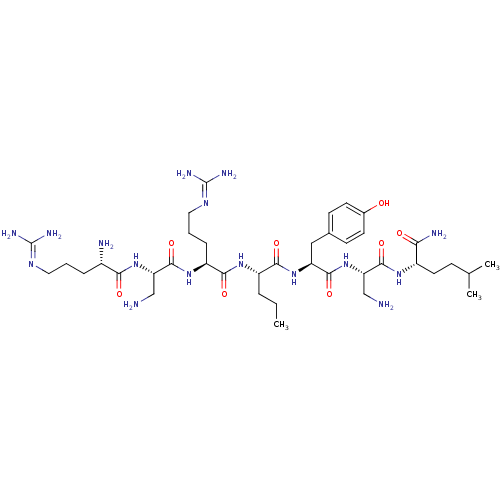
(CHEMBL1800260)Show SMILES [#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#6])-[#6])-[#6](-[#7])=O |r| Show InChI InChI=1S/C39H70N16O8/c1-4-7-26(51-34(60)27(9-6-17-49-39(46)47)52-37(63)29(19-40)54-32(58)24(42)8-5-16-48-38(44)45)33(59)53-28(18-22-11-13-23(56)14-12-22)35(61)55-30(20-41)36(62)50-25(31(43)57)15-10-21(2)3/h11-14,21,24-30,56H,4-10,15-20,40-42H2,1-3H3,(H2,43,57)(H,50,62)(H,51,60)(H,52,63)(H,53,59)(H,54,58)(H,55,61)(H4,44,45,48)(H4,46,47,49)/t24-,25-,26-,27-,28-,29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of Akt using RPRTSSF peptide and [gamma32]ATP by cell-free radioactive assay |
J Med Chem 54: 5154-64 (2011)
Article DOI: 10.1021/jm2003969
BindingDB Entry DOI: 10.7270/Q26D5TBX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50347971
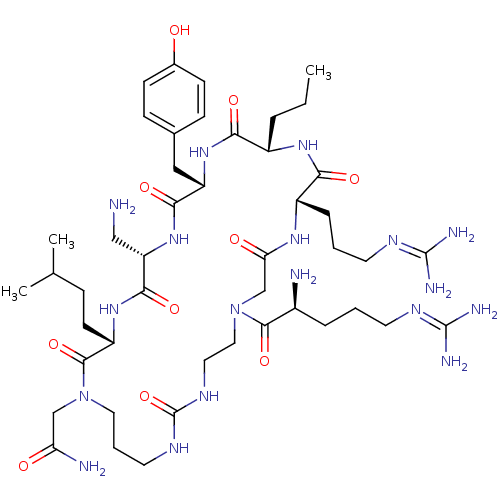
(CHEMBL1800399)Show SMILES [#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6]-[#7](-[#6]-[#6]-[#7]-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#7](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7] |r| Show InChI InChI=1S/C46H80N18O10/c1-4-8-31-38(68)61-34(23-28-12-14-29(65)15-13-28)40(70)62-35(24-47)41(71)60-33(16-11-27(2)3)43(73)63(25-36(49)66)21-7-19-56-46(74)57-20-22-64(42(72)30(48)9-5-17-54-44(50)51)26-37(67)58-32(39(69)59-31)10-6-18-55-45(52)53/h12-15,27,30-35,65H,4-11,16-26,47-48H2,1-3H3,(H2,49,66)(H,58,67)(H,59,69)(H,60,71)(H,61,68)(H,62,70)(H4,50,51,54)(H4,52,53,55)(H2,56,57,74)/t30-,31-,32-,33-,34-,35-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of Akt using RPRTSSF peptide and [gamma32]ATP by cell-free radioactive assay |
J Med Chem 54: 5154-64 (2011)
Article DOI: 10.1021/jm2003969
BindingDB Entry DOI: 10.7270/Q26D5TBX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316026

((S)-N-((6S,9S,12S,15S,18S)-1-amino-15-(aminomethyl...)Show SMILES [#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#6])-[#6])-[#6](-[#7])=O |r| Show InChI InChI=1S/C41H71N15O8/c1-4-8-28(34(59)54-30(21-24-13-15-25(57)16-14-24)36(61)55-31(22-42)37(62)51-27(33(44)58)17-12-23(2)3)52-35(60)29(10-6-19-50-41(47)48)53-38(63)32-11-7-20-56(32)39(64)26(43)9-5-18-49-40(45)46/h13-16,23,26-32,57H,4-12,17-22,42-43H2,1-3H3,(H2,44,58)(H,51,62)(H,52,60)(H,53,63)(H,54,59)(H,55,61)(H4,45,46,49)(H4,47,48,50)/t26-,27-,28-,29-,30-,31-,32-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of Akt using RPRTSSF peptide and [gamma32]ATP by cell-free radioactive assay |
J Med Chem 54: 5154-64 (2011)
Article DOI: 10.1021/jm2003969
BindingDB Entry DOI: 10.7270/Q26D5TBX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50347958
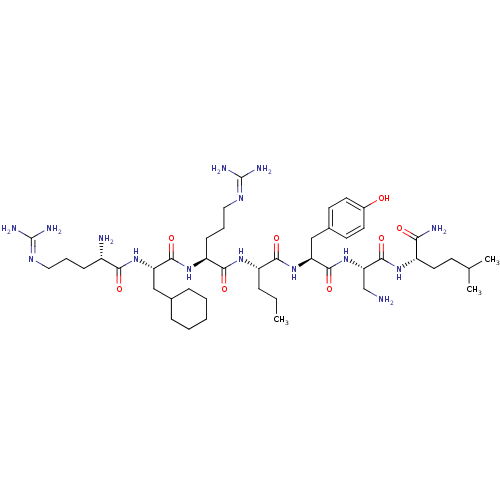
(CHEMBL1800146)Show SMILES [#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1)-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#6])-[#6])-[#6](-[#7])=O |r| Show InChI InChI=1S/C45H79N15O8/c1-4-10-32(39(64)59-35(24-28-16-18-29(61)19-17-28)42(67)60-36(25-46)43(68)55-31(37(48)62)20-15-26(2)3)56-40(65)33(14-9-22-54-45(51)52)57-41(66)34(23-27-11-6-5-7-12-27)58-38(63)30(47)13-8-21-53-44(49)50/h16-19,26-27,30-36,61H,4-15,20-25,46-47H2,1-3H3,(H2,48,62)(H,55,68)(H,56,65)(H,57,66)(H,58,63)(H,59,64)(H,60,67)(H4,49,50,53)(H4,51,52,54)/t30-,31-,32-,33-,34-,35-,36-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of Akt using RPRTSSF peptide and [gamma32]ATP by cell-free radioactive assay |
J Med Chem 54: 5154-64 (2011)
Article DOI: 10.1021/jm2003969
BindingDB Entry DOI: 10.7270/Q26D5TBX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50347960

