

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Homo sapiens (Human)) | BDBM50167296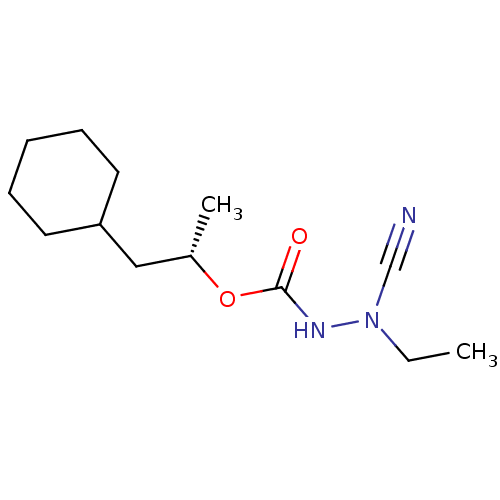 ((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-ethylhyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50167295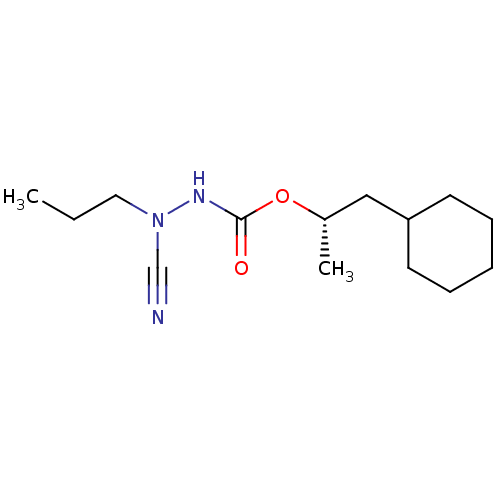 ((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-propylhy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50167302 ((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-isobutyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50167298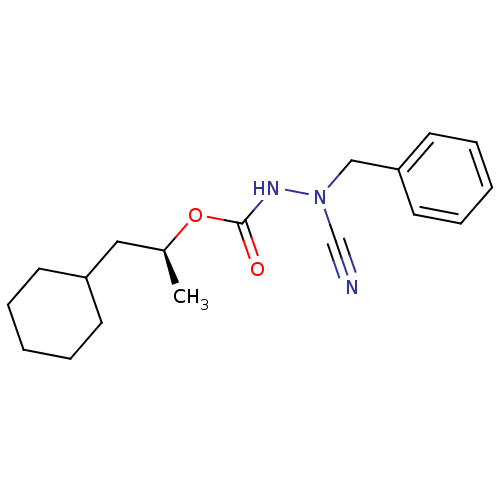 ((1S)-2-cyclohexyl-1-methylethyl 2-benzyl-2-cyanohy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50167303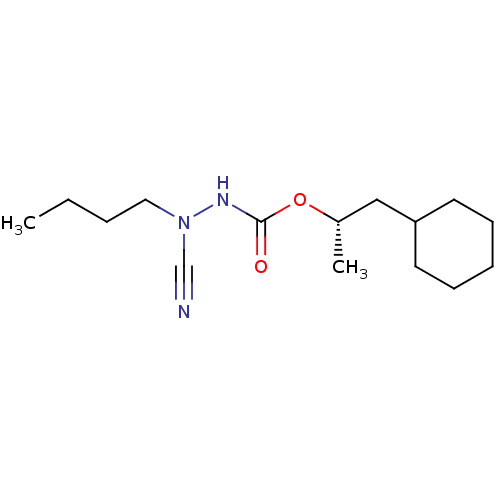 ((1S)-2-cyclohexyl-1-methylethyl 2-butyl-2-cyanohyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50167289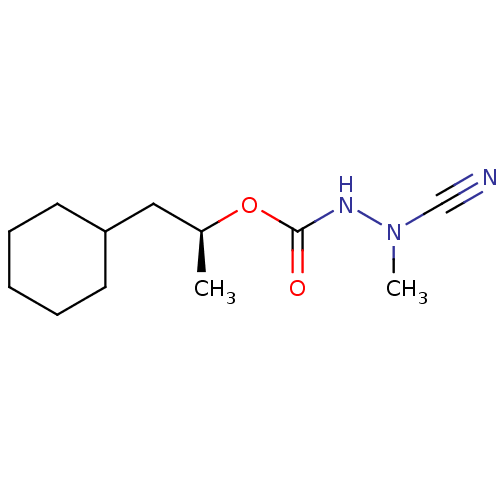 ((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-methylhy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50325767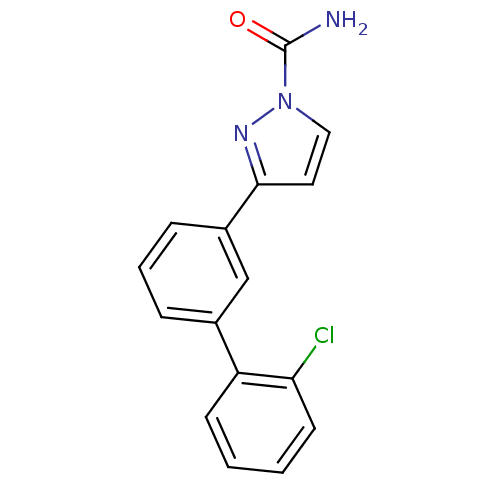 (3-(2'-chlorobiphenyl-3-yl)-1H-pyrazole-1-carboxami...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human Nav 1.7 channel by electrophysiology | Bioorg Med Chem Lett 20: 5480-3 (2010) Article DOI: 10.1016/j.bmcl.2010.07.080 BindingDB Entry DOI: 10.7270/Q2PV6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50167290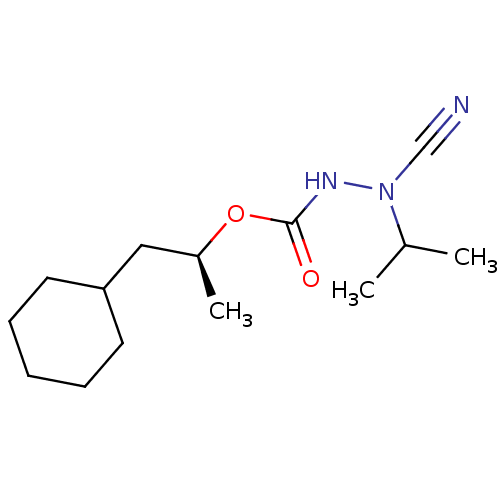 ((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-isopropy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50167289 ((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-methylhy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin L using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085666 ((2S,3S,4R,5R)-5-{6-(2,2-Diphenyl-ethylamino)-2-[2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50521464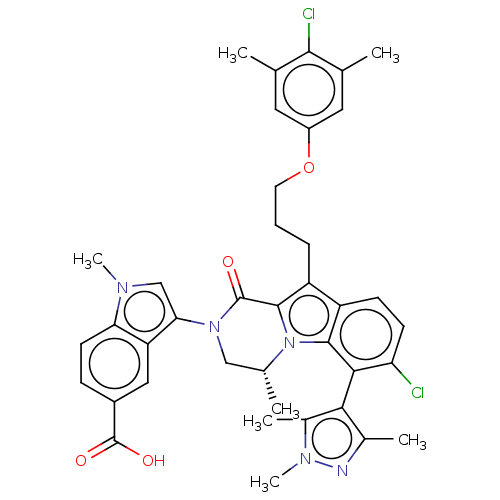 (CHEMBL4469869 | US11596639, Example 78) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of FITC-labeled Bak peptide binding to MBP-fused Mcl-1 (unknown origin) measured after 3 hrs by TR-FRET assay | J Med Chem 62: 3971-3988 (2019) Article DOI: 10.1021/acs.jmedchem.8b01991 BindingDB Entry DOI: 10.7270/Q2V69P04 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50521464 (CHEMBL4469869 | US11596639, Example 78) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of FITC-labeled Bak peptide binding to MBP-fused Mcl-1 (unknown origin) measured after 3 hrs by TR-FRET assay | J Med Chem 62: 3971-3988 (2019) Article DOI: 10.1021/acs.jmedchem.8b01991 BindingDB Entry DOI: 10.7270/Q2V69P04 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50521464 (CHEMBL4469869 | US11596639, Example 78) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of FITC-labeled Bak peptide binding to MBP-fused Mcl-1 (unknown origin) measured after 3 hrs by TR-FRET assay | J Med Chem 62: 3971-3988 (2019) Article DOI: 10.1021/acs.jmedchem.8b01991 BindingDB Entry DOI: 10.7270/Q2V69P04 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50167288 ((1S)-1-benzylpropyl 2-cyano-2-methylhydrazinecarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50167288 ((1S)-1-benzylpropyl 2-cyano-2-methylhydrazinecarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin L using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166333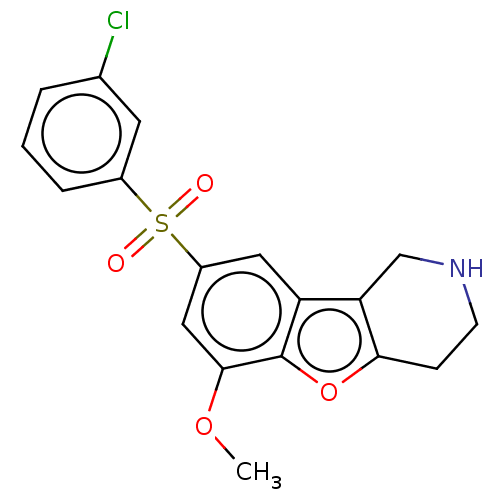 (US9067949, 190) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463596 (US10781204, Compound I-194 | US10781204, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nimbus Lakshmi, Inc. US Patent | Assay Description The caliper machine employs an off chip mobility shift assay to detect phosphorylated peptide substrates from kinase assays, using microfluidics tech... | US Patent US10781204 (2020) BindingDB Entry DOI: 10.7270/Q21G0Q9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085674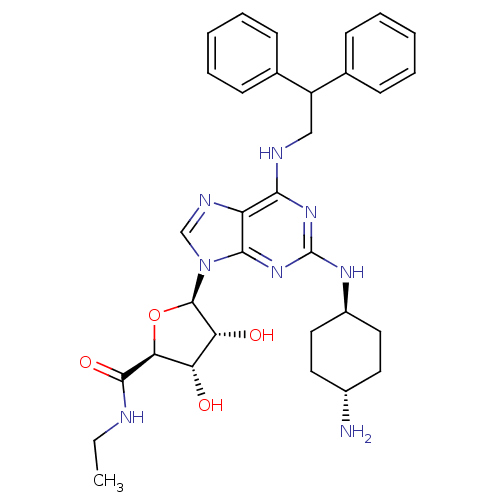 ((2S,3S,4R,5R)-5-[2-(4-Amino-cyclohexylamino)-6-(2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463736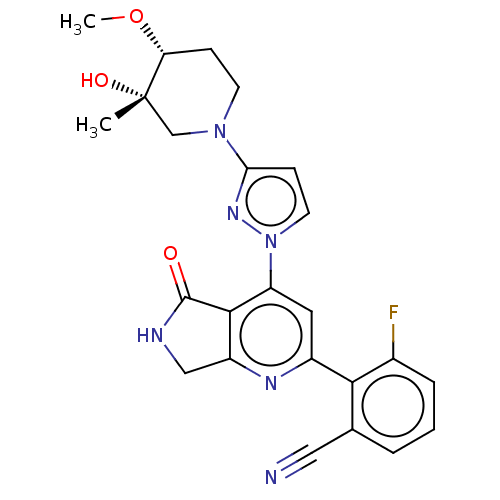 (US10781204, Compound I-213 | US10781204, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463752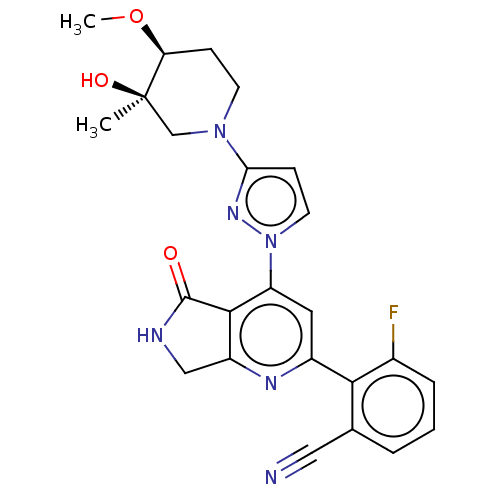 (US10781204, Compound I-229 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463696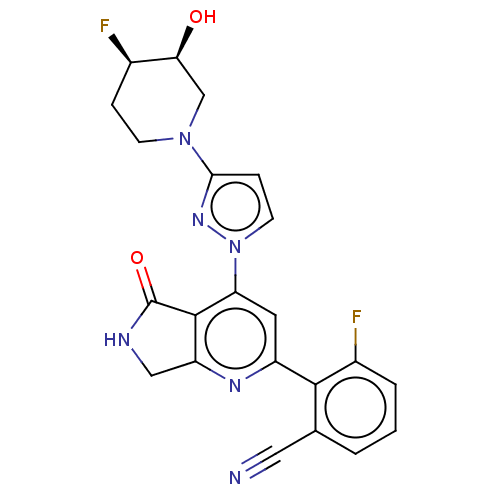 (US10781204, Compound I-173 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463710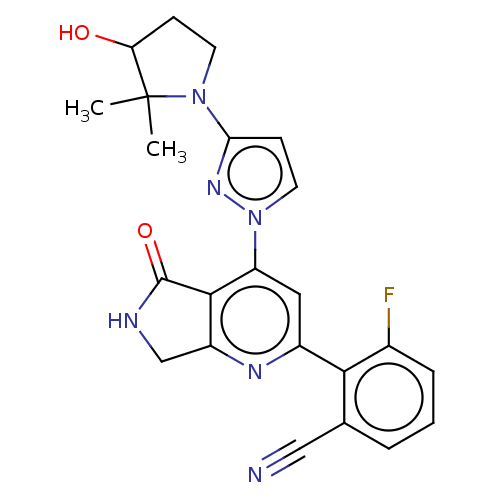 (US10781204, Compound I-187 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463711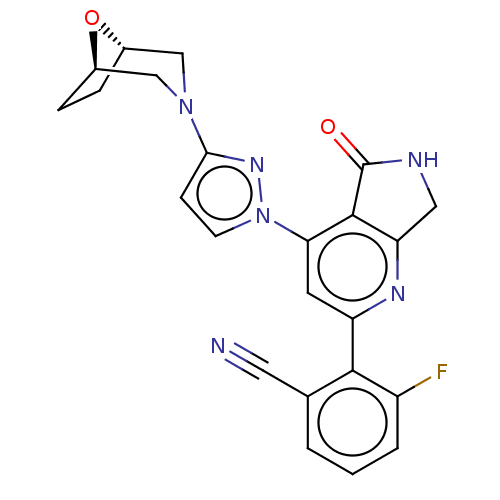 (US10781204, Compound I-188 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463662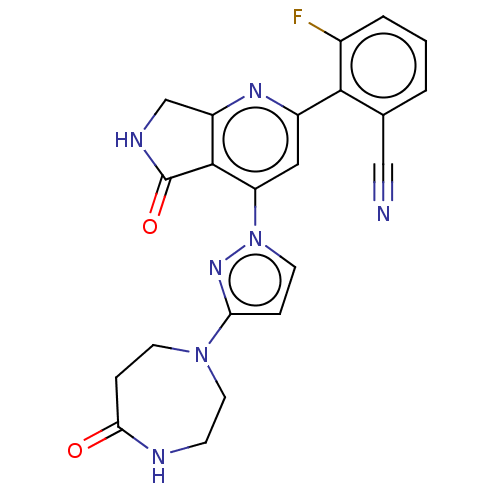 (US10781204, Compound I-141 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463600 (US10781204, Compound I-142 | US10781204, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463671 (US10781204, Compound I-149 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463672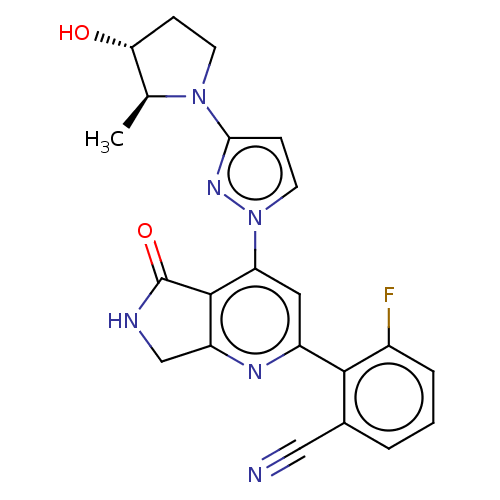 (US10781204, Compound I-150 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463682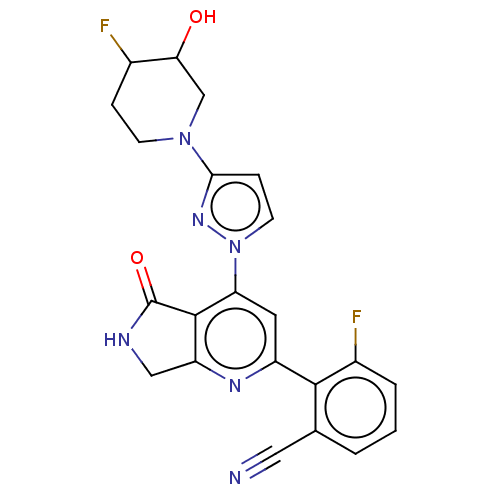 (US10781204, Compound I-160 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463684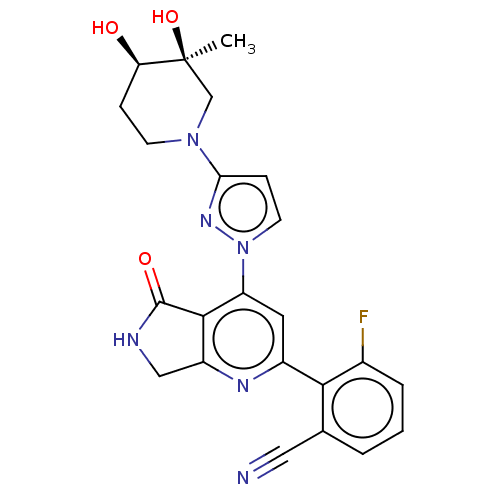 (US10781204, Compound I-162 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463689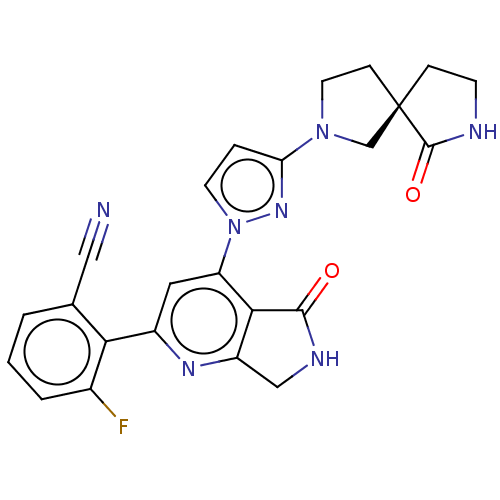 (US10781204, Compound I-166 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463639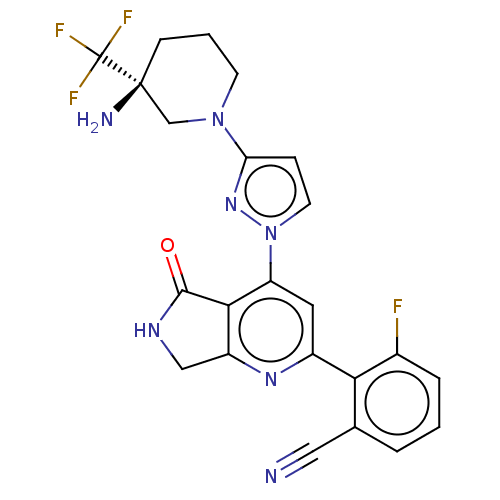 (US10781204, Compound I-118 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463640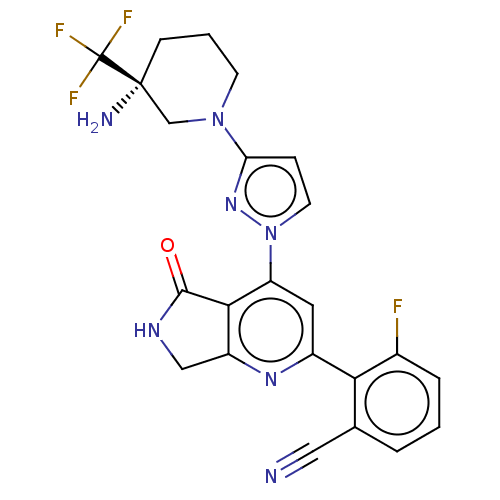 (US10781204, Compound I-119 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463657 (US10781204, Compound I-136 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463605 (US10781204, Compound I-84 | US11434240, Compound I...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463609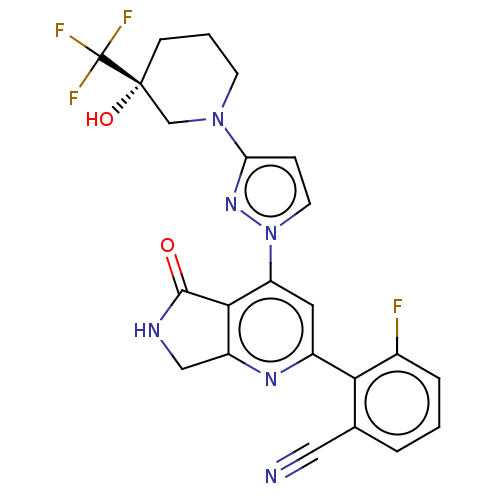 (US10781204, Compound I-88 | US11434240, Compound I...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463621 (US10781204, Compound I-100 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM570426 (US11434240, Compound I-62) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463591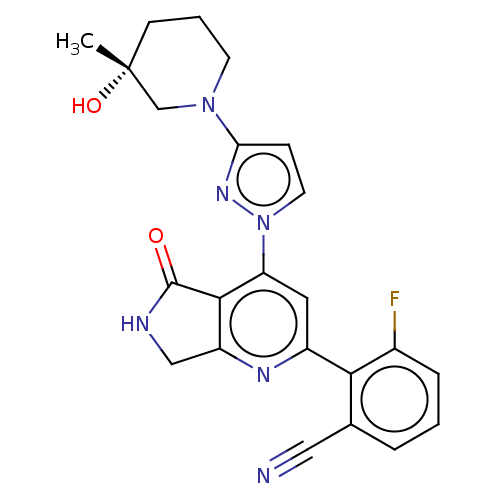 (US10781204, Compound I-111 | US10781204, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463596 (US10781204, Compound I-194 | US10781204, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Peptide substrate, [KKSRGDYMTMQIG], (20 μM) is prepared in reaction buffer (20 mM Hepes pH 7.5, 10 mM MgCl2, 1 mM EGTA, 0.02% Brij35, 0.02 mg/mL... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QV3QRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463736 (US10781204, Compound I-213 | US10781204, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nimbus Lakshmi, Inc. US Patent | Assay Description The caliper machine employs an off chip mobility shift assay to detect phosphorylated peptide substrates from kinase assays, using microfluidics tech... | US Patent US10781204 (2020) BindingDB Entry DOI: 10.7270/Q21G0Q9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463752 (US10781204, Compound I-229 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nimbus Lakshmi, Inc. US Patent | Assay Description The caliper machine employs an off chip mobility shift assay to detect phosphorylated peptide substrates from kinase assays, using microfluidics tech... | US Patent US10781204 (2020) BindingDB Entry DOI: 10.7270/Q21G0Q9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463710 (US10781204, Compound I-187 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nimbus Lakshmi, Inc. US Patent | Assay Description The caliper machine employs an off chip mobility shift assay to detect phosphorylated peptide substrates from kinase assays, using microfluidics tech... | US Patent US10781204 (2020) BindingDB Entry DOI: 10.7270/Q21G0Q9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463711 (US10781204, Compound I-188 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nimbus Lakshmi, Inc. US Patent | Assay Description The caliper machine employs an off chip mobility shift assay to detect phosphorylated peptide substrates from kinase assays, using microfluidics tech... | US Patent US10781204 (2020) BindingDB Entry DOI: 10.7270/Q21G0Q9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463671 (US10781204, Compound I-149 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nimbus Lakshmi, Inc. US Patent | Assay Description The caliper machine employs an off chip mobility shift assay to detect phosphorylated peptide substrates from kinase assays, using microfluidics tech... | US Patent US10781204 (2020) BindingDB Entry DOI: 10.7270/Q21G0Q9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463672 (US10781204, Compound I-150 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nimbus Lakshmi, Inc. US Patent | Assay Description The caliper machine employs an off chip mobility shift assay to detect phosphorylated peptide substrates from kinase assays, using microfluidics tech... | US Patent US10781204 (2020) BindingDB Entry DOI: 10.7270/Q21G0Q9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463682 (US10781204, Compound I-160 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nimbus Lakshmi, Inc. US Patent | Assay Description The caliper machine employs an off chip mobility shift assay to detect phosphorylated peptide substrates from kinase assays, using microfluidics tech... | US Patent US10781204 (2020) BindingDB Entry DOI: 10.7270/Q21G0Q9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463684 (US10781204, Compound I-162 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nimbus