 Found 103 hits with Last Name = 'shao' and Initial = 'z'
Found 103 hits with Last Name = 'shao' and Initial = 'z' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50572947

(CHEMBL4871014)Show SMILES C[C@H](N1C2CCC1CC(C2)C(O)=O)c1ccc2ccc(O[C@H]3CC[C@H](CC3)C(F)(F)F)c(c2c1)C(F)(F)F |r,wU:21.22,24.29,1.0,(69.55,-51.11,;69.5,-49.57,;70.79,-48.72,;72.16,-49.41,;72.75,-48.34,;72.21,-47.11,;70.69,-47.18,;71.98,-46.34,;73.36,-47.03,;73.44,-48.57,;74.65,-46.19,;74.56,-44.65,;76.02,-46.88,;68.17,-48.8,;68.16,-47.25,;66.82,-46.49,;65.5,-47.27,;64.16,-46.5,;62.83,-47.27,;62.83,-48.82,;61.5,-49.59,;60.16,-48.82,;58.84,-49.58,;57.5,-48.8,;57.51,-47.26,;58.85,-46.5,;60.17,-47.27,;56.18,-46.48,;56.19,-44.94,;54.84,-47.25,;54.83,-45.71,;64.17,-49.59,;65.5,-48.81,;66.84,-49.58,;64.17,-51.13,;62.84,-51.9,;65.51,-51.9,;64.16,-52.66,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ATX assessed as reduction in LPA formation using FS-3 as substrate preincubated for 30 mins followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00211
BindingDB Entry DOI: 10.7270/Q2Q81HVP |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50572945

(CHEMBL4850429)Show SMILES C[C@H](N1C2CCC1CC(C2)C(O)=O)c1ccc2ccc(O[C@@H]3CC[C@H](C)CC3)c(c2c1)C(F)(F)F |r,wU:21.22,24.26,1.0,(16.92,-51.39,;16.87,-49.85,;18.15,-49,;19.53,-49.7,;20.11,-48.63,;19.58,-47.39,;18.06,-47.47,;19.35,-46.63,;20.73,-47.32,;20.81,-48.86,;22.01,-46.48,;21.93,-44.94,;23.39,-47.17,;15.54,-49.09,;15.53,-47.54,;14.19,-46.78,;12.87,-47.55,;11.53,-46.79,;10.2,-47.56,;10.2,-49.1,;8.86,-49.87,;7.53,-49.1,;7.54,-47.55,;6.22,-46.78,;4.88,-47.55,;3.55,-46.77,;4.87,-49.09,;6.21,-49.87,;11.53,-49.88,;12.87,-49.1,;14.2,-49.86,;11.54,-51.42,;10.21,-52.19,;12.87,-52.18,;11.53,-52.95,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ATX assessed as reduction in LPA formation using FS-3 as substrate preincubated for 30 mins followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00211
BindingDB Entry DOI: 10.7270/Q2Q81HVP |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50313806
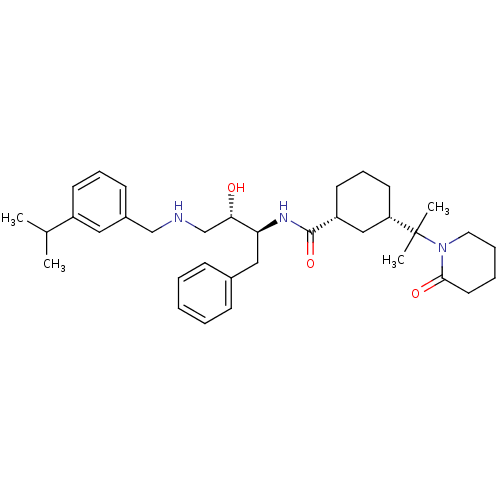
((1R,3S)-N-((2S,3S)-3-hydroxy-4-(3-isopropylbenzyla...)Show SMILES CC(C)c1cccc(CNC[C@H](O)[C@H](Cc2ccccc2)NC(=O)[C@@H]2CCC[C@@H](C2)C(C)(C)N2CCCCC2=O)c1 |r| Show InChI InChI=1S/C35H51N3O3/c1-25(2)28-15-10-14-27(20-28)23-36-24-32(39)31(21-26-12-6-5-7-13-26)37-34(41)29-16-11-17-30(22-29)35(3,4)38-19-9-8-18-33(38)40/h5-7,10,12-15,20,25,29-32,36,39H,8-9,11,16-19,21-24H2,1-4H3,(H,37,41)/t29-,30+,31+,32+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50572943

(CHEMBL4872157)Show SMILES C[C@H](N1C2CCCC1CC(C2)C(O)=O)c1ccc2ccc(O[C@@H]3CC[C@H](C)CC3)c(c2c1)C(F)(F)F |r,wU:22.23,25.27,1.0,(40.97,-38.74,;40.93,-37.21,;42.21,-36.36,;43.58,-37.05,;44.28,-35.68,;42.73,-35.77,;43.64,-34.75,;42.11,-34.82,;43.4,-33.98,;44.78,-34.67,;44.87,-36.21,;46.07,-33.83,;45.98,-32.29,;47.44,-34.52,;39.59,-36.44,;39.58,-34.89,;38.25,-34.13,;36.92,-34.91,;35.59,-34.14,;34.26,-34.91,;34.26,-36.46,;32.92,-37.23,;31.59,-36.46,;31.6,-34.91,;30.27,-34.14,;28.93,-34.9,;27.6,-34.12,;28.93,-36.44,;30.26,-37.22,;35.59,-37.23,;36.92,-36.45,;38.26,-37.22,;35.59,-38.77,;34.26,-39.54,;36.93,-39.54,;35.58,-40.3,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ATX assessed as reduction in LPA formation using FS-3 as substrate preincubated for 30 mins followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00211
BindingDB Entry DOI: 10.7270/Q2Q81HVP |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50572941
(CHEMBL4871067)Show SMILES C[C@H]1CC[C@H](CC1)Oc1ccc2ccc(CN3C4CCCC3CC(C4)C(O)=O)cc2c1C(F)(F)F |r,wU:4.7,1.0,(54.02,-21,;55.35,-21.78,;56.69,-21.02,;58.02,-21.79,;58.01,-23.34,;56.68,-24.1,;55.35,-23.32,;59.34,-24.11,;60.68,-23.34,;60.68,-21.79,;62.01,-21.02,;63.34,-21.79,;64.67,-21.01,;66.01,-21.77,;66.02,-23.32,;67.35,-24.09,;68.63,-23.24,;70.01,-23.93,;70.7,-22.56,;69.16,-22.65,;70.06,-21.63,;68.53,-21.7,;69.83,-20.86,;71.2,-21.55,;71.29,-23.09,;72.49,-20.71,;72.41,-19.17,;73.87,-21.4,;64.68,-24.1,;63.35,-23.33,;62.01,-24.11,;62.02,-25.65,;60.68,-26.42,;63.35,-26.42,;62,-27.18,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ATX assessed as reduction in LPA formation using FS-3 as substrate preincubated for 30 mins followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00211
BindingDB Entry DOI: 10.7270/Q2Q81HVP |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50313798

