

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50005854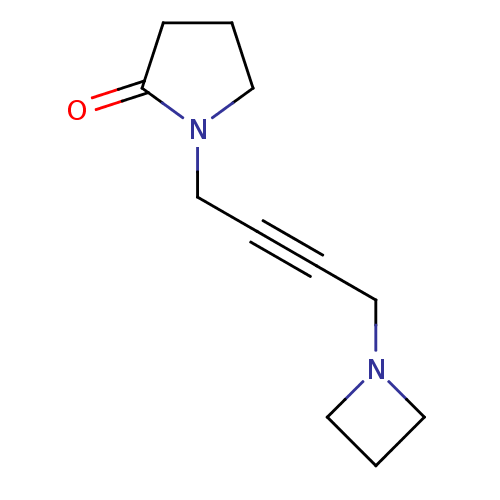 (1-(4-Azetidin-1-yl-but-2-ynyl)-pyrrolidin-2-one | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards muscarinic acetylcholine receptor M2 in rat brainstem | J Med Chem 35: 1550-7 (1992) BindingDB Entry DOI: 10.7270/Q2MK6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50004665 ((oxotremorine)1-(4-Pyrrolidin-1-yl-but-2-ynyl)-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards muscarinic acetylcholine receptor M2 in rat brainstem | J Med Chem 35: 1550-7 (1992) BindingDB Entry DOI: 10.7270/Q2MK6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50004665 ((oxotremorine)1-(4-Pyrrolidin-1-yl-but-2-ynyl)-pyr...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 196 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards muscarinic acetylcholine receptor in rat cortex | J Med Chem 35: 1550-7 (1992) BindingDB Entry DOI: 10.7270/Q2MK6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50455987 (CHEMBL2115360) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 636 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards M2 receptor in rat brainstem | J Med Chem 35: 1550-7 (1992) BindingDB Entry DOI: 10.7270/Q2MK6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50005851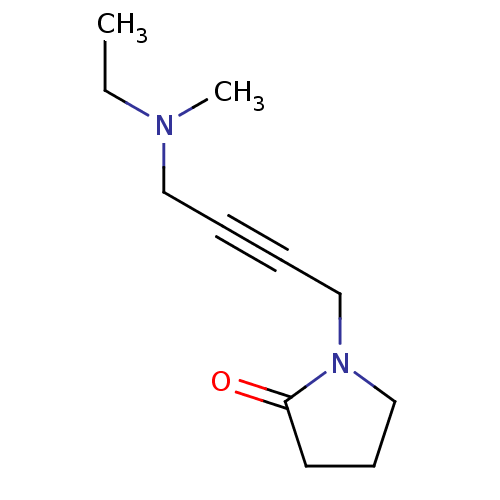 (1-[4-(Ethyl-methyl-amino)-but-2-ynyl]-pyrrolidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 813 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards muscarinic acetylcholine receptor M2 in rat brainstem | J Med Chem 35: 1550-7 (1992) BindingDB Entry DOI: 