(CHEMBL1800262)Show SMILES [#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#6])-[#6])-[#6](-[#7])=O |r| Show InChI InChI=1S/C42H76N16O8/c1-4-9-29(36(62)57-32(22-25-14-16-26(59)17-15-25)39(65)58-33(23-44)40(66)53-28(34(46)60)18-13-24(2)3)55-38(64)31(12-8-21-52-42(49)50)56-37(63)30(11-5-6-19-43)54-35(61)27(45)10-7-20-51-41(47)48/h14-17,24,27-33,59H,4-13,18-23,43-45H2,1-3H3,(H2,46,60)(H,53,66)(H,54,61)(H,55,64)(H,56,63)(H,57,62)(H,58,65)(H4,47,48,51)(H4,49,50,52)/t27-,28-,29-,30-,31-,32-,33-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of Akt using RPRTSSF peptide and [gamma32]ATP by cell-free radioactive assay |
J Med Chem 54: 5154-64 (2011)
Article DOI: 10.1021/jm2003969
BindingDB Entry DOI: 10.7270/Q26D5TBX |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50481235
(WFVLLTITVLR)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(C)C)[C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C68H109N15O14/c1-15-39(12)54(64(93)83-56(41(14)85)65(94)80-53(38(10)11)63(92)77-48(28-34(2)3)58(87)74-47(67(96)97)26-21-27-72-68(70)71)81-66(95)55(40(13)84)82-60(89)50(30-36(6)7)76-59(88)49(29-35(4)5)78-62(91)52(37(8)9)79-61(90)51(31-42-22-17-16-18-23-42)75-57(86)45(69)32-43-33-73-46-25-20-19-24-44(43)46/h16-20,22-25,33-41,45,47-56,73,84-85H,15,21,26-32,69H2,1-14H3,(H,74,87)(H,75,86)(H,76,88)(H,77,92)(H,78,91)(H,79,90)(H,80,94)(H,81,95)(H,82,89)(H,83,93)(H,96,97)(H4,70,71,72)/t39-,40+,41+,45-,47-,48-,49-,50-,51-,52-,53-,54-,55-,56-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Binding affinity to histidine-tagged HIV1 full length integrase by fluorescence anisotropy |
Bioorg Med Chem 17: 7635-42 (2009)
Article DOI: 10.1016/j.bmc.2009.09.053
BindingDB Entry DOI: 10.7270/Q2ZG6W22 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50481234

(CHEMBL603517)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CS)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)[C@@H](C)O)[C@@H](C)O)C(C)C)[C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C110H180N30O26S/c1-21-59(16)85(103(160)140-89(63(20)144)106(163)135-84(58(14)15)102(159)129-73(39-52(2)3)93(150)126-72(108(165)166)34-28-38-119-110(115)116)136-107(164)88(62(19)143)139-96(153)75(41-54(6)7)127-94(151)74(40-53(4)5)130-101(158)83(57(12)13)134-99(156)78(44-64-29-23-22-24-30-64)123-82(146)49-121-91(148)70(33-27-37-118-109(113)114)125-95(152)79(46-66-48-117-51-122-66)132-105(162)87(61(18)142)138-98(155)77(43-56(10)11)131-104(161)86(60(17)141)137-97(154)76(42-55(8)9)128-100(157)80(50-167)133-92(149)71(35-36-81(112)145)124-90(147)68(111)45-65-47-120-69-32-26-25-31-67(65)69/h22-26,29-32,47-48,51-63,68,70-80,83-89,120,141-144,167H,21,27-28,33-46,49-50,111H2,1-20H3,(H2,112,145)(H,117,122)(H,121,148)(H,123,146)(H,124,147)(H,125,152)(H,126,150)(H,127,151)(H,128,157)(H,129,159)(H,130,158)(H,131,161)(H,132,162)(H,133,149)(H,134,156)(H,135,163)(H,136,164)(H,137,154)(H,138,155)(H,139,153)(H,140,160)(H,165,166)(H4,113,114,118)(H4,115,116,119)/t59-,60+,61+,62+,63+,68-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,83-,84-,85-,86-,87-,88-,89-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Binding affinity to tetrameric histidine-tagged HIV1 full length integrase by fluorescence anisotropy |
Bioorg Med Chem 17: 7635-42 (2009)
Article DOI: 10.1016/j.bmc.2009.09.053
BindingDB Entry DOI: 10.7270/Q2ZG6W22 |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50481233

(CHEMBL593680)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CS)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(O)=O |r| Show InChI InChI=1S/C53H83N17O14S/c1-25(2)16-36(65-50(82)39(23-85)68-46(78)35(13-14-40(55)73)63-44(76)32(54)18-29-20-60-33-11-8-7-10-31(29)33)48(80)69-42(27(5)71)51(83)66-37(17-26(3)4)49(81)70-43(28(6)72)52(84)67-38(19-30-21-58-24-62-30)47(79)64-34(12-9-15-59-53(56)57)45(77)61-22-41(74)75/h7-8,10-11,20-21,24-28,32,34-39,42-43,60,71-72,85H,9,12-19,22-23,54H2,1-6H3,(H2,55,73)(H,58,62)(H,61,77)(H,63,76)(H,64,79)(H,65,82)(H,66,83)(H,67,84)(H,68,78)(H,69,80)(H,70,81)(H,74,75)(H4,56,57,59)/t27-,28-,32+,34+,35+,36+,37+,38+,39+,42+,43+/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Binding affinity to histidine-tagged HIV1 full length integrase by fluorescence anisotropy |
Bioorg Med Chem 17: 7635-42 (2009)
Article DOI: 10.1016/j.bmc.2009.09.053
BindingDB Entry DOI: 10.7270/Q2ZG6W22 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Mus musculus) | BDBM50374670