((2R,4S,5S)-5-((S)-2-((S)-2-acetamido-4-methylpenta...)Show SMILES CCCCNC(=O)[C@H](C)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(C)C)NC(C)=O |r| Show InChI InChI=1S/C27H52N4O5S/c1-9-10-12-28-25(34)19(6)16-24(33)22(14-17(2)3)31-26(35)21(11-13-37-8)30-27(36)23(15-18(4)5)29-20(7)32/h17-19,21-24,33H,9-16H2,1-8H3,(H,28,34)(H,29,32)(H,30,36)(H,31,35)/t19-,21+,22+,23+,24+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of Cathepsin D |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50313805
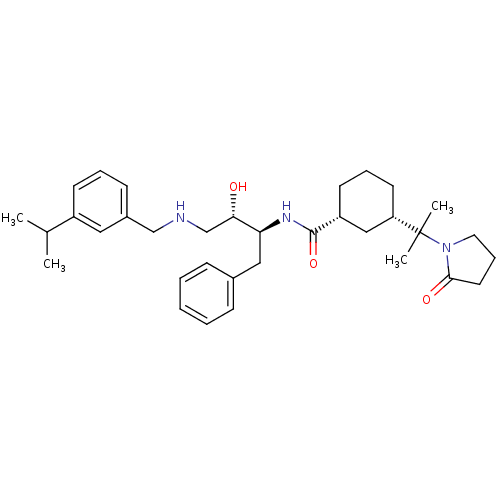
((1R,3S)-N-((2S,3S)-3-hydroxy-4-(3-isopropylbenzyla...)Show SMILES CC(C)c1cccc(CNC[C@H](O)[C@H](Cc2ccccc2)NC(=O)[C@@H]2CCC[C@@H](C2)C(C)(C)N2CCCC2=O)c1 |r| Show InChI InChI=1S/C34H49N3O3/c1-24(2)27-14-8-13-26(19-27)22-35-23-31(38)30(20-25-11-6-5-7-12-25)36-33(40)28-15-9-16-29(21-28)34(3,4)37-18-10-17-32(37)39/h5-8,11-14,19,24,28-31,35,38H,9-10,15-18,20-23H2,1-4H3,(H,36,40)/t28-,29+,30+,31+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50572936
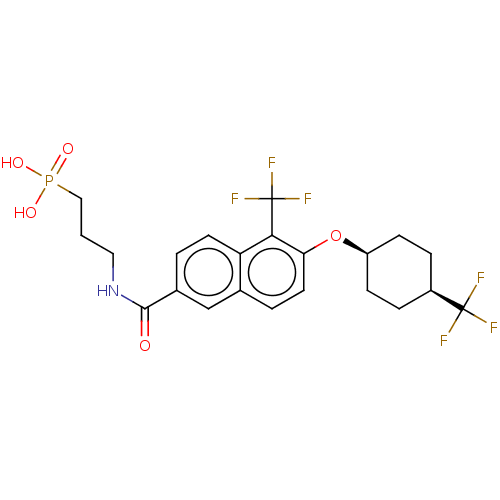
(CHEMBL4858196)Show SMILES OP(O)(=O)CCCNC(=O)c1ccc2c(c(O[C@H]3CC[C@H](CC3)C(F)(F)F)ccc2c1)C(F)(F)F |r,wU:17.16,20.23,(25.17,-6.03,;25.18,-7.57,;26.51,-6.79,;25.17,-9.11,;23.84,-6.81,;22.51,-7.59,;21.17,-6.82,;19.84,-7.6,;18.51,-6.84,;18.5,-5.3,;17.18,-7.62,;17.19,-9.17,;15.85,-9.94,;14.52,-9.17,;13.18,-9.95,;11.85,-9.18,;10.51,-9.95,;9.18,-9.18,;7.85,-9.94,;6.52,-9.17,;6.53,-7.62,;7.86,-6.86,;9.19,-7.63,;5.2,-6.85,;5.2,-5.31,;3.86,-7.61,;3.85,-6.07,;11.85,-7.64,;13.18,-6.87,;14.51,-7.63,;15.84,-6.86,;13.19,-11.49,;11.85,-12.27,;14.52,-12.26,;13.18,-13.03,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ATX assessed as reduction in LPA formation using FS-3 as substrate preincubated for 30 mins followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00211
BindingDB Entry DOI: 10.7270/Q2Q81HVP |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50313796
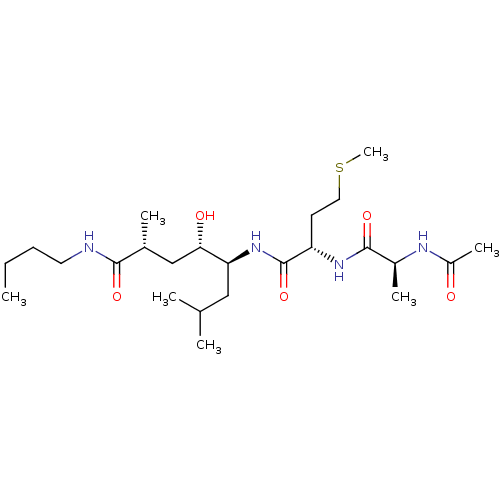
((2R,4S,5S)-5-((S)-2-((S)-2-acetamidopropanamido)-4...)Show SMILES CCCCNC(=O)[C@H](C)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CCSC)NC(=O)[C@H](C)NC(C)=O |r| Show InChI InChI=1S/C24H46N4O5S/c1-8-9-11-25-22(31)16(4)14-21(30)20(13-15(2)3)28-24(33)19(10-12-34-7)27-23(32)17(5)26-18(6)29/h15-17,19-21,30H,8-14H2,1-7H3,(H,25,31)(H,26,29)(H,27,32)(H,28,33)/t16-,17+,19+,20+,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of Cathepsin D |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50572940
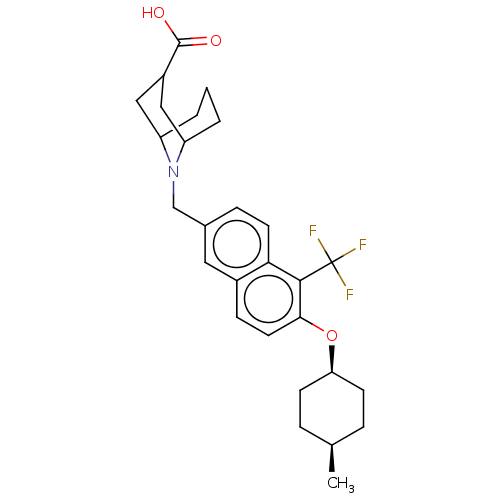
(CHEMBL4868783)Show SMILES C[C@H]1CC[C@H](CC1)Oc1ccc2cc(CN3C4CCCC3CC(C4)C(O)=O)ccc2c1C(F)(F)F |r,wU:4.7,1.0,(28.35,-20.89,;29.68,-21.67,;31.02,-20.91,;32.34,-21.68,;32.34,-23.23,;31.01,-23.99,;29.67,-23.21,;33.67,-24,;35,-23.23,;35,-21.68,;36.33,-20.91,;37.67,-21.68,;38.99,-20.9,;40.33,-21.66,;41.66,-20.89,;43,-21.65,;43,-23.19,;44.54,-23.19,;43.76,-21.85,;45.08,-22.19,;44.32,-20.87,;45.66,-21.64,;45.67,-23.18,;44.33,-23.95,;47,-23.94,;48.33,-23.17,;47,-25.48,;40.34,-23.21,;39.01,-23.99,;37.67,-23.22,;36.34,-24,;36.34,-25.54,;35.01,-26.31,;37.68,-26.31,;36.33,-27.07,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ATX assessed as reduction in LPA formation using FS-3 as substrate preincubated for 30 mins followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00211
BindingDB Entry DOI: 10.7270/Q2Q81HVP |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Rattus norvegicus) | BDBM50572947

