

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuraminidase (Influenza A virus) | BDBM4994 ((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Thailand/1(KAN-1)/2004(H5N1)) neuraminidase by by Michaelis Menten equation analysis | Antimicrob Agents Chemother 53: 3088-96 (2009) Article DOI: 10.1128/AAC.01667-08 BindingDB Entry DOI: 10.7270/Q2VM4CHC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/chicken/Yogjakarta/BBVet-IX/2004(H5N1)) neuraminidase by Michaelis Menten equation analysis | Antimicrob Agents Chemother 53: 3088-96 (2009) Article DOI: 10.1128/AAC.01667-08 BindingDB Entry DOI: 10.7270/Q2VM4CHC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Laos/25/2006(H5N1)) neuraminidase by Michaelis Menten equation analysis | Antimicrob Agents Chemother 53: 3088-96 (2009) Article DOI: 10.1128/AAC.01667-08 BindingDB Entry DOI: 10.7270/Q2VM4CHC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Thailand/1(KAN-1)/2004(H5N1)) neuraminidase by by Michaelis Menten equation analysis | Antimicrob Agents Chemother 53: 3088-96 (2009) Article DOI: 10.1128/AAC.01667-08 BindingDB Entry DOI: 10.7270/Q2VM4CHC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Turkey/651242/2006(H5N1)) neuraminidase by Michaelis Menten equation analysis | Antimicrob Agents Chemother 53: 3088-96 (2009) Article DOI: 10.1128/AAC.01667-08 BindingDB Entry DOI: 10.7270/Q2VM4CHC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM4994 ((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Laos/25/2006(H5N1)) neuraminidase by Michaelis Menten equation analysis | Antimicrob Agents Chemother 53: 3088-96 (2009) Article DOI: 10.1128/AAC.01667-08 BindingDB Entry DOI: 10.7270/Q2VM4CHC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM4994 ((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/chicken/Yogjakarta/BBVet-IX/2004(H5N1)) neuraminidase by Michaelis Menten equation analysis | Antimicrob Agents Chemother 53: 3088-96 (2009) Article DOI: 10.1128/AAC.01667-08 BindingDB Entry DOI: 10.7270/Q2VM4CHC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM4994 ((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Turkey/651242/2006(H5N1)) neuraminidase by Michaelis Menten equation analysis | Antimicrob Agents Chemother 53: 3088-96 (2009) Article DOI: 10.1128/AAC.01667-08 BindingDB Entry DOI: 10.7270/Q2VM4CHC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM50591389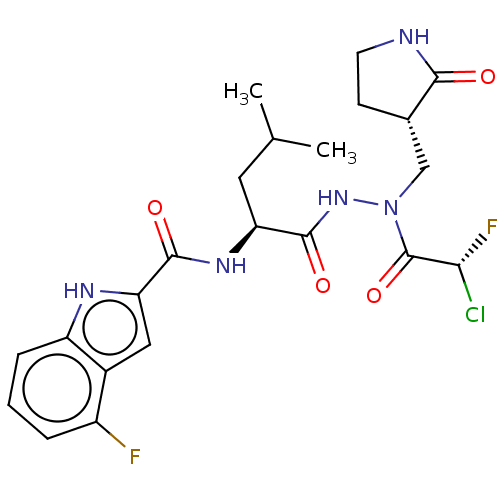 (CHEMBL5179778) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01081 BindingDB Entry DOI: 10.7270/Q2NK3K0J | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262661 (CHEMBL4075825) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM50591390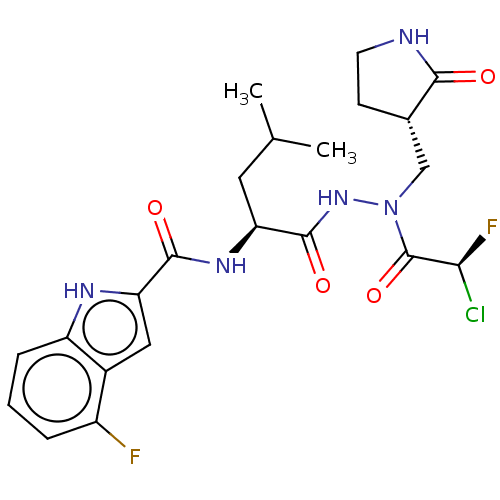 (CHEMBL5190754) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01081 BindingDB Entry DOI: 10.7270/Q2NK3K0J | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500766 (CHEMBL3754409) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated fo... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262660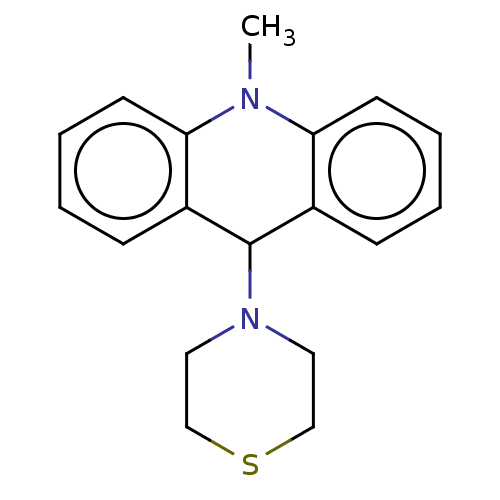 (CHEMBL4065259) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500766 (CHEMBL3754409) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate assessed as steady state inhibition constant pre... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262687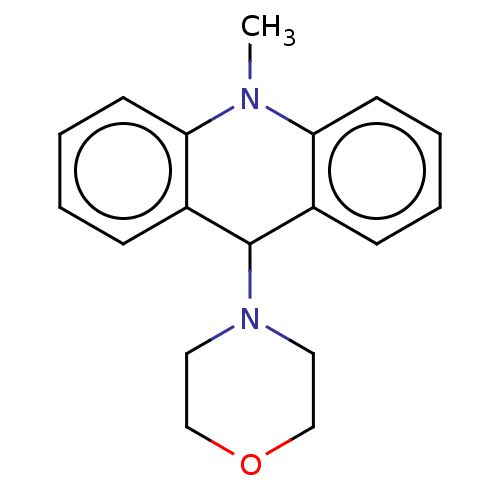 (CHEMBL4077169) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500752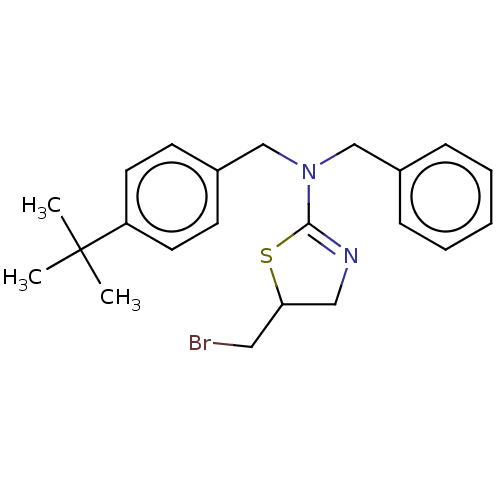 (CHEMBL3754327) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate assessed as steady state inhibition constant pre... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262637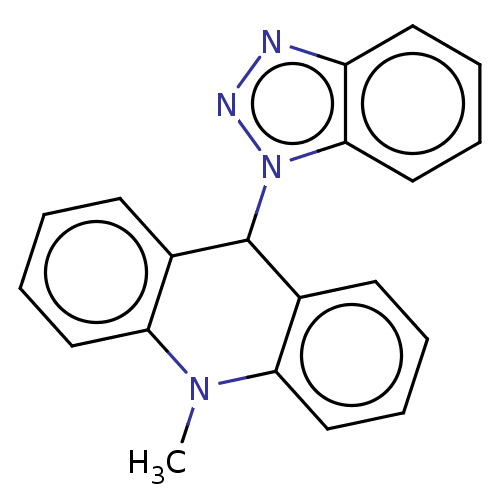 (CHEMBL4088659) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500752 (CHEMBL3754327) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated fo... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500760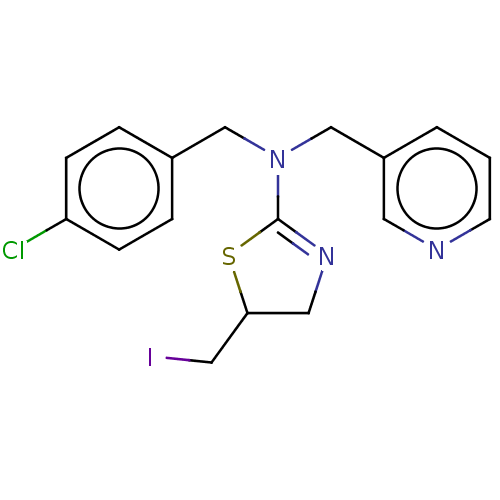 (CHEMBL3754622) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated fo... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262651 (CHEMBL4080726) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500766 (CHEMBL3754409) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500755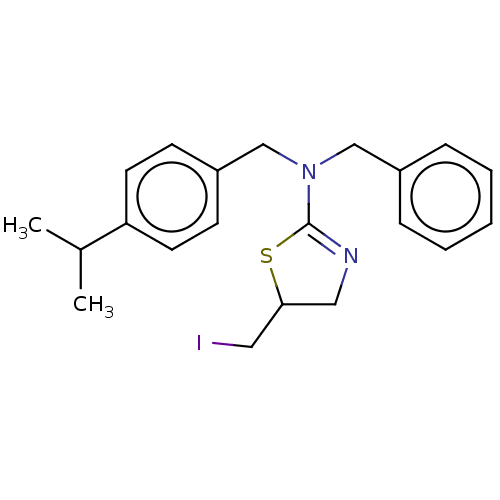 (CHEMBL3752466) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated fo... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262656 (CHEMBL4067342) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500756 (CHEMBL3752908) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500755 (CHEMBL3752466) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate assessed as steady state inhibition constant pre... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50262666 (CHEMBL4104952) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by Lineweaver-Burk double re... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50262661 (CHEMBL4075825) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by Lineweaver-Burk double re... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500757 (CHEMBL3752682) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate assessed as steady state inhibition constant pre... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500760 (CHEMBL3754622) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50262660 (CHEMBL4065259) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by Lineweaver-Burk double re... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50262687 (CHEMBL4077169) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by Lineweaver-Burk double re... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50262637 (CHEMBL4088659) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by Lineweaver-Burk double re... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500758 (CHEMBL3753216) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate assessed as steady state inhibition constant pre... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500755 (CHEMBL3752466) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50500748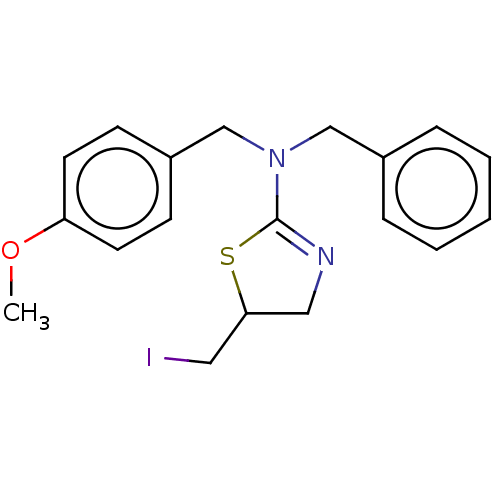 (CHEMBL3753156) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 7.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of porcine liver carboxylesterase using 4-nitrophenol acetate as substrate assessed as steady state inhibition constant pre... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500746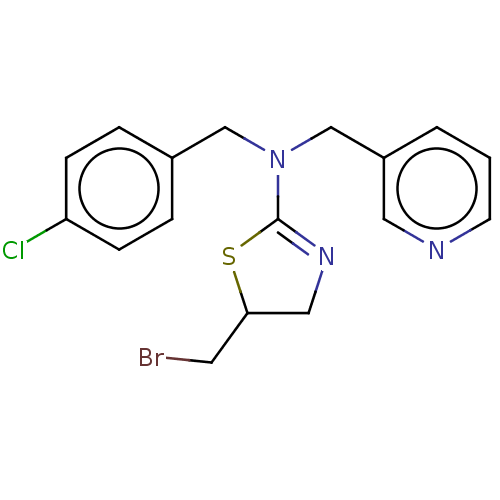 (CHEMBL3753531) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated fo... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50262651 (CHEMBL4080726) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by Lineweaver-Burk double re... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETD7 (Homo sapiens (Human)) | BDBM50017721 (1-Methyl-4-(5H-dibenzo(a,d)cycloheptenylidene)pipe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Curated by ChEMBL | Assay Description Inhibition of recombinant Set7/9 (unknown origin) expressed in Escherichia coli BL21 (DE3) using Ac-KRSK-MCA peptide/SAM as substrate preincubated fo... | J Med Chem 59: 3650-60 (2016) Article DOI: 10.1021/acs.jmedchem.5b01732 BindingDB Entry DOI: 10.7270/Q2X068Z4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262666 (CHEMBL4104952) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500746 (CHEMBL3753531) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences Curated by ChEMBL | Assay Description Noncompetitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate assessed as steady state inhibition constant preincubated... | Bioorg Med Chem 24: 1050-62 (2016) Article DOI: 10.1016/j.bmc.2016.01.031 BindingDB Entry DOI: 10.7270/Q2B85C4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM4994 ((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Laos/25/2006(H5N1)) neuraminidase isolated from virus-infected BALB/c mouse by fluorometric assay | Antimicrob Agents Chemother 53: 3088-96 (2009) Article DOI: 10.1128/AAC.01667-08 BindingDB Entry DOI: 10.7270/Q2VM4CHC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM4994 ((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Laos/25/2006(H5N1)) neuraminidase by fluorometric assay | Antimicrob Agents Chemother 53: 3088-96 (2009) Article DOI: 10.1128/AAC.01667-08 BindingDB Entry DOI: 10.7270/Q2VM4CHC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM4994 ((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Thailand/1(KAN-1)/2004(H5N1)) neuraminidase