 Found 126 hits with Last Name = 'simonnet' and Initial = 'y'
Found 126 hits with Last Name = 'simonnet' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598905
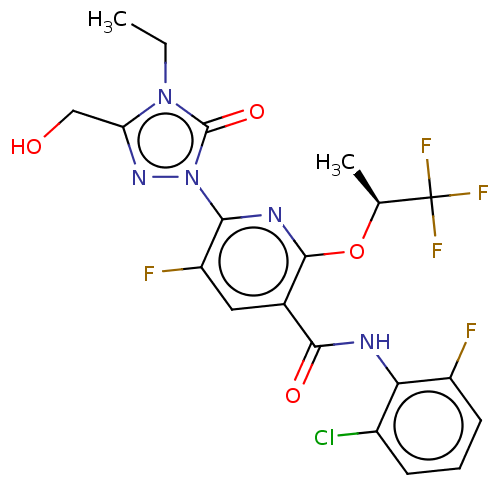
(CHEMBL5180161)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(F)cccc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598923
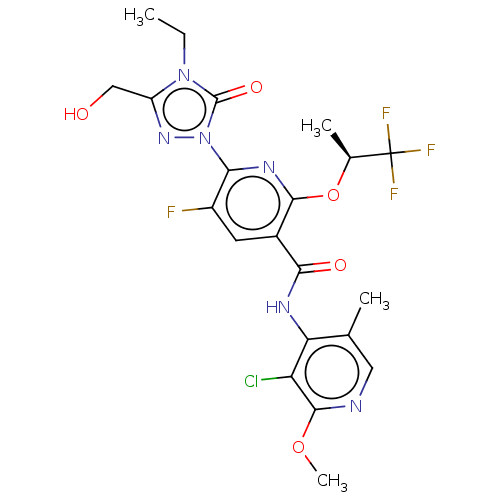
(CHEMBL5193821)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(C)cnc(OC)c2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598917
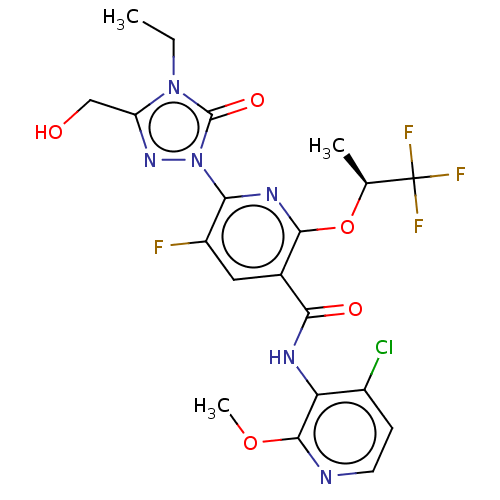
(CHEMBL5171223)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(Cl)ccnc2OC)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598902

(CHEMBL5187184)Show SMILES CCn1c(CO)nn(-c2nc(OC(C)C)c(cc2F)C(=O)Nc2c(F)cccc2Cl)c1=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50163592
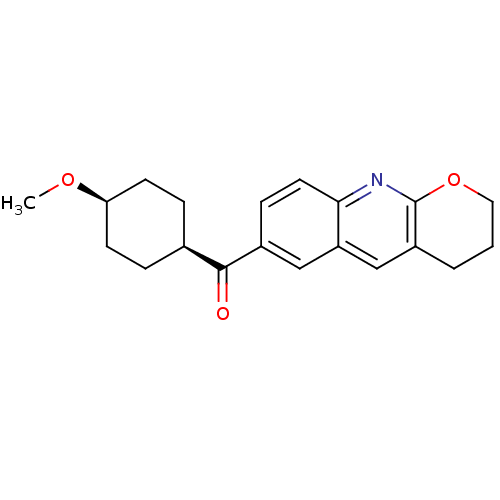
((3,4-Dihydro-2H-1-oxa-9-aza-anthracen-6-yl)-(4-met...)Show SMILES CO[C@H]1CC[C@H](CC1)C(=O)c1ccc2nc3OCCCc3cc2c1 |wU:5.8,2.1,(-8.6,-.89,;-7.27,-1.66,;-5.94,-.89,;-5.94,.65,;-4.61,1.42,;-3.28,.65,;-3.28,-.89,;-4.61,-1.66,;-1.95,1.42,;-1.98,2.96,;-.62,.67,;-.62,-.87,;.72,-1.64,;2.05,-.85,;3.39,-1.62,;4.72,-.82,;6.07,-1.57,;7.4,-.8,;7.38,.76,;6.03,1.51,;4.7,.72,;3.36,1.49,;2.04,.69,;.71,1.44,)| Show InChI InChI=1S/C20H23NO3/c1-23-17-7-4-13(5-8-17)19(22)14-6-9-18-16(11-14)12-15-3-2-10-24-20(15)21-18/h6,9,11-13,17H,2-5,7-8,10H2,1H3/t13-,17+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human metabotropic glutamate receptor |
J Med Chem 48: 2134-53 (2005)
Article DOI: 10.1021/jm049499o
BindingDB Entry DOI: 10.7270/Q2RR2017 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50163592