(CHEMBL4871014)Show SMILES C[C@H](N1C2CCC1CC(C2)C(O)=O)c1ccc2ccc(O[C@H]3CC[C@H](CC3)C(F)(F)F)c(c2c1)C(F)(F)F |r,wU:21.22,24.29,1.0,(69.55,-51.11,;69.5,-49.57,;70.79,-48.72,;72.16,-49.41,;72.75,-48.34,;72.21,-47.11,;70.69,-47.18,;71.98,-46.34,;73.36,-47.03,;73.44,-48.57,;74.65,-46.19,;74.56,-44.65,;76.02,-46.88,;68.17,-48.8,;68.16,-47.25,;66.82,-46.49,;65.5,-47.27,;64.16,-46.5,;62.83,-47.27,;62.83,-48.82,;61.5,-49.59,;60.16,-48.82,;58.84,-49.58,;57.5,-48.8,;57.51,-47.26,;58.85,-46.5,;60.17,-47.27,;56.18,-46.48,;56.19,-44.94,;54.84,-47.25,;54.83,-45.71,;64.17,-49.59,;65.5,-48.81,;66.84,-49.58,;64.17,-51.13,;62.84,-51.9,;65.51,-51.9,;64.16,-52.66,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LPA in rat plasma after 18 hrs by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00211
BindingDB Entry DOI: 10.7270/Q2Q81HVP |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50313797
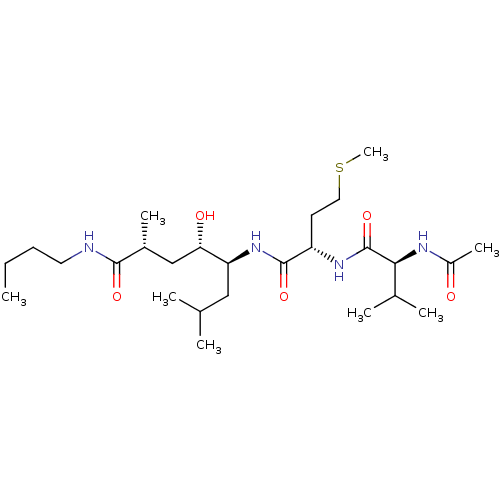
((2R,4S,5S)-5-((S)-2-((S)-2-acetamido-3-methylbutan...)Show SMILES CCCCNC(=O)[C@H](C)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CCSC)NC(=O)[C@@H](NC(C)=O)C(C)C |r| Show InChI InChI=1S/C26H50N4O5S/c1-9-10-12-27-24(33)18(6)15-22(32)21(14-16(2)3)30-25(34)20(11-13-36-8)29-26(35)23(17(4)5)28-19(7)31/h16-18,20-23,32H,9-15H2,1-8H3,(H,27,33)(H,28,31)(H,29,35)(H,30,34)/t18-,20+,21+,22+,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of Cathepsin D |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50313798

((2R,4S,5S)-5-((S)-2-((S)-2-acetamido-4-methylpenta...)Show SMILES CCCCNC(=O)[C@H](C)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(C)C)NC(C)=O |r| Show InChI InChI=1S/C27H52N4O5S/c1-9-10-12-28-25(34)19(6)16-24(33)22(14-17(2)3)31-26(35)21(11-13-37-8)30-27(36)23(15-18(4)5)29-20(7)32/h17-19,21-24,33H,9-16H2,1-8H3,(H,28,34)(H,29,32)(H,30,36)(H,31,35)/t19-,21+,22+,23+,24+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM50572947

(CHEMBL4871014)Show SMILES C[C@H](N1C2CCC1CC(C2)C(O)=O)c1ccc2ccc(O[C@H]3CC[C@H](CC3)C(F)(F)F)c(c2c1)C(F)(F)F |r,wU:21.22,24.29,1.0,(69.55,-51.11,;69.5,-49.57,;70.79,-48.72,;72.16,-49.41,;72.75,-48.34,;72.21,-47.11,;70.69,-47.18,;71.98,-46.34,;73.36,-47.03,;73.44,-48.57,;74.65,-46.19,;74.56,-44.65,;76.02,-46.88,;68.17,-48.8,;68.16,-47.25,;66.82,-46.49,;65.5,-47.27,;64.16,-46.5,;62.83,-47.27,;62.83,-48.82,;61.5,-49.59,;60.16,-48.82,;58.84,-49.58,;57.5,-48.8,;57.51,-47.26,;58.85,-46.5,;60.17,-47.27,;56.18,-46.48,;56.19,-44.94,;54.84,-47.25,;54.83,-45.71,;64.17,-49.59,;65.5,-48.81,;66.84,-49.58,;64.17,-51.13,;62.84,-51.9,;65.51,-51.9,;64.16,-52.66,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of LPA in human plasma after 18 hrs by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00211
BindingDB Entry DOI: 10.7270/Q2Q81HVP |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50313806

((1R,3S)-N-((2S,3S)-3-hydroxy-4-(3-isopropylbenzyla...)Show SMILES CC(C)c1cccc(CNC[C@H](O)[C@H](Cc2ccccc2)NC(=O)[C@@H]2CCC[C@@H](C2)C(C)(C)N2CCCCC2=O)c1 |r| Show InChI InChI=1S/C35H51N3O3/c1-25(2)28-15-10-14-27(20-28)23-36-24-32(39)31(21-26-12-6-5-7-13-26)37-34(41)29-16-11-17-30(22-29)35(3,4)38-19-9-8-18-33(38)40/h5-7,10,12-15,20,25,29-32,36,39H,8-9,11,16-19,21-24H2,1-4H3,(H,37,41)/t29-,30+,31+,32+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 expressed in CHO cells co-transfected with human APP assessed as inhibition of cellular release of Abeta 40 after 24 hrs |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50572942
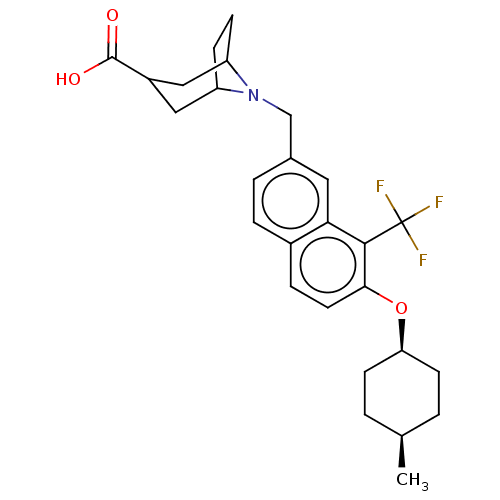
(CHEMBL4859055)Show SMILES C[C@H]1CC[C@H](CC1)Oc1ccc2ccc(CN3C4CCC3CC(C4)C(O)=O)cc2c1C(F)(F)F |r,wU:4.7,1.0,(2.38,-33.53,;3.71,-34.31,;5.05,-33.55,;6.37,-34.32,;6.37,-35.87,;5.04,-36.63,;3.71,-35.85,;7.7,-36.64,;9.03,-35.87,;9.03,-34.32,;10.36,-33.55,;11.7,-34.32,;13.03,-33.54,;14.36,-34.3,;14.37,-35.85,;15.7,-36.62,;16.99,-35.77,;18.36,-36.46,;18.95,-35.39,;18.41,-34.16,;16.89,-34.23,;18.18,-33.39,;19.56,-34.08,;19.64,-35.62,;20.85,-33.24,;20.76,-31.7,;22.22,-33.93,;13.04,-36.63,;11.7,-35.86,;10.37,-36.64,;10.37,-38.18,;9.04,-38.95,;11.71,-38.94,;10.36,-39.71,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ATX assessed as reduction in LPA formation using FS-3 as substrate preincubated for 30 mins followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00211
BindingDB Entry DOI: 10.7270/Q2Q81HVP |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50572948
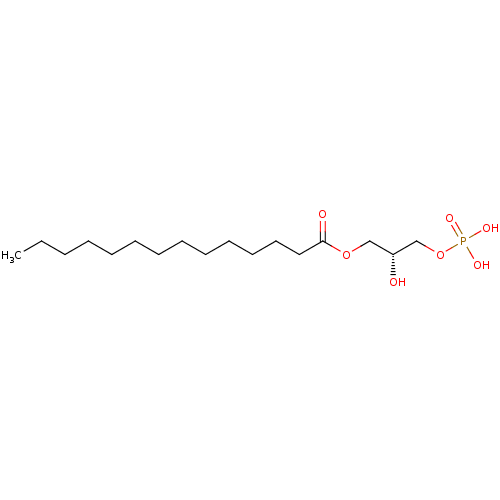
(CHEBI:62833 | CHEMBL1615121)Show SMILES CCCCCCCCCCCCCC(=O)OC[C@@H](O)COP(O)(O)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ATX assessed as reduction in LPA formation using FS-3 as substrate preincubated for 30 mins followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00211
BindingDB Entry DOI: 10.7270/Q2Q81HVP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM16077