10.7270/Q2MK6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50005856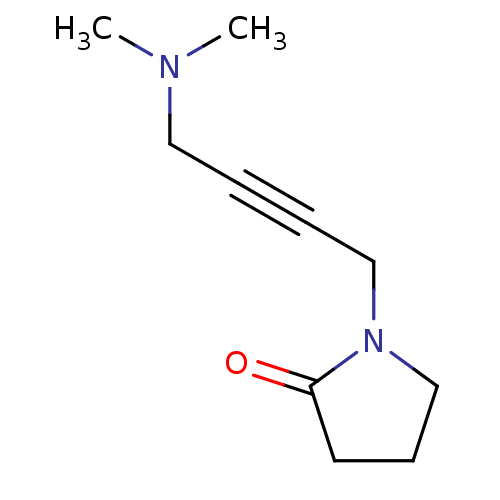 (1-(4-Dimethylamino-but-2-ynyl)-pyrrolidin-2-one | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 926 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards Muscarinic acetylcholine receptor M2 in rat brainstem | J Med Chem 35: 1550-7 (1992) BindingDB Entry DOI: 10.7270/Q2MK6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50455986 (CHEMBL2115361) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards M1 receptor in rat cortex | J Med Chem 35: 1550-7 (1992) BindingDB Entry DOI: 10.7270/Q2MK6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50005854 (1-(4-Azetidin-1-yl-but-2-ynyl)-pyrrolidin-2-one | ...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards muscarinic acetylcholine receptor in rat cortex | J Med Chem 35: 1550-7 (1992) BindingDB Entry DOI: 10.7270/Q2MK6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50455988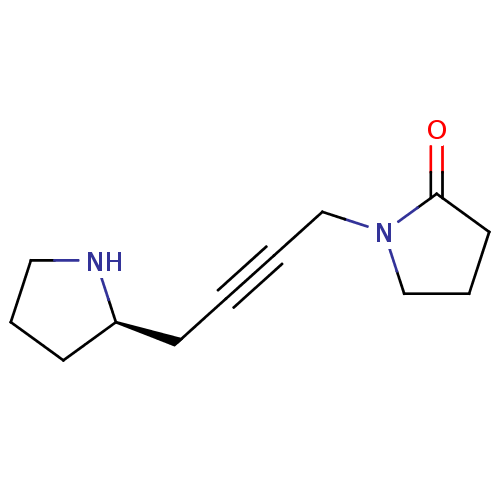 (CHEMBL2115359) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards M2 receptor in rat brainstem | J Med Chem 35: 1550-7 (1992) BindingDB Entry DOI: 10.7270/Q2MK6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50005851 (1-[4-(Ethyl-methyl-amino)-but-2-ynyl]-pyrrolidin-2...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards muscarinic acetylcholine receptor in rat cortex | J Med Chem 35: 1550-7 (1992) BindingDB Entry DOI: 10.7270/Q2MK6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50455987 (CHEMBL2115360) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards M1 receptor in rat cortex | J Med Chem 35: 1550-7 (1992) BindingDB Entry DOI: 10.7270/Q2MK6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50455988 (CHEMBL2115359) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards M1 receptor in rat cortex | J Med Chem 35: 1550-7 (1992) BindingDB Entry DOI: 10.7270/Q2MK6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50455986 (CHEMBL2115361) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards M2 receptor in rat brainstem | J Med Chem 35: 1550-7 (1992) BindingDB Entry DOI: 10.7270/Q2MK6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50455982 (CHEMBL2114427) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards M1 receptor in rat cortex | J Med Chem 35: 1550-7 (1992) BindingDB Entry DOI: 10.7270/Q2MK6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50455985 (CHEMBL2115362) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards M1 receptor in rat cortex | J Med Chem 35: 1550-7 (1992) BindingDB Entry DOI: 10.7270/Q2MK6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50455983 (CHEMBL2114425) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards M2 receptor in rat brainstem | J Med Chem 35: 1550-7 (1992) BindingDB Entry DOI: 10.7270/Q2MK6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50455985 (CHEMBL2115362) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards M2 receptor in rat brainstem | J Med Chem 35: 1550-7 (1992) BindingDB Entry DOI: 10.7270/Q2MK6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50455982 (CHEMBL2114427) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards M2 receptor in rat brainstem | J Med Chem 35: 1550-7 (1992) BindingDB Entry DOI: 10.7270/Q2MK6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50455984 (CHEMBL2114430) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards M1 receptor in rat cortex | J Med Chem 35: 1550-7 (1992) BindingDB Entry DOI: 10.7270/Q2MK6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50455984 (CHEMBL2114430) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards M2 receptor in rat brainstem | J Med Chem 35: 1550-7 (1992) BindingDB Entry DOI: 10.7270/Q2MK6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50455981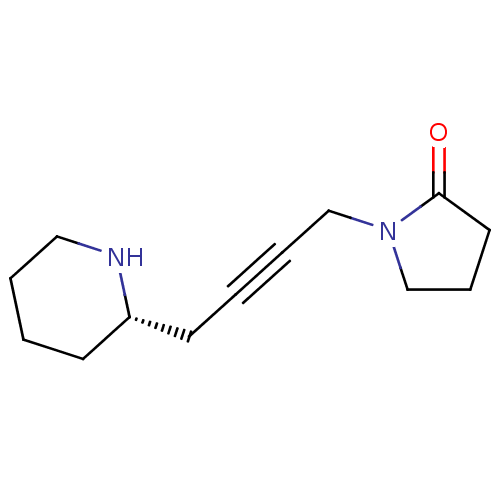 (CHEMBL2114426) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards M2 receptor in rat brainstem | J Med Chem 35: 1550-7 (1992) BindingDB Entry DOI: 10.7270/Q2MK6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50005856 (1-(4-Dimethylamino-but-2-ynyl)-pyrrolidin-2-one | ...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards Muscarinic acetylcholine receptor M1 in rat cortex | J Med Chem 35: 1550-7 (1992) BindingDB Entry DOI: 10.7270/Q2MK6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50455981 (CHEMBL2114426) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards M1 receptor in rat cortex | J Med Chem 35: 1550-7 (1992) BindingDB Entry DOI: 10.7270/Q2MK6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50005855 (1-(4-Methylamino-but-2-ynyl)-pyrrolidin-2-one | CH...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards muscarinic acetylcholine receptor M2 in rat brainstem | J Med Chem 35: 1550-7 (1992) BindingDB Entry DOI: 10.7270/Q2MK6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50455983 (CHEMBL2114425) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards M1 receptor in rat cortex | J Med Chem 35: 1550-7 (1992) BindingDB Entry DOI: 10.7270/Q2MK6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50005855 (1-(4-Methylamino-but-2-ynyl)-pyrrolidin-2-one | CH...