((3,4-Dihydro-2H-1-oxa-9-aza-anthracen-6-yl)-(4-met...)Show SMILES CO[C@H]1CC[C@H](CC1)C(=O)c1ccc2nc3OCCCc3cc2c1 |wU:5.8,2.1,(-8.6,-.89,;-7.27,-1.66,;-5.94,-.89,;-5.94,.65,;-4.61,1.42,;-3.28,.65,;-3.28,-.89,;-4.61,-1.66,;-1.95,1.42,;-1.98,2.96,;-.62,.67,;-.62,-.87,;.72,-1.64,;2.05,-.85,;3.39,-1.62,;4.72,-.82,;6.07,-1.57,;7.4,-.8,;7.38,.76,;6.03,1.51,;4.7,.72,;3.36,1.49,;2.04,.69,;.71,1.44,)| Show InChI InChI=1S/C20H23NO3/c1-23-17-7-4-13(5-8-17)19(22)14-6-9-18-16(11-14)12-15-3-2-10-24-20(15)21-18/h6,9,11-13,17H,2-5,7-8,10H2,1H3/t13-,17+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human metabotropic glutamate receptor |
J Med Chem 48: 2134-53 (2005)
Article DOI: 10.1021/jm049499o
BindingDB Entry DOI: 10.7270/Q2RR2017 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50163613
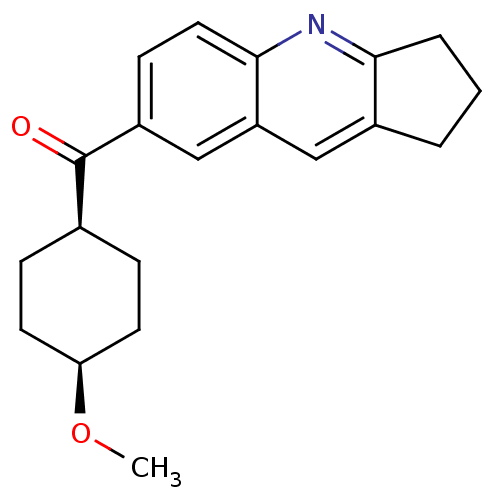
((2,3-Dihydro-1H-cyclopenta[b]quinolin-7-yl)-(4-met...)Show SMILES CO[C@H]1CC[C@H](CC1)C(=O)c1ccc2nc3CCCc3cc2c1 |wU:5.8,2.1,(-8.28,-.89,;-6.95,-1.66,;-5.62,-.89,;-5.62,.65,;-4.31,1.42,;-2.96,.65,;-2.96,-.89,;-4.31,-1.66,;-1.63,1.42,;-1.65,2.96,;-.3,.67,;-.3,-.87,;1.03,-1.64,;2.36,-.85,;3.72,-1.62,;5.05,-.82,;6.52,-1.29,;7.43,-.03,;6.49,1.23,;5.02,.74,;3.67,1.49,;2.36,.69,;1.01,1.44,)| Show InChI InChI=1S/C20H23NO2/c1-23-17-8-5-13(6-9-17)20(22)15-7-10-19-16(12-15)11-14-3-2-4-18(14)21-19/h7,10-13,17H,2-6,8-9H2,1H3/t13-,17+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human metabotropic glutamate receptor |
J Med Chem 48: 2134-53 (2005)
Article DOI: 10.1021/jm049499o
BindingDB Entry DOI: 10.7270/Q2RR2017 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598922

(CHEMBL5186161)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(C)cnc(OC)c2C)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598915

(CHEMBL5198030)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(Cl)ccnc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598926

