

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50081804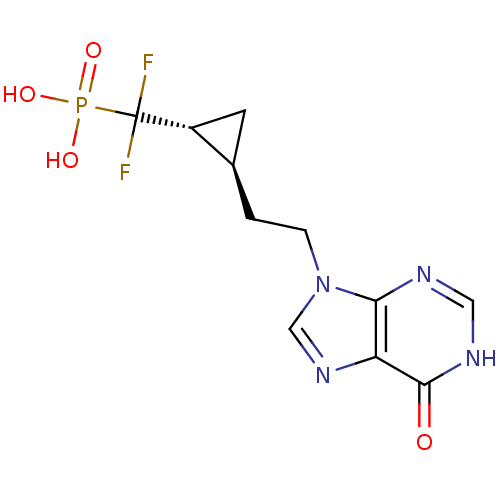 ((Difluoro-{(1R,2S)-2-[2-(6-oxo-1,6-dihydro-purin-9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in human erythrocyte | Bioorg Med Chem Lett 9: 2833-6 (1999) BindingDB Entry DOI: 10.7270/Q2JQ107B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50081803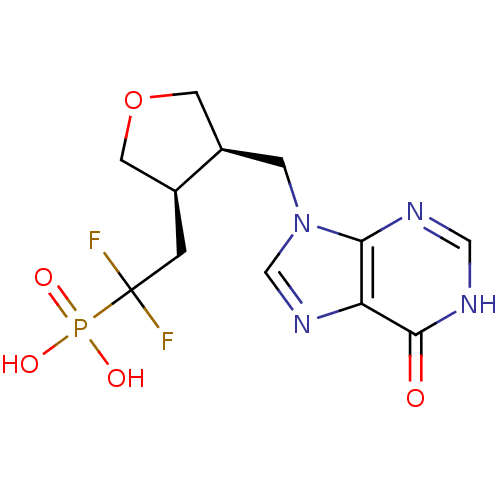 (CHEMBL94840 | {1,1-Difluoro-2-[(3R,4R)-4-(6-oxo-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in human erythrocyte | Bioorg Med Chem Lett 9: 2833-6 (1999) BindingDB Entry DOI: 10.7270/Q2JQ107B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50081807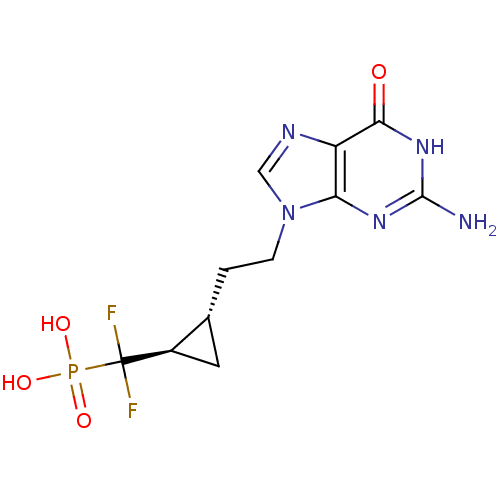 (({(1R,2S)-2-[2-(2-Amino-6-oxo-1,6-dihydro-purin-9-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in human erythrocyte | Bioorg Med Chem Lett 9: 2833-6 (1999) BindingDB Entry DOI: 10.7270/Q2JQ107B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50214705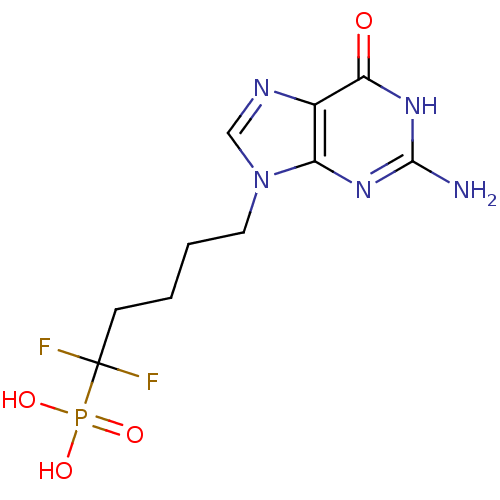 (5-(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)-1,1-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in human erythrocyte | Bioorg Med Chem Lett 9: 2833-6 (1999) BindingDB Entry DOI: 10.7270/Q2JQ107B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingomyelin phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50122309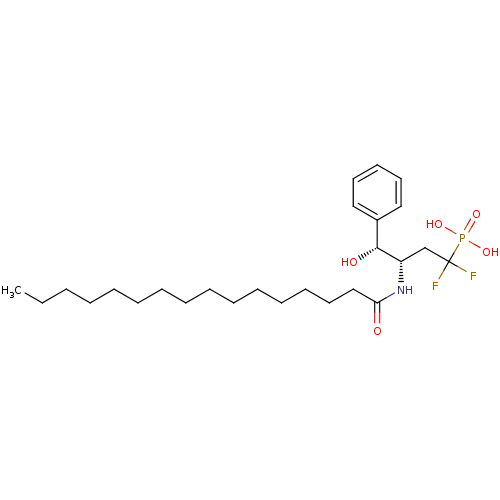 (((3S,4R)-1,1-Difluoro-3-hexadecanoylamino-4-hydrox...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Inhibitory activity of the compound against schyphostatin of neutral sphingomyelinase (N-SMase) from bovine brain microsome | Bioorg Med Chem Lett 13: 229-36 (2002) BindingDB Entry DOI: 10.7270/Q2K64HFH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingomyelin phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50122310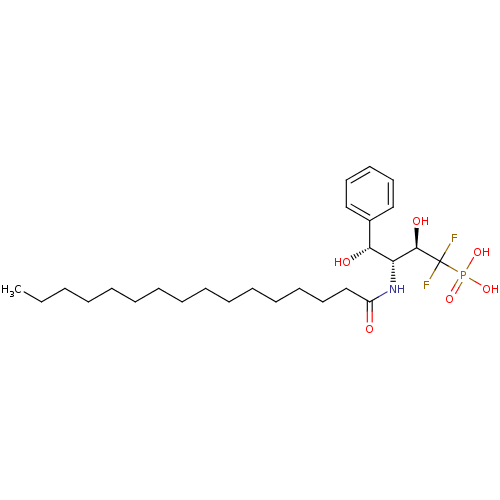 (((2R,3R,4R)-1,1-Difluoro-3-hexadecanoylamino-2,4-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.53E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Inhibitory activity against neutral sphingomyelinase (N-SMase) from bovine brain microsomes | Bioorg Med Chem Lett 13: 229-36 (2002) BindingDB Entry DOI: 10.7270/Q2K64HFH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50222711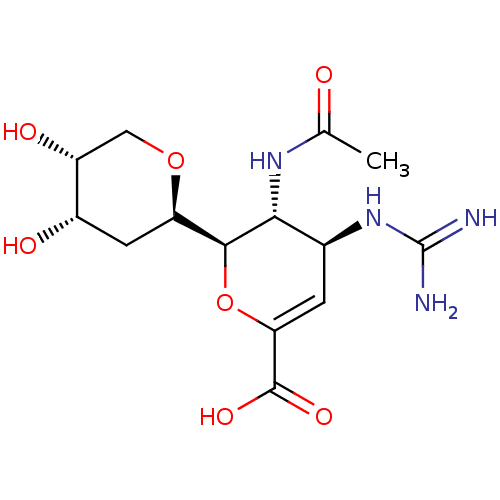 (CHEMBL350985) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.84 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against influenza A virus sialidase A/PR/8/34 | Bioorg Med Chem Lett 13: 669-73 (2003) BindingDB Entry DOI: 10.7270/Q2P84F3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50222713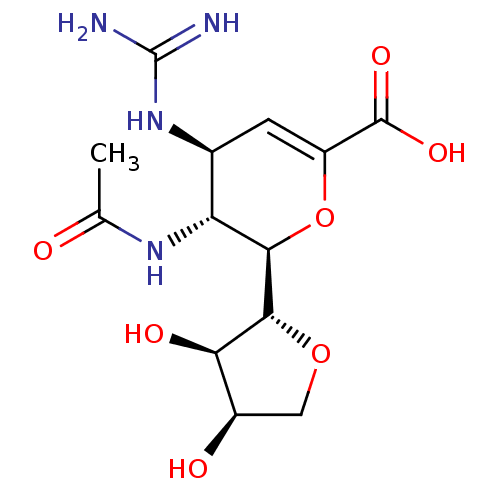 (CHEMBL423972) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 17.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against influenza A virus sialidase A/PR/8/34 | Bioorg Med Chem Lett 13: 669-73 (2003) BindingDB Entry DOI: 10.7270/Q2P84F3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50222710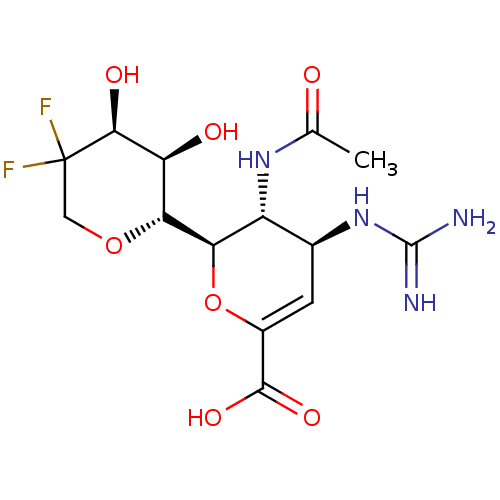 (CHEMBL349349) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 27.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against influenza A virus sialidase A/PR/8/34 | Bioorg Med Chem Lett 13: 669-73 (2003) BindingDB Entry DOI: 10.7270/Q2P84F3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50222706 (CHEMBL159225) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 28.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against influenza A virus sialidase A/PR/8/34 | Bioorg Med Chem Lett 13: 669-73 (2003) BindingDB Entry DOI: 10.7270/Q2P84F3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50222712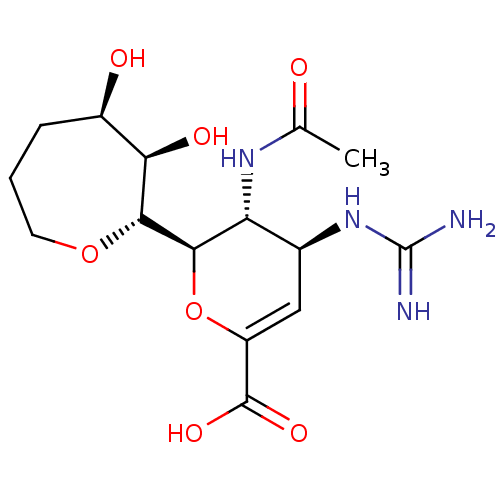 (CHEMBL351695) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 33.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against influenza A virus sialidase A/PR/8/34 | Bioorg Med Chem Lett 13: 669-73 (2003) BindingDB Entry DOI: 10.7270/Q2P84F3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50081803 (CHEMBL94840 | {1,1-Difluoro-2-[(3R,4R)-4-(6-oxo-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in Cellulomonas sp | Bioorg Med Chem Lett 9: 2833-6 (1999) BindingDB Entry DOI: 10.7270/Q2JQ107B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 36.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against influenza A virus sialidase A/PR/8/34 | Bioorg Med Chem Lett 13: 669-73 (2003) BindingDB Entry DOI: 10.7270/Q2P84F3T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50081806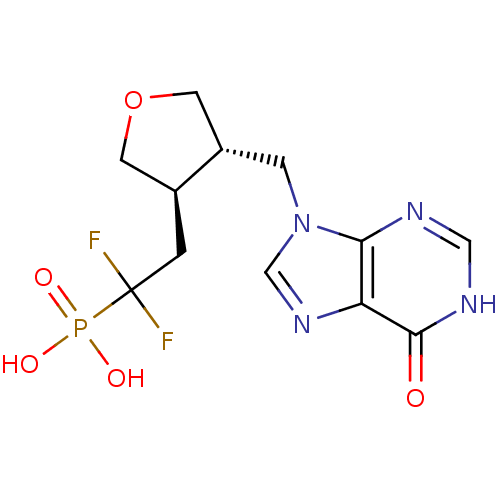 (CHEMBL94920 | {1,1-Difluoro-2-[(3R,4S)-4-(6-oxo-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in Cellulomonas sp | Bioorg Med Chem Lett 9: 2833-6 (1999) BindingDB Entry DOI: 10.7270/Q2JQ107B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50081804 ((Difluoro-{(1R,2S)-2-[2-(6-oxo-1,6-dihydro-purin-9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in Cellulomonas sp | Bioorg Med Chem Lett 9: 2833-6 (1999) BindingDB Entry DOI: 10.7270/Q2JQ107B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50081803 (CHEMBL94840 | {1,1-Difluoro-2-[(3R,4R)-4-(6-oxo-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in human erythrocyte | Bioorg Med Chem Lett 9: 2833-6 (1999) BindingDB Entry DOI: 10.7270/Q2JQ107B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50081806 (CHEMBL94920 | {1,1-Difluoro-2-[(3R,4S)-4-(6-oxo-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in human erythrocyte | Bioorg Med Chem Lett 9: 2833-6 (1999) BindingDB Entry DOI: 10.7270/Q2JQ107B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50081807 (({(1R,2S)-2-[2-(2-Amino-6-oxo-1,6-dihydro-purin-9-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in human erythrocyte | Bioorg Med Chem Lett 9: 2833-6 (1999) BindingDB Entry DOI: 10.7270/Q2JQ107B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50081804 ((Difluoro-{(1R,2S)-2-[2-(6-oxo-1,6-dihydro-purin-9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in Cellulomonas sp | Bioorg Med Chem Lett 9: 2833-6 (1999) BindingDB Entry DOI: 10.7270/Q2JQ107B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50214705 (5-(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)-1,1-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in human erythrocyte | Bioorg Med Chem Lett 9: 2833-6 (1999) BindingDB Entry DOI: 10.7270/Q2JQ107B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50081807 (({(1R,2S)-2-[2-(2-Amino-6-oxo-1,6-dihydro-purin-9-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in human erythrocyte | Bioorg Med Chem Lett 9: 2833-6 (1999) BindingDB Entry DOI: 10.7270/Q2JQ107B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50214705 (5-(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)-1,1-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in Cellulomonas sp | Bioorg Med Chem Lett 9: 2833-6 (1999) BindingDB Entry DOI: 10.7270/Q2JQ107B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50222715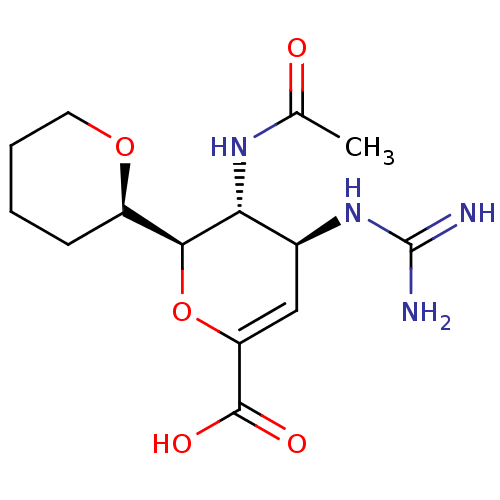 (CHEMBL161713) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 735 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against influenza A virus sialidase A/PR/8/34 | Bioorg Med Chem Lett 13: 669-73 (2003) BindingDB Entry DOI: 10.7270/Q2P84F3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingomyelin phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50100341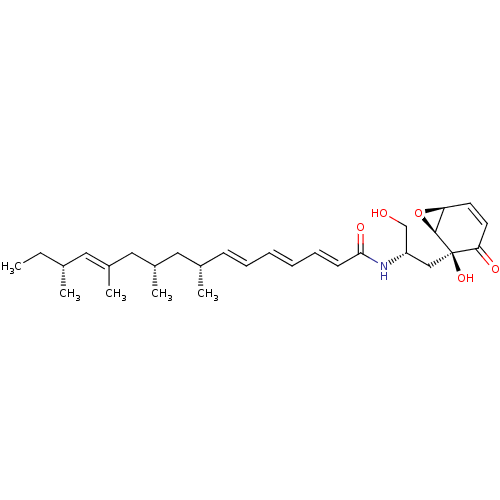 ((2E,4E,6E,12E)-(8R,10S,14R)-8,10,12,14-Tetramethyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Inhibitory activity of the compound against neutral sphingomyelinase (N-SMase) from bovine brain microsomes | Bioorg Med Chem Lett 13: 229-36 (2002) BindingDB Entry DOI: 10.7270/Q2K64HFH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingomyelin phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50122309 (((3S,4R)-1,1-Difluoro-3-hexadecanoylamino-4-hydrox...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Inhibitory activity of the compound against neutral sphingomyelinase (N-SMase) from bovine brain microsomes | Bioorg Med Chem Lett 13: 229-36 (2002) BindingDB Entry DOI: 10.7270/Q2K64HFH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingomyelin phosphodiesterase 2 (Rattus norvegicus) | BDBM50100341 ((2E,4E,6E,12E)-(8R,10S,14R)-8,10,12,14-Tetramethyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Inhibitory concentration against neutral sphingomyelinase (N-Smase) from rat brain microsomes | Bioorg Med Chem Lett 11: 1277-80 (2001) BindingDB Entry DOI: 10.7270/Q2B56J1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50222707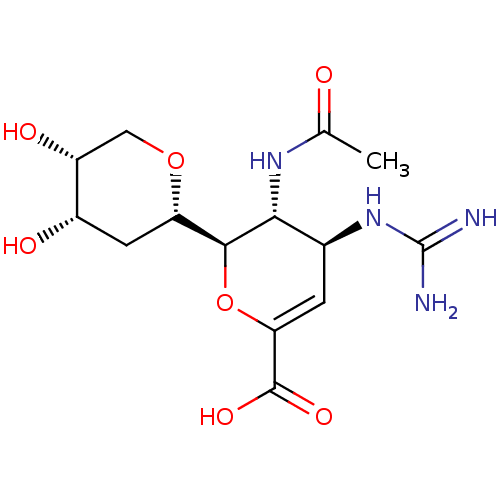 (CHEMBL346983) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against influenza A virus sialidase A/PR/8/34 | Bioorg Med Chem Lett 13: 669-73 (2003) BindingDB Entry DOI: 10.7270/Q2P84F3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50222708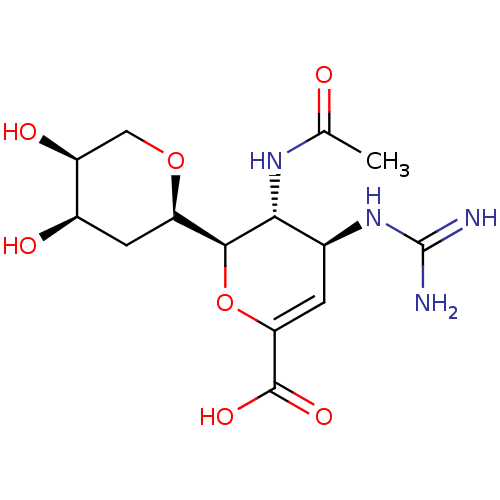 (CHEMBL161355) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against influenza A virus sialidase A/PR/8/34 | Bioorg Med Chem Lett 13: 669-73 (2003) BindingDB Entry DOI: 10.7270/Q2P84F3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50222714 (CHEMBL351043) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against influenza A virus sialidase A/PR/8/34 | Bioorg Med Chem Lett 13: 669-73 (2003) BindingDB Entry DOI: 10.7270/Q2P84F3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50075302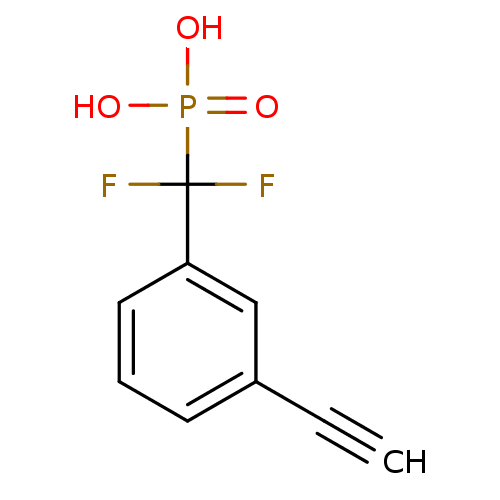 ((3-ethynylphenyl)(difluoro)methylphosphonate | CHE...