(CHEMBL270923)Show SMILES NC(=O)CN1CCNC(=O)c2ccccc2C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:30.32,wD:52.55,19.20,41.44,(6.67,-17.07,;5.13,-17.07,;4.36,-15.74,;4.37,-18.41,;2.83,-18.41,;2.05,-17.08,;.51,-17.09,;-.25,-18.42,;-1.79,-18.43,;-2.56,-19.76,;-2.57,-17.1,;-1.81,-15.76,;-2.59,-14.43,;-4.14,-14.45,;-4.89,-15.78,;-4.11,-17.1,;-4.87,-18.43,;-6.41,-18.44,;-4.1,-19.76,;-5.43,-20.54,;-6.76,-19.76,;-8.09,-20.54,;-9.42,-19.75,;-10.76,-20.53,;-10.76,-22.07,;-9.41,-22.83,;-8.09,-22.06,;-5.43,-22.08,;-6.76,-22.84,;-4.1,-22.84,;-4.1,-24.38,;-5.43,-25.16,;-5.43,-26.7,;-6.77,-27.46,;-6.77,-28.99,;-5.43,-29.77,;-4.1,-28.99,;-4.1,-27.45,;-2.76,-25.16,;-2.76,-26.7,;-1.42,-24.38,;-.09,-25.16,;-.09,-26.7,;1.24,-27.46,;1.24,-29,;2.58,-29.78,;2.58,-31.32,;3.91,-32.08,;1.24,-32.08,;1.24,-24.38,;2.58,-25.16,;1.24,-22.84,;2.58,-22.08,;3.91,-22.84,;5.24,-22.08,;5.41,-20.53,;6.91,-20.22,;7.67,-21.56,;9.18,-21.89,;9.64,-23.36,;8.6,-24.49,;7.1,-24.16,;6.63,-22.7,;2.06,-19.75,;.52,-19.75,)| Show InChI InChI=1S/C47H53N11O7/c48-40(59)28-58-23-22-51-41(60)33-17-7-8-18-34(33)42(61)55-37(24-29-12-3-1-4-13-29)45(64)56-38(25-30-14-5-2-6-15-30)44(63)54-36(20-11-21-52-47(49)50)43(62)57-39(46(58)65)26-31-27-53-35-19-10-9-16-32(31)35/h1-10,12-19,27,36-39,53H,11,20-26,28H2,(H2,48,59)(H,51,60)(H,54,63)(H,55,61)(H,56,64)(H,57,62)(H4,49,50,52)/t36-,37-,38+,39-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 550 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC1R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Mus musculus) | BDBM50374671

(CHEMBL272660)Show SMILES NC(=O)CN1CCCNC(=O)CCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:27.28,wD:16.16,49.51,38.40,(28.48,-20.35,;27.16,-19.57,;27.17,-18.03,;25.81,-20.32,;25.8,-21.86,;23.64,-21.92,;23.15,-20.32,;24.48,-19.55,;24.48,-18.01,;23.15,-17.24,;23.15,-15.7,;21.81,-18.01,;21.81,-19.55,;20.48,-20.32,;19.15,-19.55,;20.48,-21.86,;19.15,-22.63,;17.81,-21.86,;16.48,-22.63,;15.15,-21.85,;13.82,-22.62,;13.81,-24.16,;15.16,-24.93,;16.48,-24.16,;19.15,-24.17,;17.81,-24.94,;20.48,-24.94,;20.48,-26.48,;19.15,-27.25,;19.15,-28.79,;17.81,-29.56,;17.8,-31.09,;19.14,-31.87,;20.48,-31.09,;20.47,-29.55,;21.81,-27.25,;21.81,-28.79,;23.15,-26.48,;24.48,-27.25,;24.48,-28.79,;25.81,-29.56,;25.81,-31.1,;27.15,-31.87,;27.15,-33.41,;28.48,-34.18,;25.81,-34.18,;25.81,-26.48,;27.15,-27.25,;25.81,-24.94,;27.15,-24.17,;28.48,-24.94,;29.82,-24.17,;29.98,-22.63,;31.49,-22.31,;32.24,-23.66,;33.75,-23.99,;34.21,-25.46,;33.18,-26.59,;31.67,-26.26,;31.21,-24.8,;27.15,-22.63,;28.48,-21.86,)| Show InChI InChI=1S/C44H55N11O7/c45-37(56)27-55-22-10-21-48-38(57)18-19-39(58)51-34(23-28-11-3-1-4-12-28)41(60)53-35(24-29-13-5-2-6-14-29)42(61)52-33(17-9-20-49-44(46)47)40(59)54-36(43(55)62)25-30-26-50-32-16-8-7-15-31(30)32/h1-8,11-16,26,33-36,50H,9-10,17-25,27H2,(H2,45,56)(H,48,57)(H,51,58)(H,52,61)(H,53,60)(H,54,59)(H4,46,47,49)/t33-,34-,35+,36-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC1R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Mus musculus) | BDBM50121900

(Ac-Ser-Try-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pr...)Show SMILES CCCC[C@H](NC(=O)C(CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@@H]1C(=O)N[C@H](C(C)C)C(N)=O Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61?,62+,65+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC1R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50374672