(CHEMBL5206111)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(C)cc(OC)nc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50163596

((2-Amino-3-ethyl-quinolin-6-yl)-(4-methoxy-cyclohe...)Show SMILES CCc1cc2cc(ccc2nc1N)C(=O)C1CCC(CC1)OC |(7.7,.65,;6.37,1.42,;5.03,.65,;3.7,1.4,;2.37,.63,;1.04,1.4,;-.3,.63,;-.3,-.91,;1.04,-1.68,;2.37,-.91,;3.7,-1.68,;5.03,-.91,;6.39,-1.68,;-1.63,1.42,;-1.63,2.96,;-2.96,.65,;-4.3,1.42,;-5.63,.65,;-5.63,-.9,;-4.3,-1.67,;-2.96,-.9,;-6.96,-1.67,;-8.3,-.9,)| Show InChI InChI=1S/C19H24N2O2/c1-3-12-10-15-11-14(6-9-17(15)21-19(12)20)18(22)13-4-7-16(23-2)8-5-13/h6,9-11,13,16H,3-5,7-8H2,1-2H3,(H2,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human metabotropic glutamate receptor |
J Med Chem 48: 2134-53 (2005)
Article DOI: 10.1021/jm049499o
BindingDB Entry DOI: 10.7270/Q2RR2017 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598913

(CHEMBL5193591)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(C)ccnc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598920

(CHEMBL5209113)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(F)cnc(OC)c2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM470454
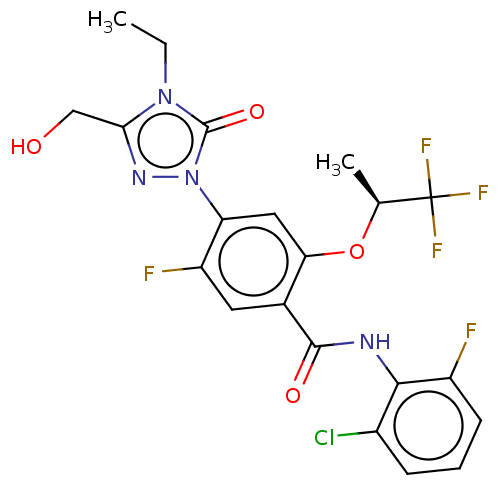
(N-(2-chloro-6-fluorophenyl)-4-[4-ethyl-3-(hydroxym...)Show SMILES CCn1c(CO)nn(-c2cc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(F)cccc2Cl)c1=O |r| Show InChI InChI=1S/C21H18ClF5N4O4/c1-3-30-17(9-32)29-31(20(30)34)15-8-16(35-10(2)21(25,26)27)11(7-14(15)24)19(33)28-18-12(22)5-4-6-13(18)23/h4-8,10,32H,3,9H2,1-2H3,(H,28,33)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598914
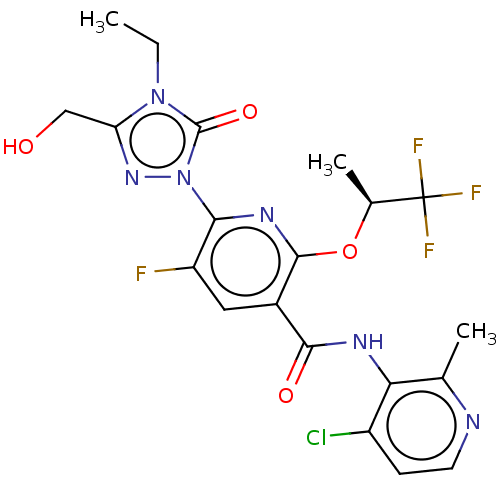
(CHEMBL5197263)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(C)nccc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50163617

((2,3-Dihydro-thieno[2,3-b]quinolin-6-yl)-(4-methox...)Show SMILES CO[C@H]1CC[C@H](CC1)C(=O)c1ccc2nc3SCCc3cc2c1 |wU:5.8,2.1,(-8.35,-.82,;-7.02,-1.59,;-5.67,-.82,;-5.67,.72,;-4.36,1.49,;-3.03,.72,;-3.03,-.82,;-4.36,-1.59,;-1.68,1.49,;-1.7,3.03,;-.34,.74,;-.34,-.82,;.98,-1.57,;2.31,-.78,;3.66,-1.56,;4.98,-.75,;6.32,-1.5,;7.65,-.71,;7.65,.83,;4.97,.79,;3.62,1.54,;2.31,.75,;.96,1.51,)| Show InChI InChI=1S/C19H21NO2S/c1-22-16-5-2-12(3-6-16)18(21)13-4-7-17-15(10-13)11-14-8-9-23-19(14)20-17/h4,7,10-12,16H,2-3,5-6,8-9H2,1H3/t12-,16+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human metabotropic glutamate receptor |
J Med Chem 48: 2134-53 (2005)
Article DOI: 10.1021/jm049499o
BindingDB Entry DOI: 10.7270/Q2RR2017 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50163617