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Inhibitory potency of the compound for the protein tyrosine phosphatase(PTP 1B)-catalyzed hydrolysis of p-nitrophenol phosphate | Bioorg Med Chem Lett 9: 529-32 (1999) BindingDB Entry DOI: 10.7270/Q29K49DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingomyelin phosphodiesterase [1-45,48-631] (Homo sapiens (Human)) | BDBM50122309 (((3S,4R)-1,1-Difluoro-3-hexadecanoylamino-4-hydrox...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Inhibitory activity of the compound against Acid sphingomyelinase from bovine brain microsome | Bioorg Med Chem Lett 13: 229-36 (2002) BindingDB Entry DOI: 10.7270/Q2K64HFH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingomyelin phosphodiesterase 2 (Rattus norvegicus) | BDBM50100341 ((2E,4E,6E,12E)-(8R,10S,14R)-8,10,12,14-Tetramethyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Inhibitory concentration against lysosomal neutral sphingomyelinase (N-Smase) | Bioorg Med Chem Lett 11: 1277-80 (2001) BindingDB Entry DOI: 10.7270/Q2B56J1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50075306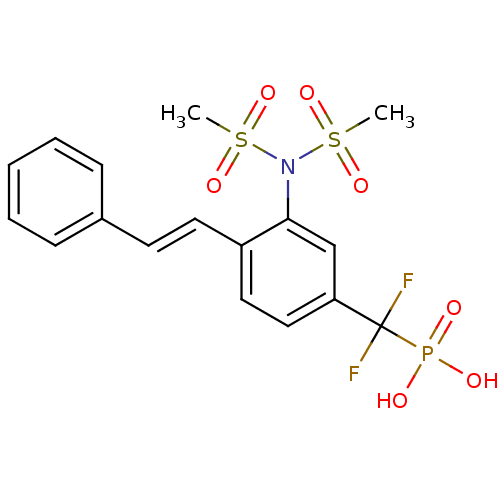 (CHEMBL151494 | {3-[bis(methylsulfonyl)amino]-4-[(E...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Inhibitory potency of the compound for the protein tyrosine phosphatase(PTP 1B)-catalyzed hydrolysis of p-nitrophenol phosphate | Bioorg Med Chem Lett 9: 529-32 (1999) BindingDB Entry DOI: 10.7270/Q29K49DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50075313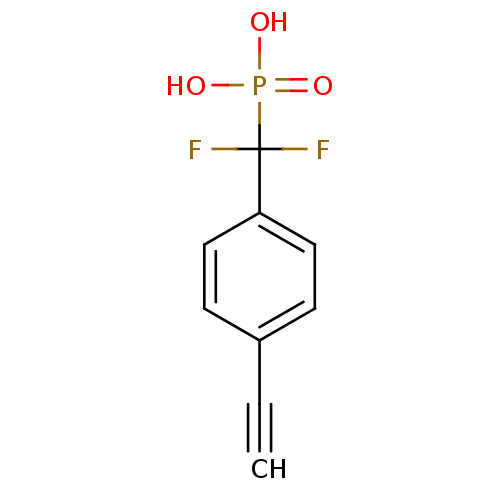 (CHEMBL150915 | [(4-Ethynyl-phenyl)-difluoro-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Inhibitory potency of the compound for the protein tyrosine phosphatase(PTP 1B)-catalyzed hydrolysis of p-nitrophenol phosphate | Bioorg Med Chem Lett 9: 529-32 (1999) BindingDB Entry DOI: 10.7270/Q29K49DW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50075310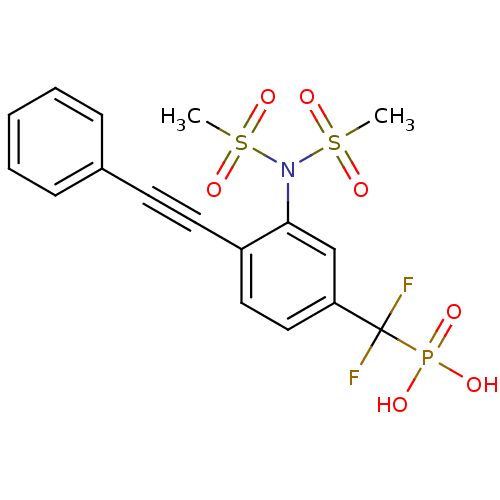 (CHEMBL357094 | [3-[bis(methylsulfonyl)amino]-4-(ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Inhibitory potency of the compound for the protein tyrosine