((2R,4S,5S)-N-butyl-5-[(2S)-2-[(2S)-2-acetamido-4-m...)Show SMILES CCCCNC(=O)[C@H](C)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(C)=O |r| Show InChI InChI=1S/C25H48N4O5/c1-9-10-11-26-23(32)17(6)14-22(31)20(12-15(2)3)29-24(33)18(7)27-25(34)21(13-16(4)5)28-19(8)30/h15-18,20-22,31H,9-14H2,1-8H3,(H,26,32)(H,27,34)(H,28,30)(H,29,33)/t17-,18+,20+,21+,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50313806

((1R,3S)-N-((2S,3S)-3-hydroxy-4-(3-isopropylbenzyla...)Show SMILES CC(C)c1cccc(CNC[C@H](O)[C@H](Cc2ccccc2)NC(=O)[C@@H]2CCC[C@@H](C2)C(C)(C)N2CCCCC2=O)c1 |r| Show InChI InChI=1S/C35H51N3O3/c1-25(2)28-15-10-14-27(20-28)23-36-24-32(39)31(21-26-12-6-5-7-13-26)37-34(41)29-16-11-17-30(22-29)35(3,4)38-19-9-8-18-33(38)40/h5-7,10,12-15,20,25,29-32,36,39H,8-9,11,16-19,21-24H2,1-4H3,(H,37,41)/t29-,30+,31+,32+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of Cathepsin D |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50313805

((1R,3S)-N-((2S,3S)-3-hydroxy-4-(3-isopropylbenzyla...)Show SMILES CC(C)c1cccc(CNC[C@H](O)[C@H](Cc2ccccc2)NC(=O)[C@@H]2CCC[C@@H](C2)C(C)(C)N2CCCC2=O)c1 |r| Show InChI InChI=1S/C34H49N3O3/c1-24(2)27-14-8-13-26(19-27)22-35-23-31(38)30(20-25-11-6-5-7-12-25)36-33(40)28-15-9-16-29(21-28)34(3,4)37-18-10-17-32(37)39/h5-8,11-14,19,24,28-31,35,38H,9-10,15-18,20-23H2,1-4H3,(H,36,40)/t28-,29+,30+,31+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 expressed in CHO cells co-transfected with human APP assessed as inhibition of cellular release of Abeta 40 after 24 hrs |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50313806

((1R,3S)-N-((2S,3S)-3-hydroxy-4-(3-isopropylbenzyla...)Show SMILES CC(C)c1cccc(CNC[C@H](O)[C@H](Cc2ccccc2)NC(=O)[C@@H]2CCC[C@@H](C2)C(C)(C)N2CCCCC2=O)c1 |r| Show InChI InChI=1S/C35H51N3O3/c1-25(2)28-15-10-14-27(20-28)23-36-24-32(39)31(21-26-12-6-5-7-13-26)37-34(41)29-16-11-17-30(22-29)35(3,4)38-19-9-8-18-33(38)40/h5-7,10,12-15,20,25,29-32,36,39H,8-9,11,16-19,21-24H2,1-4H3,(H,37,41)/t29-,30+,31+,32+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50313804
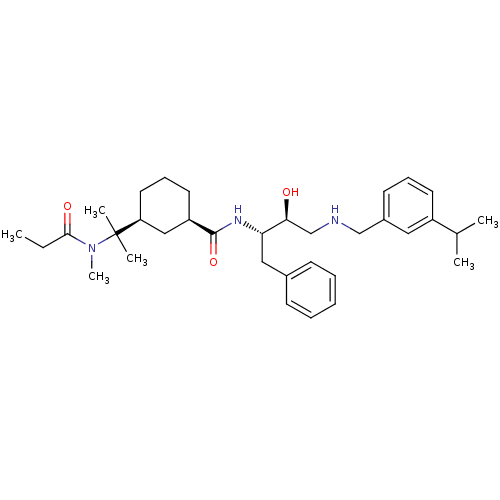
((1R,3S)-N-((2S,3S)-3-hydroxy-4-(3-isopropylbenzyla...)Show SMILES CCC(=O)N(C)C(C)(C)[C@H]1CCC[C@H](C1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)CNCc1cccc(c1)C(C)C |r| Show InChI InChI=1S/C34H51N3O3/c1-7-32(39)37(6)34(4,5)29-18-12-17-28(21-29)33(40)36-30(20-25-13-9-8-10-14-25)31(38)23-35-22-26-15-11-16-27(19-26)24(2)3/h8-11,13-16,19,24,28-31,35,38H,7,12,17-18,20-23H2,1-6H3,(H,36,40)/t28-,29+,30+,31+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50313799
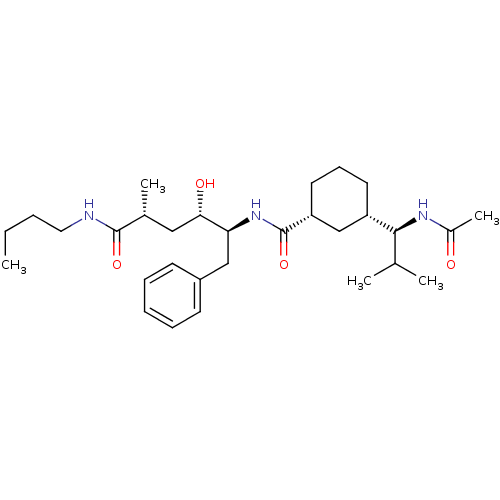
((1R,3S)-3-((S)-1-acetamido-2-methylpropyl)-N-((2S,...)Show SMILES CCCCNC(=O)[C@H](C)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCC[C@@H](C1)[C@@H](NC(C)=O)C(C)C |r| Show InChI InChI=1S/C30H49N3O4/c1-6-7-16-31-29(36)21(4)17-27(35)26(18-23-12-9-8-10-13-23)33-30(37)25-15-11-14-24(19-25)28(20(2)3)32-22(5)34/h8-10,12-13,20-21,24-28,35H,6-7,11,14-19H2,1-5H3,(H,31,36)(H,32,34)(H,33,37)/t21-,24+,25-,26+,27+,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of Cathepsin D |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50313801
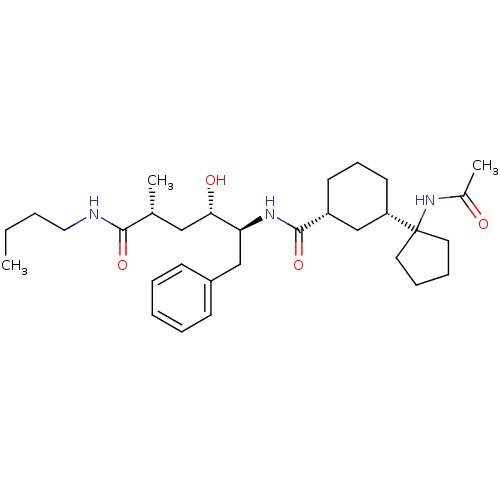
((1R,3S)-3-(1-acetamidocyclopentyl)-N-((2S,3S,5R)-6...)Show SMILES CCCCNC(=O)[C@H](C)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCC[C@@H](C1)C1(CCCC1)NC(C)=O |r| Show InChI InChI=1S/C31H49N3O4/c1-4-5-18-32-29(37)22(2)19-28(36)27(20-24-12-7-6-8-13-24)33-30(38)25-14-11-15-26(21-25)31(34-23(3)35)16-9-10-17-31/h6-8,12-13,22,25-28,36H,4-5,9-11,14-21H2,1-3H3,(H,32,37)(H,33,38)(H,34,35)/t22-,25-,26+,27+,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of Cathepsin D |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50313799