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards muscarinic acetylcholine receptor in rat cortex | J Med Chem 35: 1550-7 (1992) BindingDB Entry DOI: 10.7270/Q2MK6BT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50044039 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-[(S)-2-[(...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50044033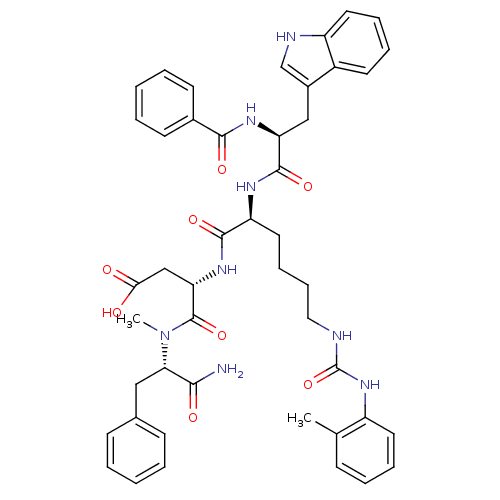 ((S)-3-[(S)-2-((S)-2-Benzoylamino-3-1H-indol-3-yl-p...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50044042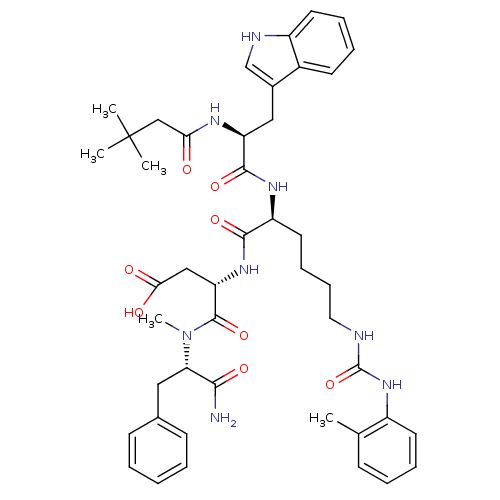 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-[(S)-2-[2...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50044028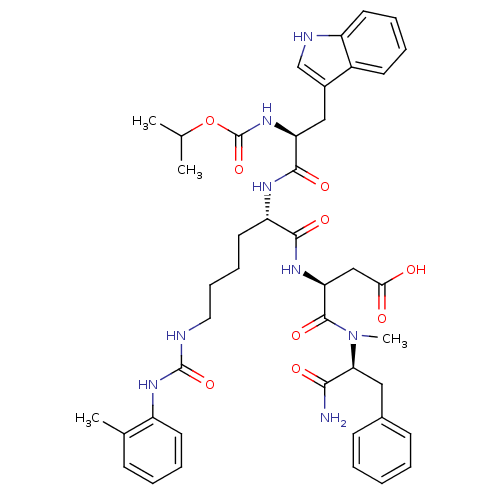 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-[(S)-2-((...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50044038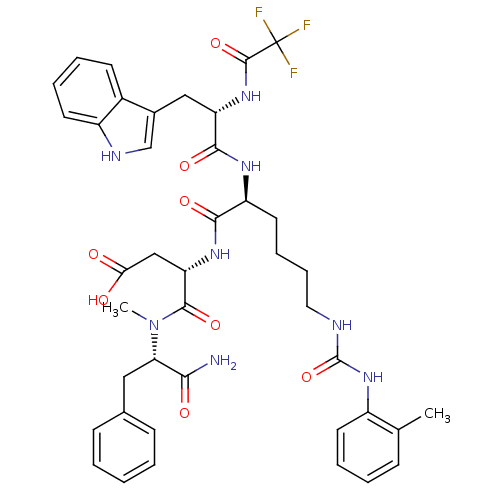 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-[(S)-2-[(...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50044044 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-[(S)-2-((...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50044032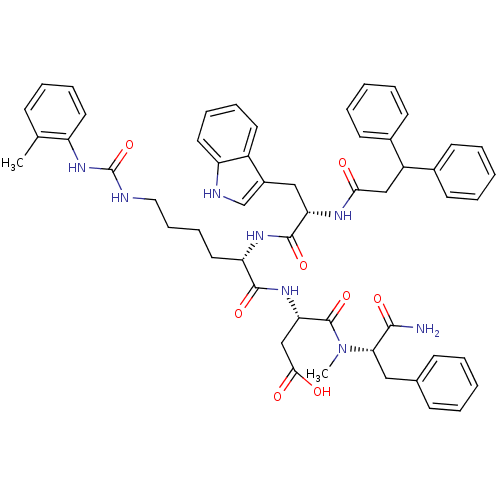 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-[(S)-2-[(...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50044037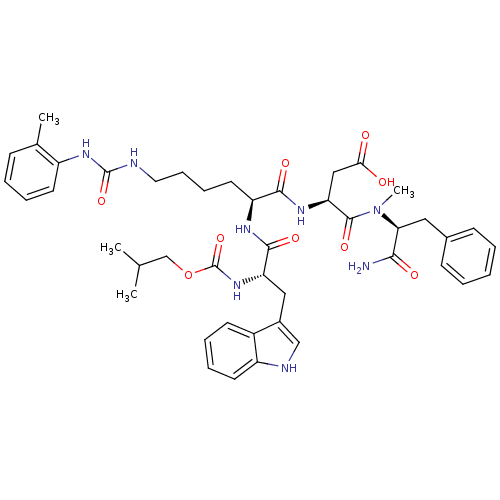 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-[(S)-2-((...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50044031 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-[(S)-2-[(...