phosphatase(PTP 1B)-catalyzed hydrolysis of p-nitrophenol phosphate | Bioorg Med Chem Lett 9: 529-32 (1999) BindingDB Entry DOI: 10.7270/Q29K49DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50075305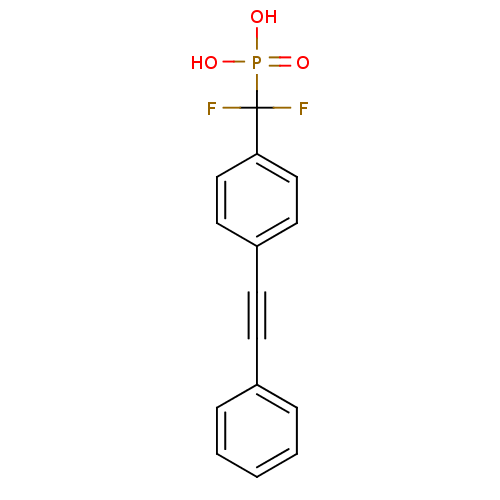 (CHEMBL149279 | [Difluoro-(4-phenylethynyl-phenyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.28E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Inhibitory potency of the compound for the protein tyrosine phosphatase(PTP 1B)-catalyzed hydrolysis of p-nitrophenol phosphate | Bioorg Med Chem Lett 9: 529-32 (1999) BindingDB Entry DOI: 10.7270/Q29K49DW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50075309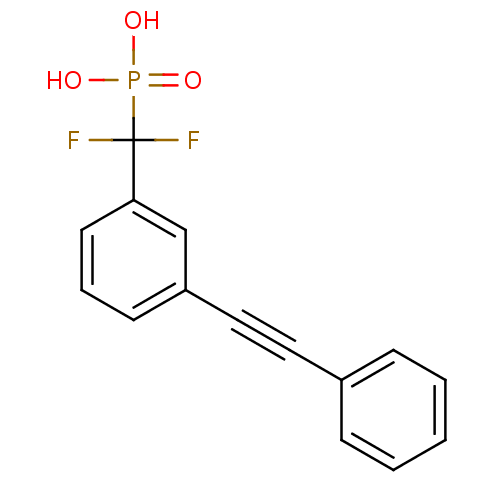 (CHEMBL150420 | [Difluoro-(3-phenylethynyl-phenyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Inhibitory potency of the compound for the protein tyrosine phosphatase(PTP 1B)-catalyzed hydrolysis of p-nitrophenol phosphate | Bioorg Med Chem Lett 9: 529-32 (1999) BindingDB Entry DOI: 10.7270/Q29K49DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50075303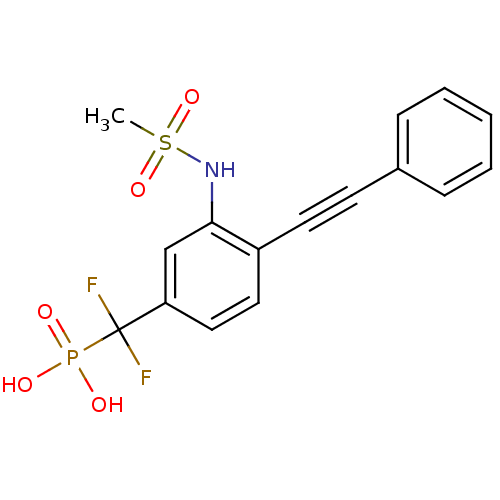 (CHEMBL150349 | [Difluoro-(3-methanesulfonylamino-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Inhibitory potency of the compound for the protein tyrosine phosphatase(PTP 1B)-catalyzed hydrolysis of p-nitrophenol phosphate | Bioorg Med Chem Lett 9: 529-32 (1999) BindingDB Entry DOI: 10.7270/Q29K49DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50075311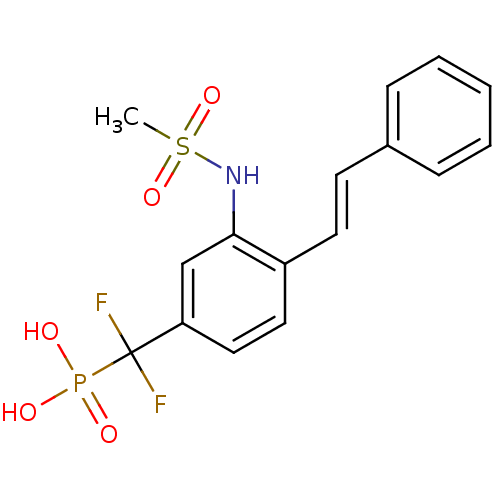 (CHEMBL150350 | [Difluoro-(3-methanesulfonylamino-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.76E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Inhibitory potency of the compound for the protein tyrosine phosphatase(PTP 1B)-catalyzed hydrolysis of p-nitrophenol phosphate | Bioorg Med Chem Lett 9: 529-32 (1999) BindingDB Entry DOI: 10.7270/Q29K49DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingomyelin phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50122309 (((3S,4R)-1,1-Difluoro-3-hexadecanoylamino-4-hydrox...