((1R,3S)-3-((S)-1-acetamido-2-methylpropyl)-N-((2S,...)Show SMILES CCCCNC(=O)[C@H](C)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCC[C@@H](C1)[C@@H](NC(C)=O)C(C)C |r| Show InChI InChI=1S/C30H49N3O4/c1-6-7-16-31-29(36)21(4)17-27(35)26(18-23-12-9-8-10-13-23)33-30(37)25-15-11-14-24(19-25)28(20(2)3)32-22(5)34/h8-10,12-13,20-21,24-28,35H,6-7,11,14-19H2,1-5H3,(H,31,36)(H,32,34)(H,33,37)/t21-,24+,25-,26+,27+,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50313804

((1R,3S)-N-((2S,3S)-3-hydroxy-4-(3-isopropylbenzyla...)Show SMILES CCC(=O)N(C)C(C)(C)[C@H]1CCC[C@H](C1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)CNCc1cccc(c1)C(C)C |r| Show InChI InChI=1S/C34H51N3O3/c1-7-32(39)37(6)34(4,5)29-18-12-17-28(21-29)33(40)36-30(20-25-13-9-8-10-14-25)31(38)23-35-22-26-15-11-16-27(19-26)24(2)3/h8-11,13-16,19,24,28-31,35,38H,7,12,17-18,20-23H2,1-6H3,(H,36,40)/t28-,29+,30+,31+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50572946
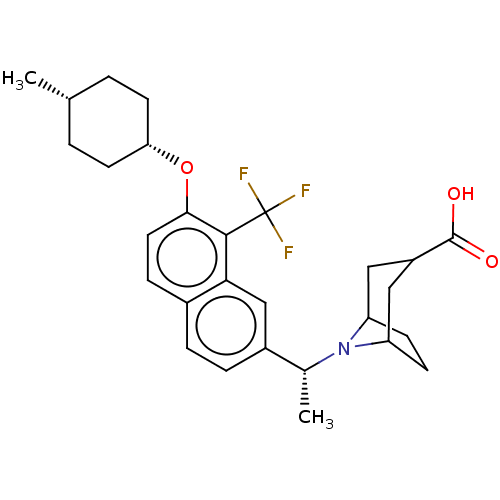
(CHEMBL4847281)Show SMILES C[C@@H](N1C2CCC1CC(C2)C(O)=O)c1ccc2ccc(O[C@@H]3CC[C@H](C)CC3)c(c2c1)C(F)(F)F |r,wU:21.22,24.26,wD:1.0,(42.97,-50.58,;42.92,-49.04,;44.21,-48.19,;45.58,-48.88,;46.17,-47.81,;45.64,-46.58,;44.11,-46.66,;45.4,-45.81,;46.78,-46.5,;46.87,-48.04,;48.07,-45.66,;47.98,-44.12,;49.44,-46.35,;41.59,-48.27,;41.58,-46.72,;40.25,-45.96,;38.92,-46.74,;37.59,-45.97,;36.26,-46.74,;36.25,-48.29,;34.92,-49.06,;33.59,-48.29,;33.6,-46.74,;32.27,-45.97,;30.93,-46.73,;29.6,-45.95,;30.93,-48.27,;32.26,-49.05,;37.59,-49.06,;38.92,-48.28,;40.26,-49.05,;37.59,-50.6,;36.26,-51.37,;38.93,-51.37,;37.58,-52.13,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ATX assessed as reduction in LPA formation using FS-3 as substrate preincubated for 30 mins followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00211
BindingDB Entry DOI: 10.7270/Q2Q81HVP |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50313800
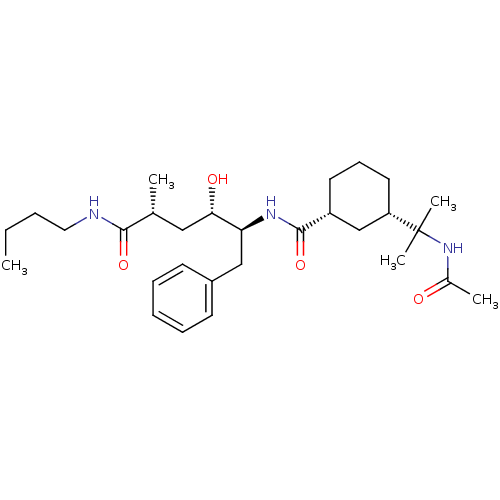
((1R,3S)-3-(2-acetamidopropan-2-yl)-N-((2S,3S,5R)-6...)Show SMILES CCCCNC(=O)[C@H](C)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCC[C@@H](C1)C(C)(C)NC(C)=O |r| Show InChI InChI=1S/C29H47N3O4/c1-6-7-16-30-27(35)20(2)17-26(34)25(18-22-12-9-8-10-13-22)31-28(36)23-14-11-15-24(19-23)29(4,5)32-21(3)33/h8-10,12-13,20,23-26,34H,6-7,11,14-19H2,1-5H3,(H,30,35)(H,31,36)(H,32,33)/t20-,23-,24+,25+,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of Cathepsin D |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM16077