(CHEMBL271586)Show SMILES NC(=O)CN1CCCNC(=O)CCCCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:29.30,wD:51.53,18.18,40.42,(30.64,-.68,;29.3,.09,;29.31,1.63,;27.97,-.68,;26.64,.1,;26.64,1.64,;27.98,2.4,;27.98,3.94,;25.3,3.95,;23.96,4.72,;23.96,6.26,;22.63,3.95,;21.3,4.72,;19.96,3.95,;19.96,2.41,;18.63,1.64,;17.29,2.41,;18.63,.1,;17.29,-.67,;15.96,.1,;14.63,-.67,;13.3,.11,;11.96,-.66,;11.96,-2.2,;13.31,-2.97,;14.63,-2.2,;17.29,-2.21,;15.96,-2.98,;18.63,-2.98,;18.63,-4.52,;17.29,-5.29,;17.29,-6.83,;15.95,-7.59,;15.95,-9.13,;17.29,-9.9,;18.63,-9.12,;18.62,-7.59,;19.96,-5.29,;19.96,-6.83,;21.3,-4.52,;22.63,-5.29,;22.63,-6.83,;23.96,-7.6,;23.96,-9.14,;25.3,-9.91,;25.3,-11.45,;26.63,-12.22,;23.96,-12.22,;23.96,-4.52,;25.3,-5.29,;23.96,-2.98,;25.3,-2.21,;26.63,-2.98,;28.17,-2.98,;29.08,-1.72,;30.54,-2.19,;30.53,-3.74,;31.67,-4.77,;31.35,-6.28,;29.88,-6.75,;28.74,-5.71,;29.07,-4.21,;25.3,-.67,;23.97,.1,)| Show InChI InChI=1S/C46H59N11O7/c47-39(58)29-57-24-12-23-50-40(59)20-9-10-21-41(60)53-36(25-30-13-3-1-4-14-30)43(62)55-37(26-31-15-5-2-6-16-31)44(63)54-35(19-11-22-51-46(48)49)42(61)56-38(45(57)64)27-32-28-52-34-18-8-7-17-33(32)34/h1-8,13-18,28,35-38,52H,9-12,19-27,29H2,(H2,47,58)(H,50,59)(H,53,60)(H,54,63)(H,55,62)(H,56,61)(H4,48,49,51)/t35-,36-,37+,38-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC5R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50374670

(CHEMBL270923)Show SMILES NC(=O)CN1CCNC(=O)c2ccccc2C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:30.32,wD:52.55,19.20,41.44,(6.67,-17.07,;5.13,-17.07,;4.36,-15.74,;4.37,-18.41,;2.83,-18.41,;2.05,-17.08,;.51,-17.09,;-.25,-18.42,;-1.79,-18.43,;-2.56,-19.76,;-2.57,-17.1,;-1.81,-15.76,;-2.59,-14.43,;-4.14,-14.45,;-4.89,-15.78,;-4.11,-17.1,;-4.87,-18.43,;-6.41,-18.44,;-4.1,-19.76,;-5.43,-20.54,;-6.76,-19.76,;-8.09,-20.54,;-9.42,-19.75,;-10.76,-20.53,;-10.76,-22.07,;-9.41,-22.83,;-8.09,-22.06,;-5.43,-22.08,;-6.76,-22.84,;-4.1,-22.84,;-4.1,-24.38,;-5.43,-25.16,;-5.43,-26.7,;-6.77,-27.46,;-6.77,-28.99,;-5.43,-29.77,;-4.1,-28.99,;-4.1,-27.45,;-2.76,-25.16,;-2.76,-26.7,;-1.42,-24.38,;-.09,-25.16,;-.09,-26.7,;1.24,-27.46,;1.24,-29,;2.58,-29.78,;2.58,-31.32,;3.91,-32.08,;1.24,-32.08,;1.24,-24.38,;2.58,-25.16,;1.24,-22.84,;2.58,-22.08,;3.91,-22.84,;5.24,-22.08,;5.41,-20.53,;6.91,-20.22,;7.67,-21.56,;9.18,-21.89,;9.64,-23.36,;8.6,-24.49,;7.1,-24.16,;6.63,-22.7,;2.06,-19.75,;.52,-19.75,)| Show InChI InChI=1S/C47H53N11O7/c48-40(59)28-58-23-22-51-41(60)33-17-7-8-18-34(33)42(61)55-37(24-29-12-3-1-4-13-29)45(64)56-38(25-30-14-5-2-6-15-30)44(63)54-36(20-11-21-52-47(49)50)43(62)57-39(46(58)65)26-31-27-53-35-19-10-9-16-32(31)35/h1-10,12-19,27,36-39,53H,11,20-26,28H2,(H2,48,59)(H,51,60)(H,54,63)(H,55,61)(H,56,64)(H,57,62)(H4,49,50,52)/t36-,37-,38+,39-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 390 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC5R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50374669

(CHEMBL406985)Show SMILES NC(=O)CN1CCNC(=O)CCCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:27.28,wD:16.16,49.51,38.40,(13.37,-36.69,;12.04,-35.93,;12.04,-34.39,;10.72,-36.69,;10.72,-38.22,;8.06,-38.19,;8.05,-36.66,;9.38,-35.89,;9.37,-34.36,;10.7,-33.58,;8.04,-33.6,;6.71,-34.37,;6.71,-35.91,;5.38,-36.68,;4.04,-35.91,;5.38,-38.22,;4.04,-38.99,;2.71,-38.22,;1.37,-38.99,;.04,-38.21,;-1.29,-38.98,;-1.29,-40.52,;.05,-41.29,;1.38,-40.52,;4.04,-40.53,;2.71,-41.3,;5.38,-41.3,;5.38,-42.84,;4.04,-43.61,;4.04,-45.15,;2.7,-45.91,;2.7,-47.45,;4.04,-48.22,;5.38,-47.44,;5.37,-45.91,;6.71,-43.61,;6.71,-45.15,;8.04,-42.84,;9.38,-43.61,;9.38,-45.15,;10.71,-45.92,;10.71,-47.46,;12.05,-48.23,;12.05,-49.77,;13.38,-50.54,;10.71,-50.54,;10.71,-42.84,;12.05,-43.61,;10.71,-41.3,;12.05,-40.53,;13.38,-41.3,;14.72,-40.53,;14.88,-38.98,;16.39,-38.67,;17.14,-40.02,;18.64,-40.35,;19.12,-41.81,;18.08,-42.95,;16.57,-42.62,;16.11,-41.15,;12.05,-38.99,;13.38,-38.22,)| Show InChI InChI=1S/C44H55N11O7/c45-37(56)27-55-22-21-48-38(57)18-9-19-39(58)51-34(23-28-11-3-1-4-12-28)41(60)53-35(24-29-13-5-2-6-14-29)42(61)52-33(17-10-20-49-44(46)47)40(59)54-36(43(55)62)25-30-26-50-32-16-8-7-15-31(30)32/h1-8,11-16,26,33-36,50H,9-10,17-25,27H2,(H2,45,56)(H,48,57)(H,51,58)(H,52,61)(H,53,60)(H,54,59)(H4,46,47,49)/t33-,34-,35+,36-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC5R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50121900