((2,3-Dihydro-thieno[2,3-b]quinolin-6-yl)-(4-methox...)Show SMILES CO[C@H]1CC[C@H](CC1)C(=O)c1ccc2nc3SCCc3cc2c1 |wU:5.8,2.1,(-8.35,-.82,;-7.02,-1.59,;-5.67,-.82,;-5.67,.72,;-4.36,1.49,;-3.03,.72,;-3.03,-.82,;-4.36,-1.59,;-1.68,1.49,;-1.7,3.03,;-.34,.74,;-.34,-.82,;.98,-1.57,;2.31,-.78,;3.66,-1.56,;4.98,-.75,;6.32,-1.5,;7.65,-.71,;7.65,.83,;4.97,.79,;3.62,1.54,;2.31,.75,;.96,1.51,)| Show InChI InChI=1S/C19H21NO2S/c1-22-16-5-2-12(3-6-16)18(21)13-4-7-17-15(10-13)11-14-8-9-23-19(14)20-17/h4,7,10-12,16H,2-3,5-6,8-9H2,1H3/t12-,16+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat metabotropic glutamate receptor 1 |
J Med Chem 48: 2134-53 (2005)
Article DOI: 10.1021/jm049499o
BindingDB Entry DOI: 10.7270/Q2RR2017 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598919

(CHEMBL5176109)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(F)cncc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598916
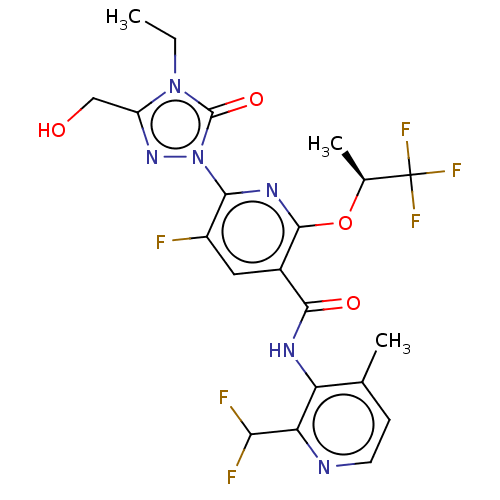
(CHEMBL5205042)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(C)ccnc2C(F)F)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598918
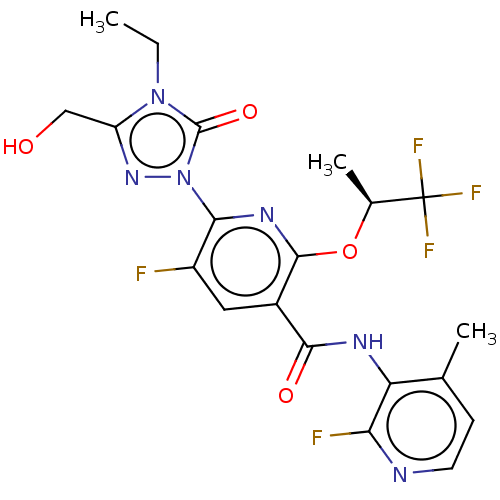
(CHEMBL5207423)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(C)ccnc2F)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598910
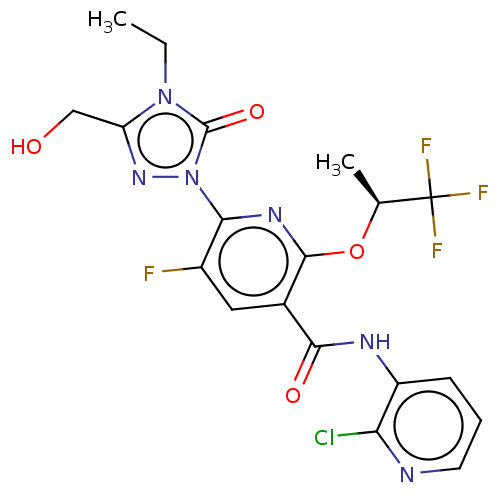
(CHEMBL5197809)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2cccnc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50163592