((2R,4S,5S)-N-butyl-5-[(2S)-2-[(2S)-2-acetamido-4-m...)Show SMILES CCCCNC(=O)[C@H](C)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(C)=O |r| Show InChI InChI=1S/C25H48N4O5/c1-9-10-11-26-23(32)17(6)14-22(31)20(12-15(2)3)29-24(33)18(7)27-25(34)21(13-16(4)5)28-19(8)30/h15-18,20-22,31H,9-14H2,1-8H3,(H,26,32)(H,27,34)(H,28,30)(H,29,33)/t17-,18+,20+,21+,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of Cathepsin D |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50572944
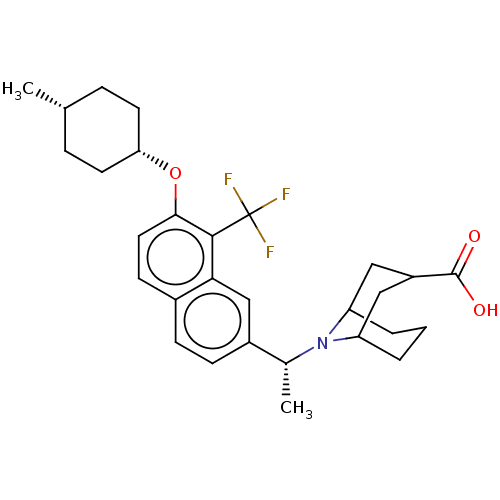
(CHEMBL4858465)Show SMILES C[C@@H](N1C2CCCC1CC(C2)C(O)=O)c1ccc2ccc(O[C@@H]3CC[C@H](C)CC3)c(c2c1)C(F)(F)F |r,wU:22.23,25.27,wD:1.0,(69.31,-38.22,;69.26,-36.68,;70.54,-35.84,;71.92,-36.53,;72.61,-35.16,;71.07,-35.25,;71.97,-34.23,;70.45,-34.3,;71.74,-33.46,;73.12,-34.15,;73.2,-35.69,;74.4,-33.31,;74.32,-31.77,;75.78,-34,;67.93,-35.92,;67.92,-34.37,;66.58,-33.61,;65.26,-34.39,;63.92,-33.62,;62.59,-34.39,;62.59,-35.94,;61.26,-36.71,;59.92,-35.93,;59.93,-34.39,;58.61,-33.62,;57.27,-34.38,;55.94,-33.6,;57.26,-35.92,;58.6,-36.7,;63.92,-36.71,;65.26,-35.93,;66.59,-36.7,;63.93,-38.25,;62.6,-39.02,;65.26,-39.01,;63.92,-39.78,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ATX assessed as reduction in LPA formation using FS-3 as substrate preincubated for 30 mins followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00211
BindingDB Entry DOI: 10.7270/Q2Q81HVP |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50313797

((2R,4S,5S)-5-((S)-2-((S)-2-acetamido-3-methylbutan...)Show SMILES CCCCNC(=O)[C@H](C)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CCSC)NC(=O)[C@@H](NC(C)=O)C(C)C |r| Show InChI InChI=1S/C26H50N4O5S/c1-9-10-12-27-24(33)18(6)15-22(32)21(14-16(2)3)30-25(34)20(11-13-36-8)29-26(35)23(17(4)5)28-19(7)31/h16-18,20-23,32H,9-15H2,1-8H3,(H,27,33)(H,28,31)(H,29,35)(H,30,34)/t18-,20+,21+,22+,23+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50313800

((1R,3S)-3-(2-acetamidopropan-2-yl)-N-((2S,3S,5R)-6...)Show SMILES CCCCNC(=O)[C@H](C)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCC[C@@H](C1)C(C)(C)NC(C)=O |r| Show InChI InChI=1S/C29H47N3O4/c1-6-7-16-30-27(35)20(2)17-26(34)25(18-22-12-9-8-10-13-22)31-28(36)23-14-11-15-24(19-23)29(4,5)32-21(3)33/h8-10,12-13,20,23-26,34H,6-7,11,14-19H2,1-5H3,(H,30,35)(H,31,36)(H,32,33)/t20-,23-,24+,25+,26+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50572939
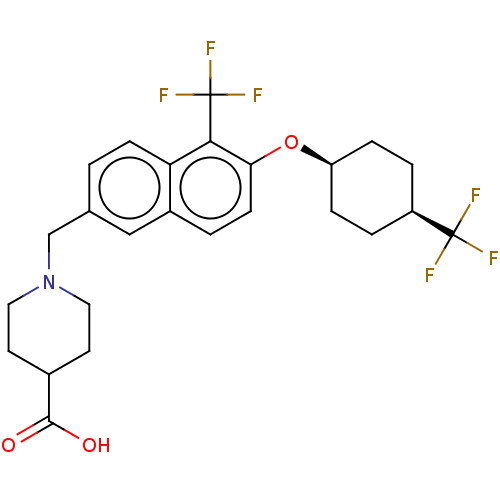
(CHEMBL4846048)Show SMILES OC(=O)C1CCN(Cc2ccc3c(c(O[C@H]4CC[C@H](CC4)C(F)(F)F)ccc3c2)C(F)(F)F)CC1 |r,wU:15.14,18.21,(24.93,-23.29,;23.6,-24.06,;23.61,-25.6,;22.27,-23.29,;20.93,-24.06,;19.6,-23.3,;19.6,-21.76,;18.26,-21,;16.93,-21.78,;16.94,-23.33,;15.61,-24.1,;14.27,-23.34,;12.94,-24.11,;11.61,-23.34,;10.27,-24.11,;8.94,-23.34,;7.61,-24.11,;6.28,-23.33,;6.28,-21.78,;7.62,-21.02,;8.95,-21.79,;4.95,-21.01,;4.96,-19.47,;3.62,-21.77,;3.61,-20.24,;11.61,-21.8,;12.94,-21.03,;14.27,-21.79,;15.6,-21.02,;12.94,-25.65,;11.61,-26.43,;14.28,-26.42,;12.93,-27.19,;20.92,-20.98,;22.26,-21.75,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ATX assessed as reduction in LPA formation using FS-3 as substrate preincubated for 30 mins followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00211
BindingDB Entry DOI: 10.7270/Q2Q81HVP |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50313804

((1R,3S)-N-((2S,3S)-3-hydroxy-4-(3-isopropylbenzyla...)Show SMILES CCC(=O)N(C)C(C)(C)[C@H]1CCC[C@H](C1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)CNCc1cccc(c1)C(C)C |r| Show InChI InChI=1S/C34H51N3O3/c1-7-32(39)37(6)34(4,5)29-18-12-17-28(21-29)33(40)36-30(20-25-13-9-8-10-14-25)31(38)23-35-22-26-15-11-16-27(19-26)24(2)3/h8-11,13-16,19,24,28-31,35,38H,7,12,17-18,20-23H2,1-6H3,(H,36,40)/t28-,29+,30+,31+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of Cathepsin D |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50313805

((1R,3S)-N-((2S,3S)-3-hydroxy-4-(3-isopropylbenzyla...)Show SMILES CC(C)c1cccc(CNC[C@H](O)[C@H](Cc2ccccc2)NC(=O)[C@@H]2CCC[C@@H](C2)C(C)(C)N2CCCC2=O)c1 |r| Show InChI InChI=1S/C34H49N3O3/c1-24(2)27-14-8-13-26(19-27)22-35-23-31(38)30(20-25-11-6-5-7-12-25)36-33(40)28-15-9-16-29(21-28)34(3,4)37-18-10-17-32(37)39/h5-8,11-14,19,24,28-31,35,38H,9-10,15-18,20-23H2,1-4H3,(H,36,40)/t28-,29+,30+,31+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of Cathepsin D |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50313803
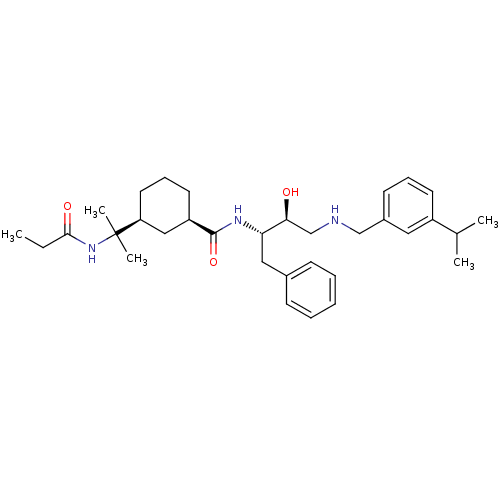
((1R,3S)-N-((2S,3S)-3-hydroxy-4-(3-isopropylbenzyla...)Show SMILES CCC(=O)NC(C)(C)[C@H]1CCC[C@H](C1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)CNCc1cccc(c1)C(C)C |r| Show InChI InChI=1S/C33H49N3O3/c1-6-31(38)36-33(4,5)28-17-11-16-27(20-28)32(39)35-29(19-24-12-8-7-9-13-24)30(37)22-34-21-25-14-10-15-26(18-25)23(2)3/h7-10,12-15,18,23,27-30,34,37H,6,11,16-17,19-22H2,1-5H3,(H,35,39)(H,36,38)/t27-,28+,29+,30+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50313804