(Ac-Ser-Try-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pr...)Show SMILES CCCC[C@H](NC(=O)C(CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@@H]1C(=O)N[C@H](C(C)C)C(N)=O Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61?,62+,65+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0780 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC5R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50374672

(CHEMBL271586)Show SMILES NC(=O)CN1CCCNC(=O)CCCCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:29.30,wD:51.53,18.18,40.42,(30.64,-.68,;29.3,.09,;29.31,1.63,;27.97,-.68,;26.64,.1,;26.64,1.64,;27.98,2.4,;27.98,3.94,;25.3,3.95,;23.96,4.72,;23.96,6.26,;22.63,3.95,;21.3,4.72,;19.96,3.95,;19.96,2.41,;18.63,1.64,;17.29,2.41,;18.63,.1,;17.29,-.67,;15.96,.1,;14.63,-.67,;13.3,.11,;11.96,-.66,;11.96,-2.2,;13.31,-2.97,;14.63,-2.2,;17.29,-2.21,;15.96,-2.98,;18.63,-2.98,;18.63,-4.52,;17.29,-5.29,;17.29,-6.83,;15.95,-7.59,;15.95,-9.13,;17.29,-9.9,;18.63,-9.12,;18.62,-7.59,;19.96,-5.29,;19.96,-6.83,;21.3,-4.52,;22.63,-5.29,;22.63,-6.83,;23.96,-7.6,;23.96,-9.14,;25.3,-9.91,;25.3,-11.45,;26.63,-12.22,;23.96,-12.22,;23.96,-4.52,;25.3,-5.29,;23.96,-2.98,;25.3,-2.21,;26.63,-2.98,;28.17,-2.98,;29.08,-1.72,;30.54,-2.19,;30.53,-3.74,;31.67,-4.77,;31.35,-6.28,;29.88,-6.75,;28.74,-5.71,;29.07,-4.21,;25.3,-.67,;23.97,.1,)| Show InChI InChI=1S/C46H59N11O7/c47-39(58)29-57-24-12-23-50-40(59)20-9-10-21-41(60)53-36(25-30-13-3-1-4-14-30)43(62)55-37(26-31-15-5-2-6-16-31)44(63)54-35(19-11-22-51-46(48)49)42(61)56-38(45(57)64)27-32-28-52-34-18-8-7-17-33(32)34/h1-8,13-18,28,35-38,52H,9-12,19-27,29H2,(H2,47,58)(H,50,59)(H,53,60)(H,54,63)(H,55,62)(H,56,61)(H4,48,49,51)/t35-,36-,37+,38-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC4R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50374671

(CHEMBL272660)Show SMILES NC(=O)CN1CCCNC(=O)CCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:27.28,wD:16.16,49.51,38.40,(28.48,-20.35,;27.16,-19.57,;27.17,-18.03,;25.81,-20.32,;25.8,-21.86,;23.64,-21.92,;23.15,-20.32,;24.48,-19.55,;24.48,-18.01,;23.15,-17.24,;23.15,-15.7,;21.81,-18.01,;21.81,-19.55,;20.48,-20.32,;19.15,-19.55,;20.48,-21.86,;19.15,-22.63,;17.81,-21.86,;16.48,-22.63,;15.15,-21.85,;13.82,-22.62,;13.81,-24.16,;15.16,-24.93,;16.48,-24.16,;19.15,-24.17,;17.81,-24.94,;20.48,-24.94,;20.48,-26.48,;19.15,-27.25,;19.15,-28.79,;17.81,-29.56,;17.8,-31.09,;19.14,-31.87,;20.48,-31.09,;20.47,-29.55,;21.81,-27.25,;21.81,-28.79,;23.15,-26.48,;24.48,-27.25,;24.48,-28.79,;25.81,-29.56,;25.81,-31.1,;27.15,-31.87,;27.15,-33.41,;28.48,-34.18,;25.81,-34.18,;25.81,-26.48,;27.15,-27.25,;25.81,-24.94,;27.15,-24.17,;28.48,-24.94,;29.82,-24.17,;29.98,-22.63,;31.49,-22.31,;32.24,-23.66,;33.75,-23.99,;34.21,-25.46,;33.18,-26.59,;31.67,-26.26,;31.21,-24.8,;27.15,-22.63,;28.48,-21.86,)| Show InChI InChI=1S/C44H55N11O7/c45-37(56)27-55-22-10-21-48-38(57)18-19-39(58)51-34(23-28-11-3-1-4-12-28)41(60)53-35(24-29-13-5-2-6-14-29)42(61)52-33(17-9-20-49-44(46)47)40(59)54-36(43(55)62)25-30-26-50-32-16-8-7-15-31(30)32/h1-8,11-16,26,33-36,50H,9-10,17-25,27H2,(H2,45,56)(H,48,57)(H,51,58)(H,52,61)(H,53,60)(H,54,59)(H4,46,47,49)/t33-,34-,35+,36-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC4R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50374669