((3,4-Dihydro-2H-1-oxa-9-aza-anthracen-6-yl)-(4-met...)Show SMILES CO[C@H]1CC[C@H](CC1)C(=O)c1ccc2nc3OCCCc3cc2c1 |wU:5.8,2.1,(-8.6,-.89,;-7.27,-1.66,;-5.94,-.89,;-5.94,.65,;-4.61,1.42,;-3.28,.65,;-3.28,-.89,;-4.61,-1.66,;-1.95,1.42,;-1.98,2.96,;-.62,.67,;-.62,-.87,;.72,-1.64,;2.05,-.85,;3.39,-1.62,;4.72,-.82,;6.07,-1.57,;7.4,-.8,;7.38,.76,;6.03,1.51,;4.7,.72,;3.36,1.49,;2.04,.69,;.71,1.44,)| Show InChI InChI=1S/C20H23NO3/c1-23-17-7-4-13(5-8-17)19(22)14-6-9-18-16(11-14)12-15-3-2-10-24-20(15)21-18/h6,9,11-13,17H,2-5,7-8,10H2,1H3/t13-,17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat metabotropic glutamate receptor 1 |
J Med Chem 48: 2134-53 (2005)
Article DOI: 10.1021/jm049499o
BindingDB Entry DOI: 10.7270/Q2RR2017 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50163590

((2,3-Diethyl-quinolin-6-yl)-(4-methoxy-cyclohexyl)...)Show SMILES CCc1cc2cc(ccc2nc1CC)C(=O)C1CCC(CC1)OC |(7.38,.69,;6.04,1.46,;4.7,.68,;3.38,1.45,;2.05,.68,;.71,1.45,;-.61,.68,;-.63,-.86,;.71,-1.63,;2.05,-.86,;3.38,-1.63,;4.72,-.86,;6.04,-1.63,;7.38,-.86,;-1.95,1.45,;-1.94,2.99,;-3.29,.68,;-3.29,-.86,;-4.61,-1.62,;-5.94,-.86,;-5.94,.68,;-4.61,1.46,;-7.28,-1.63,;-8.6,-.86,)| Show InChI InChI=1S/C21H27NO2/c1-4-14-12-17-13-16(8-11-20(17)22-19(14)5-2)21(23)15-6-9-18(24-3)10-7-15/h8,11-13,15,18H,4-7,9-10H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat metabotropic glutamate receptor 1 |
J Med Chem 48: 2134-53 (2005)
Article DOI: 10.1021/jm049499o
BindingDB Entry DOI: 10.7270/Q2RR2017 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50163590

((2,3-Diethyl-quinolin-6-yl)-(4-methoxy-cyclohexyl)...)Show SMILES CCc1cc2cc(ccc2nc1CC)C(=O)C1CCC(CC1)OC |(7.38,.69,;6.04,1.46,;4.7,.68,;3.38,1.45,;2.05,.68,;.71,1.45,;-.61,.68,;-.63,-.86,;.71,-1.63,;2.05,-.86,;3.38,-1.63,;4.72,-.86,;6.04,-1.63,;7.38,-.86,;-1.95,1.45,;-1.94,2.99,;-3.29,.68,;-3.29,-.86,;-4.61,-1.62,;-5.94,-.86,;-5.94,.68,;-4.61,1.46,;-7.28,-1.63,;-8.6,-.86,)| Show InChI InChI=1S/C21H27NO2/c1-4-14-12-17-13-16(8-11-20(17)22-19(14)5-2)21(23)15-6-9-18(24-3)10-7-15/h8,11-13,15,18H,4-7,9-10H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat metabotropic glutamate receptor 1 |
J Med Chem 48: 2134-53 (2005)
Article DOI: 10.1021/jm049499o
BindingDB Entry DOI: 10.7270/Q2RR2017 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50163592