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50040523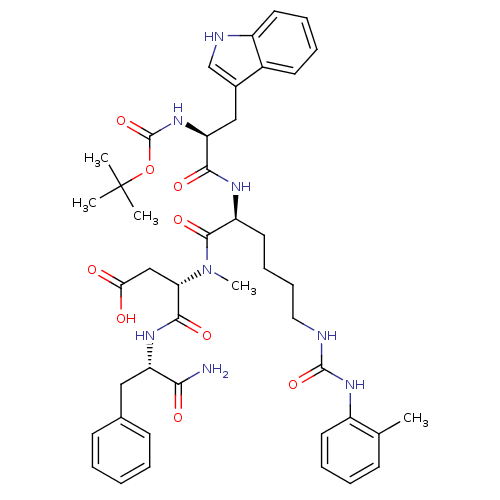 ((S)-3-{[(S)-2-[(S)-2-tert-Butoxycarbonylamino-3-(1...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50044025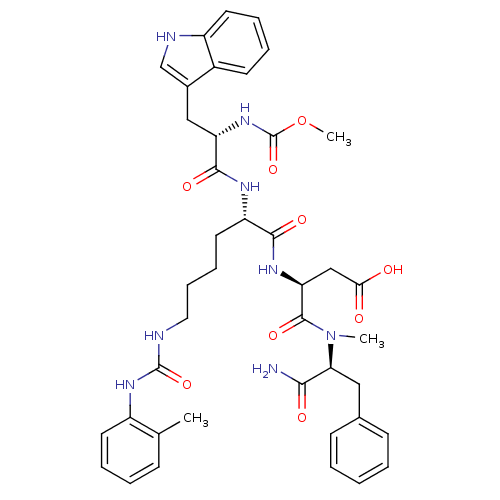 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-[(S)-2-[(...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50044043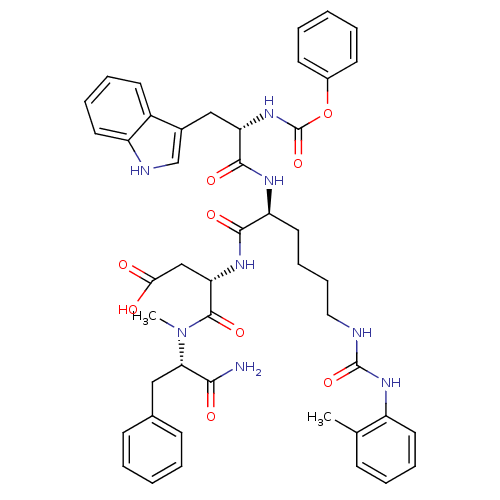 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-[(S)-2-((...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50044022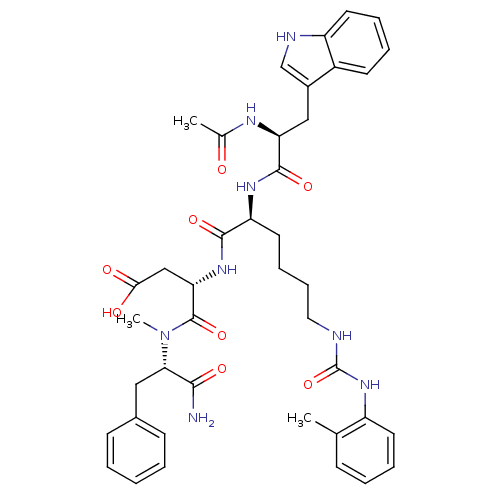 ((S)-3-[(S)-2-[(S)-2-Acetylamino-3-(1H-indol-3-yl)-...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50044024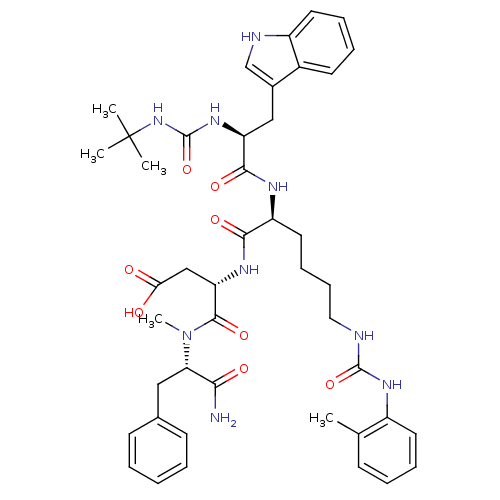 ((S)-3-[(S)-2-[2-((S)-3-tert-Butyl-ureido)-3-(1H-in...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50044040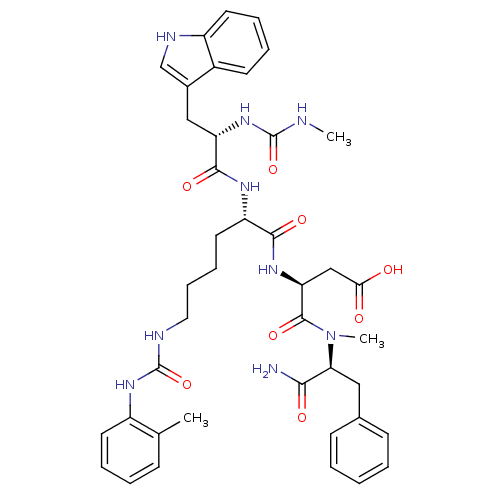 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-[(S)-2-[(...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50044036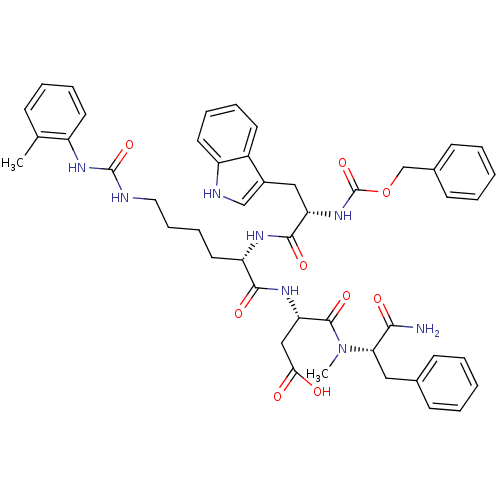 ((S)-3-[(S)-2-[(S)-2-Benzyloxycarbonylamino-3-(1H-i...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50041652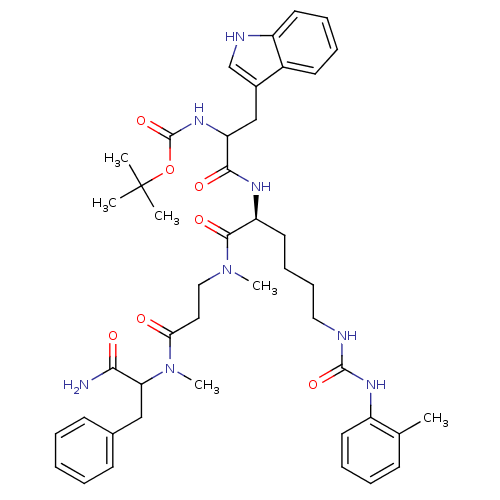 (CHEMBL41364 | [1-[(S)-1-({2-[(1-Carbamoyl-2-phenyl...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Concentration that inhibited 50% of specific binding of [125I]-Bolton-Hunter CCK-8 binding in guinea pig pancreas | J Med Chem 37: 1562-8 (1994) BindingDB Entry DOI: 10.7270/Q2WD3ZMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50044023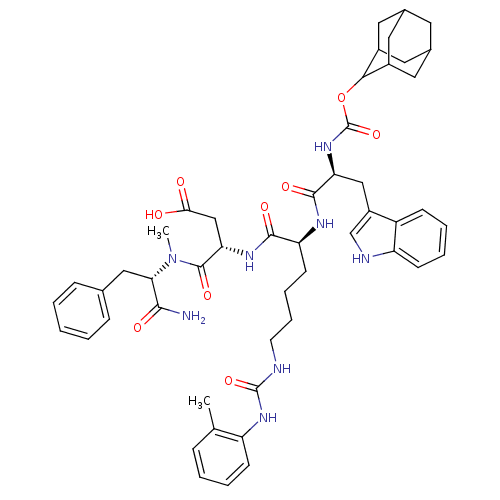 ((S)-3-[(S)-2-[(S)-2-(Adamantan-2-yloxycarbonylamin...