(CHEMBL406985)Show SMILES NC(=O)CN1CCNC(=O)CCCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:27.28,wD:16.16,49.51,38.40,(13.37,-36.69,;12.04,-35.93,;12.04,-34.39,;10.72,-36.69,;10.72,-38.22,;8.06,-38.19,;8.05,-36.66,;9.38,-35.89,;9.37,-34.36,;10.7,-33.58,;8.04,-33.6,;6.71,-34.37,;6.71,-35.91,;5.38,-36.68,;4.04,-35.91,;5.38,-38.22,;4.04,-38.99,;2.71,-38.22,;1.37,-38.99,;.04,-38.21,;-1.29,-38.98,;-1.29,-40.52,;.05,-41.29,;1.38,-40.52,;4.04,-40.53,;2.71,-41.3,;5.38,-41.3,;5.38,-42.84,;4.04,-43.61,;4.04,-45.15,;2.7,-45.91,;2.7,-47.45,;4.04,-48.22,;5.38,-47.44,;5.37,-45.91,;6.71,-43.61,;6.71,-45.15,;8.04,-42.84,;9.38,-43.61,;9.38,-45.15,;10.71,-45.92,;10.71,-47.46,;12.05,-48.23,;12.05,-49.77,;13.38,-50.54,;10.71,-50.54,;10.71,-42.84,;12.05,-43.61,;10.71,-41.3,;12.05,-40.53,;13.38,-41.3,;14.72,-40.53,;14.88,-38.98,;16.39,-38.67,;17.14,-40.02,;18.64,-40.35,;19.12,-41.81,;18.08,-42.95,;16.57,-42.62,;16.11,-41.15,;12.05,-38.99,;13.38,-38.22,)| Show InChI InChI=1S/C44H55N11O7/c45-37(56)27-55-22-21-48-38(57)18-9-19-39(58)51-34(23-28-11-3-1-4-12-28)41(60)53-35(24-29-13-5-2-6-14-29)42(61)52-33(17-10-20-49-44(46)47)40(59)54-36(43(55)62)25-30-26-50-32-16-8-7-15-31(30)32/h1-8,11-16,26,33-36,50H,9-10,17-25,27H2,(H2,45,56)(H,48,57)(H,51,58)(H,52,61)(H,53,60)(H,54,59)(H4,46,47,49)/t33-,34-,35+,36-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC4R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50374672

(CHEMBL271586)Show SMILES NC(=O)CN1CCCNC(=O)CCCCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:29.30,wD:51.53,18.18,40.42,(30.64,-.68,;29.3,.09,;29.31,1.63,;27.97,-.68,;26.64,.1,;26.64,1.64,;27.98,2.4,;27.98,3.94,;25.3,3.95,;23.96,4.72,;23.96,6.26,;22.63,3.95,;21.3,4.72,;19.96,3.95,;19.96,2.41,;18.63,1.64,;17.29,2.41,;18.63,.1,;17.29,-.67,;15.96,.1,;14.63,-.67,;13.3,.11,;11.96,-.66,;11.96,-2.2,;13.31,-2.97,;14.63,-2.2,;17.29,-2.21,;15.96,-2.98,;18.63,-2.98,;18.63,-4.52,;17.29,-5.29,;17.29,-6.83,;15.95,-7.59,;15.95,-9.13,;17.29,-9.9,;18.63,-9.12,;18.62,-7.59,;19.96,-5.29,;19.96,-6.83,;21.3,-4.52,;22.63,-5.29,;22.63,-6.83,;23.96,-7.6,;23.96,-9.14,;25.3,-9.91,;25.3,-11.45,;26.63,-12.22,;23.96,-12.22,;23.96,-4.52,;25.3,-5.29,;23.96,-2.98,;25.3,-2.21,;26.63,-2.98,;28.17,-2.98,;29.08,-1.72,;30.54,-2.19,;30.53,-3.74,;31.67,-4.77,;31.35,-6.28,;29.88,-6.75,;28.74,-5.71,;29.07,-4.21,;25.3,-.67,;23.97,.1,)| Show InChI InChI=1S/C46H59N11O7/c47-39(58)29-57-24-12-23-50-40(59)20-9-10-21-41(60)53-36(25-30-13-3-1-4-14-30)43(62)55-37(26-31-15-5-2-6-16-31)44(63)54-35(19-11-22-51-46(48)49)42(61)56-38(45(57)64)27-32-28-52-34-18-8-7-17-33(32)34/h1-8,13-18,28,35-38,52H,9-12,19-27,29H2,(H2,47,58)(H,50,59)(H,53,60)(H,54,63)(H,55,62)(H,56,61)(H4,48,49,51)/t35-,36-,37+,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.75E+3 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC3R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50374670

(CHEMBL270923)Show SMILES NC(=O)CN1CCNC(=O)c2ccccc2C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:30.32,wD:52.55,19.20,41.44,(6.67,-17.07,;5.13,-17.07,;4.36,-15.74,;4.37,-18.41,;2.83,-18.41,;2.05,-17.08,;.51,-17.09,;-.25,-18.42,;-1.79,-18.43,;-2.56,-19.76,;-2.57,-17.1,;-1.81,-15.76,;-2.59,-14.43,;-4.14,-14.45,;-4.89,-15.78,;-4.11,-17.1,;-4.87,-18.43,;-6.41,-18.44,;-4.1,-19.76,;-5.43,-20.54,;-6.76,-19.76,;-8.09,-20.54,;-9.42,-19.75,;-10.76,-20.53,;-10.76,-22.07,;-9.41,-22.83,;-8.09,-22.06,;-5.43,-22.08,;-6.76,-22.84,;-4.1,-22.84,;-4.1,-24.38,;-5.43,-25.16,;-5.43,-26.7,;-6.77,-27.46,;-6.77,-28.99,;-5.43,-29.77,;-4.1,-28.99,;-4.1,-27.45,;-2.76,-25.16,;-2.76,-26.7,;-1.42,-24.38,;-.09,-25.16,;-.09,-26.7,;1.24,-27.46,;1.24,-29,;2.58,-29.78,;2.58,-31.32,;3.91,-32.08,;1.24,-32.08,;1.24,-24.38,;2.58,-25.16,;1.24,-22.84,;2.58,-22.08,;3.91,-22.84,;5.24,-22.08,;5.41,-20.53,;6.91,-20.22,;7.67,-21.56,;9.18,-21.89,;9.64,-23.36,;8.6,-24.49,;7.1,-24.16,;6.63,-22.7,;2.06,-19.75,;.52,-19.75,)| Show InChI InChI=1S/C47H53N11O7/c48-40(59)28-58-23-22-51-41(60)33-17-7-8-18-34(33)42(61)55-37(24-29-12-3-1-4-13-29)45(64)56-38(25-30-14-5-2-6-15-30)44(63)54-36(20-11-21-52-47(49)50)43(62)57-39(46(58)65)26-31-27-53-35-19-10-9-16-32(31)35/h1-10,12-19,27,36-39,53H,11,20-26,28H2,(H2,48,59)(H,51,60)(H,54,63)(H,55,61)(H,56,64)(H,57,62)(H4,49,50,52)/t36-,37-,38+,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.05E+4 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC3R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50374671