((1R,3S)-N-((2S,3S)-3-hydroxy-4-(3-isopropylbenzyla...)Show SMILES CCC(=O)N(C)C(C)(C)[C@H]1CCC[C@H](C1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)CNCc1cccc(c1)C(C)C |r| Show InChI InChI=1S/C34H51N3O3/c1-7-32(39)37(6)34(4,5)29-18-12-17-28(21-29)33(40)36-30(20-25-13-9-8-10-14-25)31(38)23-35-22-26-15-11-16-27(19-26)24(2)3/h8-11,13-16,19,24,28-31,35,38H,7,12,17-18,20-23H2,1-6H3,(H,36,40)/t28-,29+,30+,31+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 expressed in CHO cells co-transfected with human APP assessed as inhibition of cellular release of Abeta 40 after 24 hrs |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50313799

((1R,3S)-3-((S)-1-acetamido-2-methylpropyl)-N-((2S,...)Show SMILES CCCCNC(=O)[C@H](C)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCC[C@@H](C1)[C@@H](NC(C)=O)C(C)C |r| Show InChI InChI=1S/C30H49N3O4/c1-6-7-16-31-29(36)21(4)17-27(35)26(18-23-12-9-8-10-13-23)33-30(37)25-15-11-14-24(19-25)28(20(2)3)32-22(5)34/h8-10,12-13,20-21,24-28,35H,6-7,11,14-19H2,1-5H3,(H,31,36)(H,32,34)(H,33,37)/t21-,24+,25-,26+,27+,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50313805

((1R,3S)-N-((2S,3S)-3-hydroxy-4-(3-isopropylbenzyla...)Show SMILES CC(C)c1cccc(CNC[C@H](O)[C@H](Cc2ccccc2)NC(=O)[C@@H]2CCC[C@@H](C2)C(C)(C)N2CCCC2=O)c1 |r| Show InChI InChI=1S/C34H49N3O3/c1-24(2)27-14-8-13-26(19-27)22-35-23-31(38)30(20-25-11-6-5-7-12-25)36-33(40)28-15-9-16-29(21-28)34(3,4)37-18-10-17-32(37)39/h5-8,11-14,19,24,28-31,35,38H,9-10,15-18,20-23H2,1-4H3,(H,36,40)/t28-,29+,30+,31+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50366826
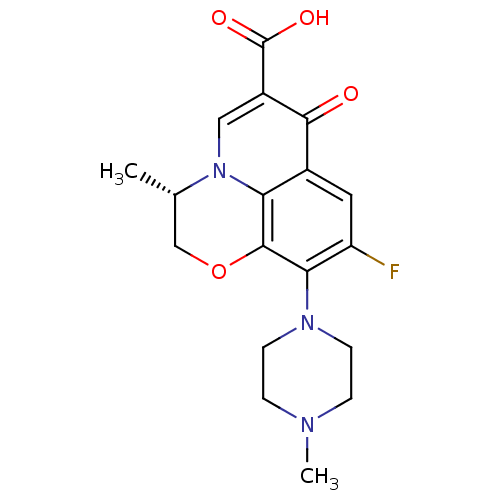
(DR-3355 | Floxacin | Iquix | LEVOFLOXACIN | Levaqu...)Show SMILES C[C@H]1COc2c(N3CCN(C)CC3)c(F)cc3c2n1cc(C(O)=O)c3=O |r| Show InChI InChI=1S/C18H20FN3O4/c1-10-9-26-17-14-11(16(23)12(18(24)25)8-22(10)14)7-13(19)15(17)21-5-3-20(2)4-6-21/h7-8,10H,3-6,9H2,1-2H3,(H,24,25)/t10-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Antibacterial activity against vancomycin-sensitive Enterococcus faecalis 12-5 after 18 hrs by broth microdilution method |
J Nat Prod 77: 1021-30 (2014)
Article DOI: 10.1021/np5000457
BindingDB Entry DOI: 10.7270/Q2280BKR |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50366826

(DR-3355 | Floxacin | Iquix | LEVOFLOXACIN | Levaqu...)Show SMILES C[C@H]1COc2c(N3CCN(C)CC3)c(F)cc3c2n1cc(C(O)=O)c3=O |r| Show InChI InChI=1S/C18H20FN3O4/c1-10-9-26-17-14-11(16(23)12(18(24)25)8-22(10)14)7-13(19)15(17)21-5-3-20(2)4-6-21/h7-8,10H,3-6,9H2,1-2H3,(H,24,25)/t10-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Antibacterial activity against vancomycin-sensitive Enterococcus faecalis ATCC 29212 after 18 hrs by broth microdilution method |
J Nat Prod 77: 1021-30 (2014)
Article DOI: 10.1021/np5000457
BindingDB Entry DOI: 10.7270/Q2280BKR |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50366826

(DR-3355 | Floxacin | Iquix | LEVOFLOXACIN | Levaqu...)Show SMILES C[C@H]1COc2c(N3CCN(C)CC3)c(F)cc3c2n1cc(C(O)=O)c3=O |r| Show InChI InChI=1S/C18H20FN3O4/c1-10-9-26-17-14-11(16(23)12(18(24)25)8-22(10)14)7-13(19)15(17)21-5-3-20(2)4-6-21/h7-8,10H,3-6,9H2,1-2H3,(H,24,25)/t10-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Antibacterial activity against vancomycin-resistant Enterococcus faecalis ATCC 51299 after 18 hrs by broth microdilution method |
J Nat Prod 77: 1021-30 (2014)
Article DOI: 10.1021/np5000457
BindingDB Entry DOI: 10.7270/Q2280BKR |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
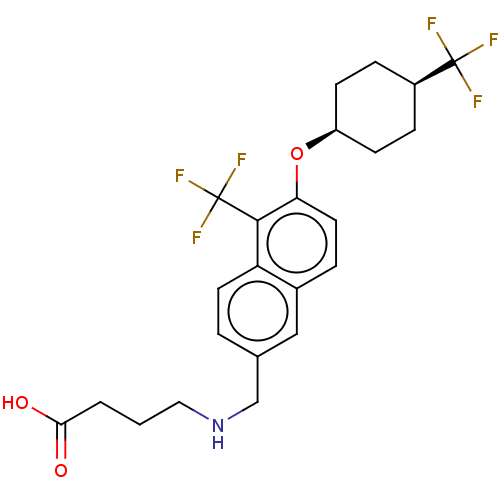
(Homo sapiens (Human)) | BDBM50572938
(CHEMBL4872976)Show SMILES OC(=O)CCCNCc1ccc2c(c(O[C@H]3CC[C@H](CC3)C(F)(F)F)ccc2c1)C(F)(F)F |r,wU:15.14,18.21,(82.34,-5.05,;81.01,-5.83,;81.02,-7.37,;79.68,-5.07,;78.35,-5.84,;77.01,-5.08,;75.68,-5.86,;74.34,-5.1,;73.01,-5.88,;73.02,-7.42,;71.69,-8.2,;70.35,-7.43,;69.02,-8.21,;67.68,-7.44,;66.35,-8.21,;65.02,-7.44,;63.69,-8.2,;62.36,-7.42,;62.36,-5.88,;63.7,-5.12,;65.02,-5.89,;61.03,-5.11,;61.04,-3.57,;59.69,-5.87,;59.68,-4.33,;67.69,-5.9,;69.01,-5.12,;70.35,-5.89,;71.68,-5.12,;69.02,-9.75,;67.69,-10.52,;70.36,-10.52,;69.01,-11.29,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ATX assessed as reduction in LPA formation using FS-3 as substrate preincubated for 30 mins followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00211
BindingDB Entry DOI: 10.7270/Q2Q81HVP |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50313801