isolated from virus-infected BALB/c mouse by fluorometric assay | Antimicrob Agents Chemother 53: 3088-96 (2009) Article DOI: 10.1128/AAC.01667-08 BindingDB Entry DOI: 10.7270/Q2VM4CHC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM4994 ((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Thailand/1(KAN-1)/2004(H5N1)) neuraminidase by fluorometric assay | Antimicrob Agents Chemother 53: 3088-96 (2009) Article DOI: 10.1128/AAC.01667-08 BindingDB Entry DOI: 10.7270/Q2VM4CHC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50051031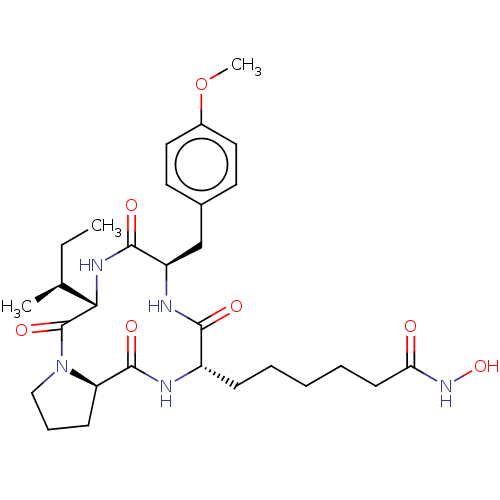 (CHEMBL2158745) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rajshahi Curated by ChEMBL | Assay Description Inhibition of human HDAC1 expressed in HEK293T cells assessed as aminomethyl coumarin release using Ac-KGLGK(Ac)-MCA) substrate after 30 mins by FLIP... | Bioorg Med Chem 22: 3862-70 (2014) Article DOI: 10.1016/j.bmc.2014.06.031 BindingDB Entry DOI: 10.7270/Q2K93953 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Mus musculus (Mouse)) | BDBM50366958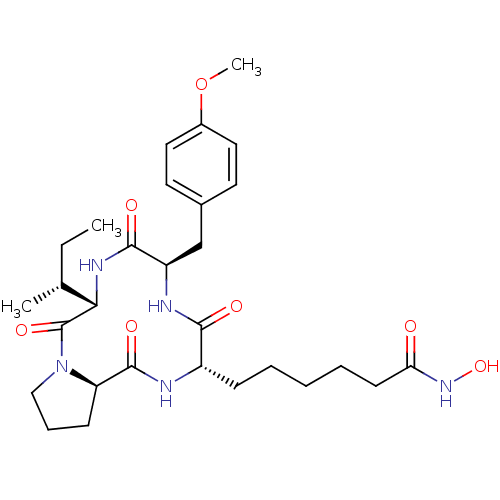 (CHEMBL1790587) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology Curated by ChEMBL | Assay Description Inhibitory activity against histone deacetylases (HDAC1) prepared from mouse melanoma B16/BL6 cells | Bioorg Med Chem Lett 14: 2427-31 (2004) Article DOI: 10.1016/j.bmcl.2004.03.018 BindingDB Entry DOI: 10.7270/Q20V8DC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM170524 (US9085540, 64) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. US Patent | Assay Description The phosphorylation activity on the peptide substrate of EGFR was investigated using LabChip (trademark) Systems (Caliper Life Sciences, Inc.). For t... | US Patent US9085540 (2015) BindingDB Entry DOI: 10.7270/Q2154FSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50452874 (CHEMBL4217620) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human recombinant BACE-1 (1 to 460 residue) using CEVNLDAEFK as substrate preincubated for 10 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116459 BindingDB Entry DOI: 10.7270/Q2057KSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM50012304 (2-{2-[2-[2-(2,2-Dimethyl-propionylamino)-3-(3H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition against Swiss 3T3 murine fibroblast cells. | J Med Chem 34: 2102-7 (1991) BindingDB Entry DOI: 10.7270/Q2X63KXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Laos/25/2006(H5N1)) neuraminidase isolated from virus-infected BALB/c mouse by fluorometric assay | Antimicrob Agents Chemother 53: 3088-96 (2009) Article DOI: 10.1128/AAC.01667-08 BindingDB Entry DOI: 10.7270/Q2VM4CHC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 771 total ) | Next | Last >> |