(CHEMBL272660)Show SMILES NC(=O)CN1CCCNC(=O)CCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:27.28,wD:16.16,49.51,38.40,(28.48,-20.35,;27.16,-19.57,;27.17,-18.03,;25.81,-20.32,;25.8,-21.86,;23.64,-21.92,;23.15,-20.32,;24.48,-19.55,;24.48,-18.01,;23.15,-17.24,;23.15,-15.7,;21.81,-18.01,;21.81,-19.55,;20.48,-20.32,;19.15,-19.55,;20.48,-21.86,;19.15,-22.63,;17.81,-21.86,;16.48,-22.63,;15.15,-21.85,;13.82,-22.62,;13.81,-24.16,;15.16,-24.93,;16.48,-24.16,;19.15,-24.17,;17.81,-24.94,;20.48,-24.94,;20.48,-26.48,;19.15,-27.25,;19.15,-28.79,;17.81,-29.56,;17.8,-31.09,;19.14,-31.87,;20.48,-31.09,;20.47,-29.55,;21.81,-27.25,;21.81,-28.79,;23.15,-26.48,;24.48,-27.25,;24.48,-28.79,;25.81,-29.56,;25.81,-31.1,;27.15,-31.87,;27.15,-33.41,;28.48,-34.18,;25.81,-34.18,;25.81,-26.48,;27.15,-27.25,;25.81,-24.94,;27.15,-24.17,;28.48,-24.94,;29.82,-24.17,;29.98,-22.63,;31.49,-22.31,;32.24,-23.66,;33.75,-23.99,;34.21,-25.46,;33.18,-26.59,;31.67,-26.26,;31.21,-24.8,;27.15,-22.63,;28.48,-21.86,)| Show InChI InChI=1S/C44H55N11O7/c45-37(56)27-55-22-10-21-48-38(57)18-19-39(58)51-34(23-28-11-3-1-4-12-28)41(60)53-35(24-29-13-5-2-6-14-29)42(61)52-33(17-9-20-49-44(46)47)40(59)54-36(43(55)62)25-30-26-50-32-16-8-7-15-31(30)32/h1-8,11-16,26,33-36,50H,9-10,17-25,27H2,(H2,45,56)(H,48,57)(H,51,58)(H,52,61)(H,53,60)(H,54,59)(H4,46,47,49)/t33-,34-,35+,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC3R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50121900

(Ac-Ser-Try-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pr...)Show SMILES CCCC[C@H](NC(=O)C(CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@@H]1C(=O)N[C@H](C(C)C)C(N)=O Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61?,62+,65+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0630 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC3R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50374673

(CHEMBL408398)Show SMILES NC(=O)CN1CCNC(=O)CCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:26.27,wD:15.15,48.50,37.39,(3.62,2.67,;2.29,1.89,;.96,2.65,;2.3,.35,;.97,-.43,;.99,1.1,;-.35,1.87,;-.35,3.41,;-1.68,4.18,;-1.68,5.72,;-3.01,3.41,;-3.01,1.87,;-4.35,1.1,;-5.68,1.87,;-4.35,-.44,;-5.68,-1.21,;-7.01,-.44,;-8.35,-1.21,;-9.68,-.43,;-11.01,-1.19,;-11.01,-2.74,;-9.67,-3.51,;-8.34,-2.73,;-5.68,-2.75,;-7.01,-3.52,;-4.35,-3.52,;-4.35,-5.06,;-5.68,-5.83,;-5.68,-7.37,;-7.02,-8.13,;-7.02,-9.67,;-5.69,-10.44,;-4.35,-9.66,;-4.36,-8.12,;-3.01,-5.83,;-3.01,-7.37,;-1.68,-5.06,;-.35,-5.83,;-.35,-7.37,;.99,-8.14,;.99,-9.68,;2.32,-10.45,;2.32,-11.99,;3.65,-12.76,;.99,-12.76,;.99,-5.06,;2.32,-5.83,;.99,-3.52,;2.32,-2.75,;3.65,-3.52,;4.99,-2.75,;5.16,-1.2,;6.66,-.89,;7.42,-2.23,;8.92,-2.56,;9.39,-4.03,;8.35,-5.17,;6.85,-4.84,;6.38,-3.37,;2.32,-1.21,;3.65,-.44,)| Show InChI InChI=1S/C43H53N11O7/c44-36(55)26-54-21-20-47-37(56)17-18-38(57)50-33(22-27-10-3-1-4-11-27)40(59)52-34(23-28-12-5-2-6-13-28)41(60)51-32(16-9-19-48-43(45)46)39(58)53-35(42(54)61)24-29-25-49-31-15-8-7-14-30(29)31/h1-8,10-15,25,32-35,49H,9,16-24,26H2,(H2,44,55)(H,47,56)(H,50,57)(H,51,60)(H,52,59)(H,53,58)(H4,45,46,48)/t32-,33-,34+,35-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC5R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Mus musculus) | BDBM50374673