((1R,3S)-3-(1-acetamidocyclopentyl)-N-((2S,3S,5R)-6...)Show SMILES CCCCNC(=O)[C@H](C)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCC[C@@H](C1)C1(CCCC1)NC(C)=O |r| Show InChI InChI=1S/C31H49N3O4/c1-4-5-18-32-29(37)22(2)19-28(36)27(20-24-12-7-6-8-13-24)33-30(38)25-14-11-15-26(21-25)31(34-23(3)35)16-9-10-17-31/h6-8,12-13,22,25-28,36H,4-5,9-11,14-21H2,1-3H3,(H,32,37)(H,33,38)(H,34,35)/t22-,25-,26+,27+,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50313801

((1R,3S)-3-(1-acetamidocyclopentyl)-N-((2S,3S,5R)-6...)Show SMILES CCCCNC(=O)[C@H](C)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCC[C@@H](C1)C1(CCCC1)NC(C)=O |r| Show InChI InChI=1S/C31H49N3O4/c1-4-5-18-32-29(37)22(2)19-28(36)27(20-24-12-7-6-8-13-24)33-30(38)25-14-11-15-26(21-25)31(34-23(3)35)16-9-10-17-31/h6-8,12-13,22,25-28,36H,4-5,9-11,14-21H2,1-3H3,(H,32,37)(H,33,38)(H,34,35)/t22-,25-,26+,27+,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50313803

((1R,3S)-N-((2S,3S)-3-hydroxy-4-(3-isopropylbenzyla...)Show SMILES CCC(=O)NC(C)(C)[C@H]1CCC[C@H](C1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)CNCc1cccc(c1)C(C)C |r| Show InChI InChI=1S/C33H49N3O3/c1-6-31(38)36-33(4,5)28-17-11-16-27(20-28)32(39)35-29(19-24-12-8-7-9-13-24)30(37)22-34-21-25-14-10-15-26(18-25)23(2)3/h7-10,12-15,18,23,27-30,34,37H,6,11,16-17,19-22H2,1-5H3,(H,35,39)(H,36,38)/t27-,28+,29+,30+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50313800

((1R,3S)-3-(2-acetamidopropan-2-yl)-N-((2S,3S,5R)-6...)Show SMILES CCCCNC(=O)[C@H](C)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCC[C@@H](C1)C(C)(C)NC(C)=O |r| Show InChI InChI=1S/C29H47N3O4/c1-6-7-16-30-27(35)20(2)17-26(34)25(18-22-12-9-8-10-13-22)31-28(36)23-14-11-15-24(19-23)29(4,5)32-21(3)33/h8-10,12-13,20,23-26,34H,6-7,11,14-19H2,1-5H3,(H,30,35)(H,31,36)(H,32,33)/t20-,23-,24+,25+,26+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM50572937
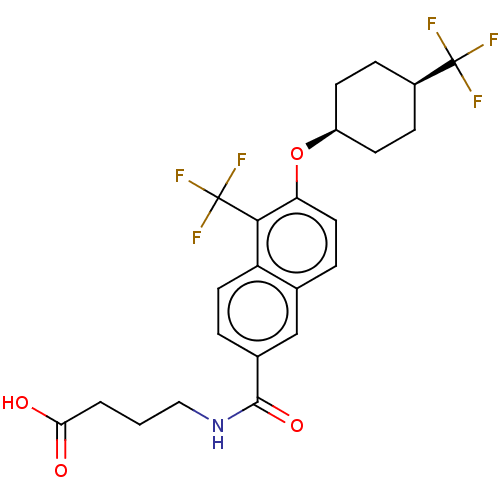
(CHEMBL4875797)Show SMILES OC(=O)CCCNC(=O)c1ccc2c(c(O[C@H]3CC[C@H](CC3)C(F)(F)F)ccc2c1)C(F)(F)F |r,wU:16.15,19.22,(53.15,-5.14,;51.82,-5.92,;51.83,-7.46,;50.48,-5.16,;49.15,-5.94,;47.81,-5.17,;46.48,-5.95,;45.14,-5.19,;45.13,-3.65,;43.81,-5.97,;43.82,-7.52,;42.49,-8.29,;41.15,-7.53,;39.82,-8.3,;38.49,-7.53,;37.15,-8.3,;35.82,-7.53,;34.49,-8.3,;33.16,-7.52,;33.16,-5.97,;34.5,-5.21,;35.83,-5.98,;31.83,-5.2,;31.84,-3.66,;30.5,-5.96,;30.49,-4.43,;38.49,-5.99,;39.82,-5.22,;41.15,-5.98,;42.48,-5.21,;39.82,-9.84,;38.49,-10.62,;41.16,-10.61,;39.81,-11.38,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ATX assessed as reduction in LPA formation using FS-3 as substrate preincubated for 30 mins followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00211
BindingDB Entry DOI: 10.7270/Q2Q81HVP |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50313802
((1R,3S)-3-(2-acetamidopropan-2-yl)-N-((2S,3S)-3-hy...)Show SMILES CC(C)c1cccc(CNC[C@H](O)[C@H](Cc2ccccc2)NC(=O)[C@@H]2CCC[C@@H](C2)C(C)(C)NC(C)=O)c1 |r| Show InChI InChI=1S/C32H47N3O3/c1-22(2)26-14-9-13-25(17-26)20-33-21-30(37)29(18-24-11-7-6-8-12-24)34-31(38)27-15-10-16-28(19-27)32(4,5)35-23(3)36/h6-9,11-14,17,22,27-30,33,37H,10,15-16,18-21H2,1-5H3,(H,34,38)(H,35,36)/t27-,28+,29+,30+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of BACE2 |
Bioorg Med Chem Lett 20: 1924-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.139
BindingDB Entry DOI: 10.7270/Q2TD9XHH |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Rattus norvegicus) | BDBM50497011
(CHEMBL3234400)Show SMILES CCCc1c(Cl)c(OC)c(Cl)c2OC(=O)c3c(CCC)c(Cl)c(O)c(Cl)c3Oc12 Show InChI InChI=1S/C20H18Cl4O5/c1-4-6-8-10-17(13(23)15(25)11(8)21)28-16-9(7-5-2)12(22)18(27-3)14(24)19(16)29-20(10)26/h25H,4-7H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Antibacterial activity against vancomycin-resistant Enterococcus faecalis 09-9 after 18 hrs by broth microdilution method |
J Nat Prod 77: 1021-30 (2014)
Article DOI: 10.1021/np5000457
BindingDB Entry DOI: 10.7270/Q2280BKR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data