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.81E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Inhibitory activity of the compound against neutral sphingomyelinase (N-SMase) from bovine brain microsomes | Bioorg Med Chem Lett 13: 229-36 (2002) BindingDB Entry DOI: 10.7270/Q2K64HFH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingomyelin phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50122309 (((3S,4R)-1,1-Difluoro-3-hexadecanoylamino-4-hydrox...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.81E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Inhibitory activity against neutral sphingomyelinase (N-SMase) from bovine brain microsomes | Bioorg Med Chem Lett 13: 229-36 (2002) BindingDB Entry DOI: 10.7270/Q2K64HFH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingomyelin phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50122310 (((2R,3R,4R)-1,1-Difluoro-3-hexadecanoylamino-2,4-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.77E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Inhibitory activity of the compound against neutral sphingomyelinase (N-SMase) from bovine brain microsomes | Bioorg Med Chem Lett 13: 229-36 (2002) BindingDB Entry DOI: 10.7270/Q2K64HFH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingomyelin phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50122310 (((2R,3R,4R)-1,1-Difluoro-3-hexadecanoylamino-2,4-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.77E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Inhibitory activity against neutral sphingomyelinase (N-SMase) from bovine brain microsomes | Bioorg Med Chem Lett 13: 229-36 (2002) BindingDB Entry DOI: 10.7270/Q2K64HFH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50075314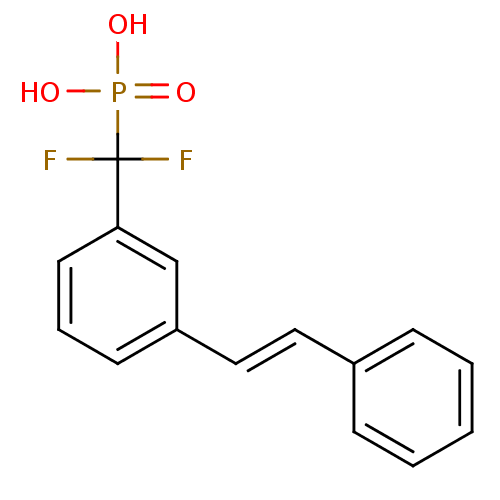 (CHEMBL151300 | difluoro{3-[(E)-2-phenylvinyl]pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.86E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Inhibitory potency of the compound for the protein tyrosine phosphatase(PTP 1B)-catalyzed hydrolysis of p-nitrophenol phosphate | Bioorg Med Chem Lett 9: 529-32 (1999) BindingDB Entry DOI: 10.7270/Q29K49DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingomyelin phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50122311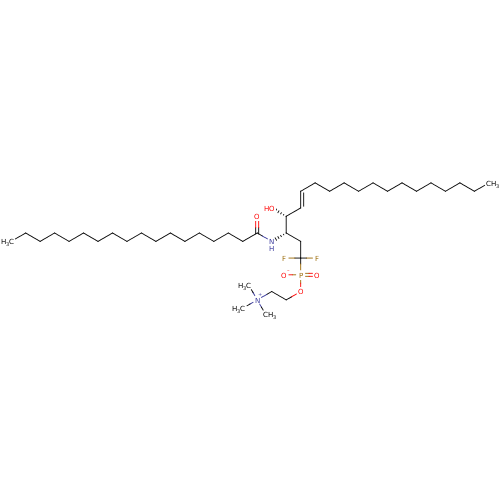 (CHEMBL77022 | {2-[((E)-(3S,4R)-1,1-Difluoro-4-hydr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Inhibitory activity of the compound against neutral sphingomyelinase (N-SMase) from bovine brain microsomes | Bioorg Med Chem