(CHEMBL408398)Show SMILES NC(=O)CN1CCNC(=O)CCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:26.27,wD:15.15,48.50,37.39,(3.62,2.67,;2.29,1.89,;.96,2.65,;2.3,.35,;.97,-.43,;.99,1.1,;-.35,1.87,;-.35,3.41,;-1.68,4.18,;-1.68,5.72,;-3.01,3.41,;-3.01,1.87,;-4.35,1.1,;-5.68,1.87,;-4.35,-.44,;-5.68,-1.21,;-7.01,-.44,;-8.35,-1.21,;-9.68,-.43,;-11.01,-1.19,;-11.01,-2.74,;-9.67,-3.51,;-8.34,-2.73,;-5.68,-2.75,;-7.01,-3.52,;-4.35,-3.52,;-4.35,-5.06,;-5.68,-5.83,;-5.68,-7.37,;-7.02,-8.13,;-7.02,-9.67,;-5.69,-10.44,;-4.35,-9.66,;-4.36,-8.12,;-3.01,-5.83,;-3.01,-7.37,;-1.68,-5.06,;-.35,-5.83,;-.35,-7.37,;.99,-8.14,;.99,-9.68,;2.32,-10.45,;2.32,-11.99,;3.65,-12.76,;.99,-12.76,;.99,-5.06,;2.32,-5.83,;.99,-3.52,;2.32,-2.75,;3.65,-3.52,;4.99,-2.75,;5.16,-1.2,;6.66,-.89,;7.42,-2.23,;8.92,-2.56,;9.39,-4.03,;8.35,-5.17,;6.85,-4.84,;6.38,-3.37,;2.32,-1.21,;3.65,-.44,)| Show InChI InChI=1S/C43H53N11O7/c44-36(55)26-54-21-20-47-37(56)17-18-38(57)50-33(22-27-10-3-1-4-11-27)40(59)52-34(23-28-12-5-2-6-13-28)41(60)51-32(16-9-19-48-43(45)46)39(58)53-35(42(54)61)24-29-25-49-31-15-8-7-14-30(29)31/h1-8,10-15,25,32-35,49H,9,16-24,26H2,(H2,44,55)(H,47,56)(H,50,57)(H,51,60)(H,52,59)(H,53,58)(H4,45,46,48)/t32-,33-,34+,35-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC4R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Mus musculus) | BDBM50374673

(CHEMBL408398)Show SMILES NC(=O)CN1CCNC(=O)CCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:26.27,wD:15.15,48.50,37.39,(3.62,2.67,;2.29,1.89,;.96,2.65,;2.3,.35,;.97,-.43,;.99,1.1,;-.35,1.87,;-.35,3.41,;-1.68,4.18,;-1.68,5.72,;-3.01,3.41,;-3.01,1.87,;-4.35,1.1,;-5.68,1.87,;-4.35,-.44,;-5.68,-1.21,;-7.01,-.44,;-8.35,-1.21,;-9.68,-.43,;-11.01,-1.19,;-11.01,-2.74,;-9.67,-3.51,;-8.34,-2.73,;-5.68,-2.75,;-7.01,-3.52,;-4.35,-3.52,;-4.35,-5.06,;-5.68,-5.83,;-5.68,-7.37,;-7.02,-8.13,;-7.02,-9.67,;-5.69,-10.44,;-4.35,-9.66,;-4.36,-8.12,;-3.01,-5.83,;-3.01,-7.37,;-1.68,-5.06,;-.35,-5.83,;-.35,-7.37,;.99,-8.14,;.99,-9.68,;2.32,-10.45,;2.32,-11.99,;3.65,-12.76,;.99,-12.76,;.99,-5.06,;2.32,-5.83,;.99,-3.52,;2.32,-2.75,;3.65,-3.52,;4.99,-2.75,;5.16,-1.2,;6.66,-.89,;7.42,-2.23,;8.92,-2.56,;9.39,-4.03,;8.35,-5.17,;6.85,-4.84,;6.38,-3.37,;2.32,-1.21,;3.65,-.44,)| Show InChI InChI=1S/C43H53N11O7/c44-36(55)26-54-21-20-47-37(56)17-18-38(57)50-33(22-27-10-3-1-4-11-27)40(59)52-34(23-28-12-5-2-6-13-28)41(60)51-32(16-9-19-48-43(45)46)39(58)53-35(42(54)61)24-29-25-49-31-15-8-7-14-30(29)31/h1-8,10-15,25,32-35,49H,9,16-24,26H2,(H2,44,55)(H,47,56)(H,50,57)(H,51,60)(H,52,59)(H,53,58)(H4,45,46,48)/t32-,33-,34+,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 770 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC3R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Mus musculus) | BDBM50374673

(CHEMBL408398)Show SMILES NC(=O)CN1CCNC(=O)CCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C1=O |wU:26.27,wD:15.15,48.50,37.39,(3.62,2.67,;2.29,1.89,;.96,2.65,;2.3,.35,;.97,-.43,;.99,1.1,;-.35,1.87,;-.35,3.41,;-1.68,4.18,;-1.68,5.72,;-3.01,3.41,;-3.01,1.87,;-4.35,1.1,;-5.68,1.87,;-4.35,-.44,;-5.68,-1.21,;-7.01,-.44,;-8.35,-1.21,;-9.68,-.43,;-11.01,-1.19,;-11.01,-2.74,;-9.67,-3.51,;-8.34,-2.73,;-5.68,-2.75,;-7.01,-3.52,;-4.35,-3.52,;-4.35,-5.06,;-5.68,-5.83,;-5.68,-7.37,;-7.02,-8.13,;-7.02,-9.67,;-5.69,-10.44,;-4.35,-9.66,;-4.36,-8.12,;-3.01,-5.83,;-3.01,-7.37,;-1.68,-5.06,;-.35,-5.83,;-.35,-7.37,;.99,-8.14,;.99,-9.68,;2.32,-10.45,;2.32,-11.99,;3.65,-12.76,;.99,-12.76,;.99,-5.06,;2.32,-5.83,;.99,-3.52,;2.32,-2.75,;3.65,-3.52,;4.99,-2.75,;5.16,-1.2,;6.66,-.89,;7.42,-2.23,;8.92,-2.56,;9.39,-4.03,;8.35,-5.17,;6.85,-4.84,;6.38,-3.37,;2.32,-1.21,;3.65,-.44,)| Show InChI InChI=1S/C43H53N11O7/c44-36(55)26-54-21-20-47-37(56)17-18-38(57)50-33(22-27-10-3-1-4-11-27)40(59)52-34(23-28-12-5-2-6-13-28)41(60)51-32(16-9-19-48-43(45)46)39(58)53-35(42(54)61)24-29-25-49-31-15-8-7-14-30(29)31/h1-8,10-15,25,32-35,49H,9,16-24,26H2,(H2,44,55)(H,47,56)(H,50,57)(H,51,60)(H,52,59)(H,53,58)(H4,45,46,48)/t32-,33-,34+,35-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 64 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC1R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data