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50044029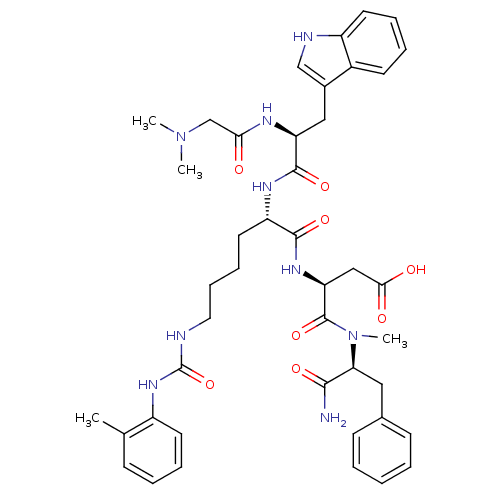 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-[(S)-2-[(...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50044030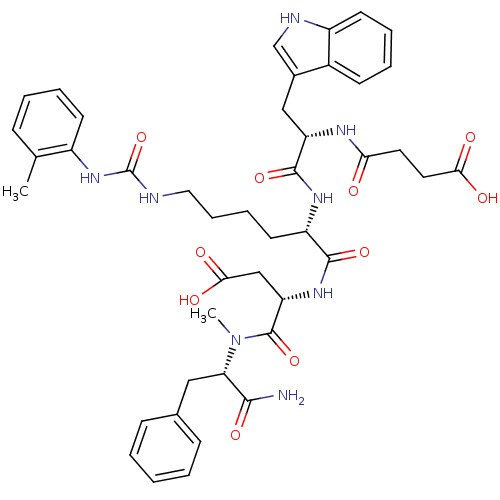 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-[(S)-2-[(...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50041650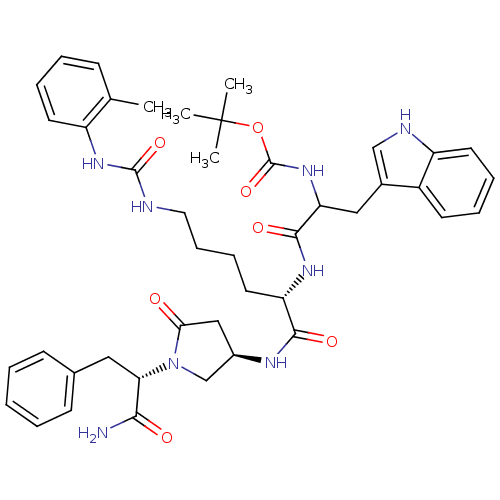 (CHEMBL39310 | [1-[(S)-1-[(R)-1-((S)-1-Carbamoyl-2-...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Concentration that inhibited 50% of specific binding of [125I]-Bolton-Hunter CCK-8 binding in guinea pig pancreas | J Med Chem 37: 1562-8 (1994) BindingDB Entry DOI: 10.7270/Q2WD3ZMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50044034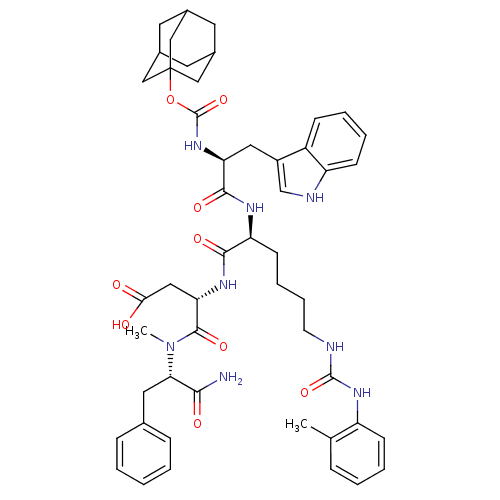 ((S)-3-[(S)-2-[(S)-2-(Adamantan-1-yloxycarbonylamin...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50041636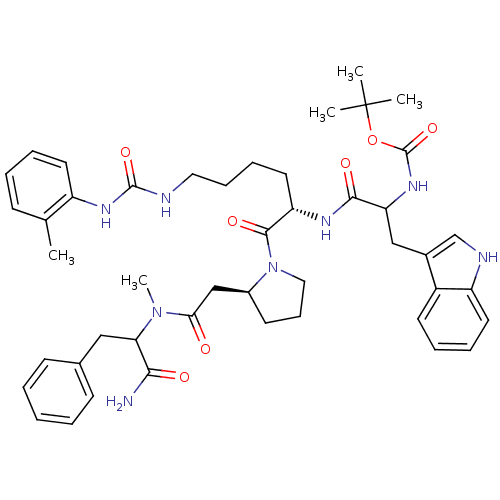 (CHEMBL289804 | [1-[(S)-1-((S)-2-{[(1-Carbamoyl-2-p...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Concentration that inhibited 50% of specific binding of [125I]-Bolton-Hunter CCK-8 binding in guinea pig pancreas | J Med Chem 37: 1562-8 (1994) BindingDB Entry DOI: 10.7270/Q2WD3ZMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50044026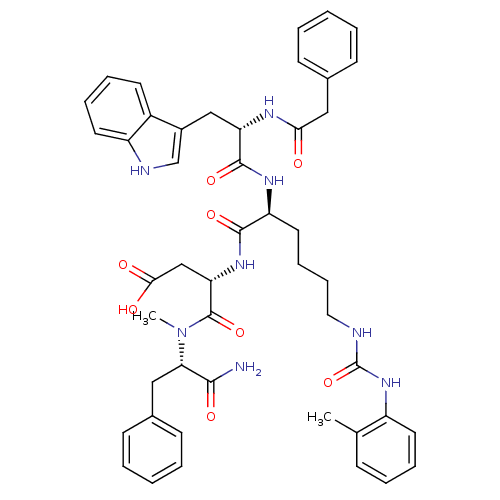 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-[(S)-2-((...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Bolton-Hunter CCK-8 binding to cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 309-13 (1994) BindingDB Entry DOI: 10.7270/Q2445N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 90 total ) | Next | Last >> |