Lett 13: 229-36 (2002) BindingDB Entry DOI: 10.7270/Q2K64HFH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50075304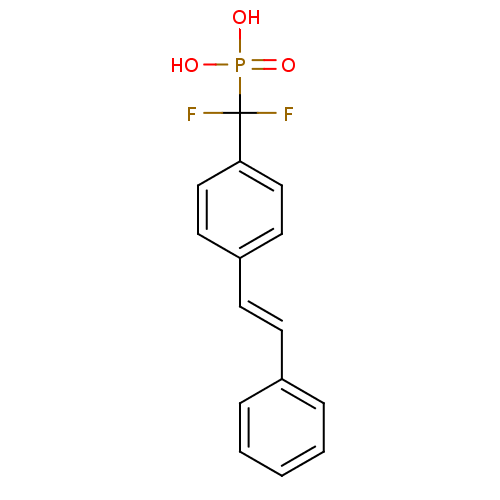 (CHEMBL150295 | {Difluoro-[4-((E)-styryl)-phenyl]-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Inhibitory potency of the compound for the protein tyrosine phosphatase(PTP 1B)-catalyzed hydrolysis of p-nitrophenol phosphate | Bioorg Med Chem Lett 9: 529-32 (1999) BindingDB Entry DOI: 10.7270/Q29K49DW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50075307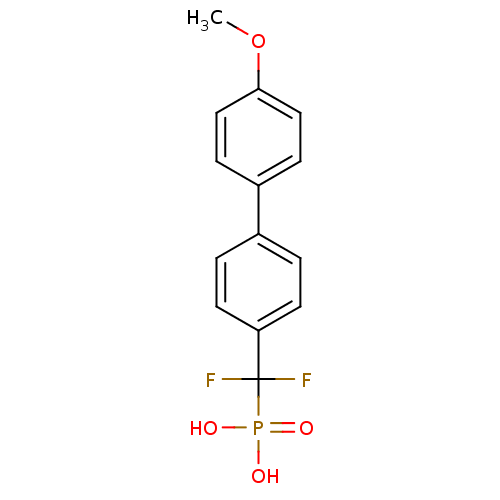 (CHEMBL150202 | [Difluoro-(4'-methoxy-biphenyl-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.51E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Inhibitory potency of the compound for the protein tyrosine phosphatase(PTP 1B)-catalyzed hydrolysis of p-nitrophenol phosphate | Bioorg Med Chem Lett 9: 529-32 (1999) BindingDB Entry DOI: 10.7270/Q29K49DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50075312 ((Difluoro-naphthalen-2-yl-methyl)-phosphonic acid ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Inhibitory potency of the compound for the protein tyrosine phosphatase(PTP 1B)-catalyzed hydrolysis of p-nitrophenol phosphate | Bioorg Med Chem Lett 9: 529-32 (1999) BindingDB Entry DOI: 10.7270/Q29K49DW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50075308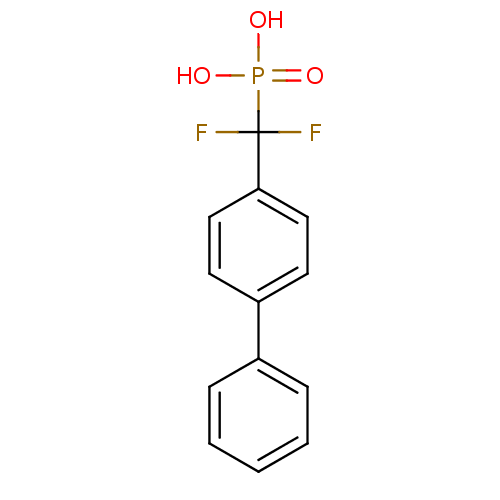 ((Biphenyl-4-yl-difluoro-methyl)-phosphonic acid | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.79E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science Curated by ChEMBL | Assay Description Inhibitory potency of the compound for the protein tyrosine phosphatase(PTP 1B)-catalyzed hydrolysis of p-nitrophenol phosphate | Bioorg Med Chem Lett 9: 529-32 (1999) BindingDB Entry DOI: 10.7270/Q29K49DW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Probable G-protein coupled receptor 142 (Homo sapiens (Human)) | BDBM50437420 (CHEMBL2409014) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Agonist activity at human GPR142 transfected in HEK293 cells after 1 hr by inositol phosphate accumulation assay | ACS Med Chem Lett 4: 790-4 (2013) Article DOI: 10.1021/ml400186z BindingDB Entry DOI: 10.7270/Q24J0GHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 74 total ) | Next | Last >> |