Lakshmi, Inc. US Patent | Assay Description The caliper machine employs an off chip mobility shift assay to detect phosphorylated peptide substrates from kinase assays, using microfluidics tech... | US Patent US10781204 (2020) BindingDB Entry DOI: 10.7270/Q21G0Q9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463689 (US10781204, Compound I-166 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nimbus Lakshmi, Inc. US Patent | Assay Description The caliper machine employs an off chip mobility shift assay to detect phosphorylated peptide substrates from kinase assays, using microfluidics tech... | US Patent US10781204 (2020) BindingDB Entry DOI: 10.7270/Q21G0Q9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463696 (US10781204, Compound I-173 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nimbus Lakshmi, Inc. US Patent | Assay Description The caliper machine employs an off chip mobility shift assay to detect phosphorylated peptide substrates from kinase assays, using microfluidics tech... | US Patent US10781204 (2020) BindingDB Entry DOI: 10.7270/Q21G0Q9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM463639 (US10781204, Compound I-118 | US11434240, Compound ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nimbus Lakshmi, Inc. US Patent | Assay Description The caliper machine employs an off chip mobility shift assay to detect phosphorylated peptide substrates from kinase assays, using microfluidics tech... | US Patent US10781204 (2020) BindingDB Entry DOI: 10.7270/Q21G0Q9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 23490 total ) | Next | Last >> |