((3,4-Dihydro-2H-1-oxa-9-aza-anthracen-6-yl)-(4-met...)Show SMILES CO[C@H]1CC[C@H](CC1)C(=O)c1ccc2nc3OCCCc3cc2c1 |wU:5.8,2.1,(-8.6,-.89,;-7.27,-1.66,;-5.94,-.89,;-5.94,.65,;-4.61,1.42,;-3.28,.65,;-3.28,-.89,;-4.61,-1.66,;-1.95,1.42,;-1.98,2.96,;-.62,.67,;-.62,-.87,;.72,-1.64,;2.05,-.85,;3.39,-1.62,;4.72,-.82,;6.07,-1.57,;7.4,-.8,;7.38,.76,;6.03,1.51,;4.7,.72,;3.36,1.49,;2.04,.69,;.71,1.44,)| Show InChI InChI=1S/C20H23NO3/c1-23-17-7-4-13(5-8-17)19(22)14-6-9-18-16(11-14)12-15-3-2-10-24-20(15)21-18/h6,9,11-13,17H,2-5,7-8,10H2,1H3/t13-,17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat metabotropic glutamate receptor 1 |
J Med Chem 48: 2134-53 (2005)
Article DOI: 10.1021/jm049499o
BindingDB Entry DOI: 10.7270/Q2RR2017 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50163589
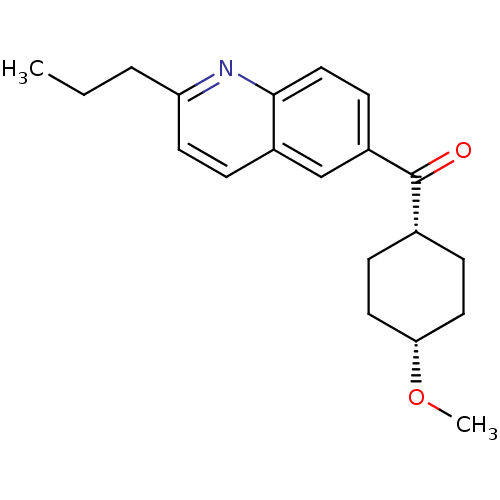
((4-Methoxy-cyclohexyl)-(2-propyl-quinolin-6-yl)-me...)Show SMILES CCCc1ccc2cc(ccc2n1)C(=O)[C@@H]1CC[C@@H](CC1)OC |wU:15.16,18.23,(8.94,-1.38,;7.59,-.61,;6.26,-1.41,;4.92,-.66,;4.91,.9,;3.55,1.65,;2.24,.86,;.89,1.61,;-.41,.83,;-.41,-.71,;.92,-1.48,;2.25,-.68,;3.6,-1.45,;-1.74,1.58,;-1.77,3.12,;-3.07,.81,;-4.43,1.58,;-5.73,.81,;-5.73,-.73,;-4.43,-1.5,;-3.07,-.73,;-7.06,-1.5,;-8.4,-.73,)| Show InChI InChI=1S/C20H25NO2/c1-3-4-17-9-5-15-13-16(8-12-19(15)21-17)20(22)14-6-10-18(23-2)11-7-14/h5,8-9,12-14,18H,3-4,6-7,10-11H2,1-2H3/t14-,18+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human metabotropic glutamate receptor |
J Med Chem 48: 2134-53 (2005)
Article DOI: 10.1021/jm049499o
BindingDB Entry DOI: 10.7270/Q2RR2017 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50163607

(3-ethyl-6-[(4-methoxycyclohexyl)carbonyl]quinoline...)Show SMILES CCc1cnc2ccc(cc2c1)C(=O)C1CCC(CC1)OC |(8,.58,;6.65,1.35,;5.33,.56,;5.34,-.98,;3.98,-1.75,;2.65,-.98,;1.32,-1.75,;-.02,-.98,;,.56,;1.33,1.33,;2.65,.56,;3.98,1.33,;-1.35,1.33,;-1.32,2.88,;-2.68,.56,;-2.68,-.98,;-4.01,-1.74,;-5.33,-.98,;-5.33,.56,;-4.01,1.35,;-6.68,-1.75,;-8.01,-.98,)| Show InChI InChI=1S/C19H23NO2/c1-3-13-10-16-11-15(6-9-18(16)20-12-13)19(21)14-4-7-17(22-2)8-5-14/h6,9-12,14,17H,3-5,7-8H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat metabotropic glutamate receptor 1 |
J Med Chem 48: 2134-53 (2005)
Article DOI: 10.1021/jm049499o
BindingDB Entry DOI: 10.7270/Q2RR2017 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50163613

((2,3-Dihydro-1H-cyclopenta[b]quinolin-7-yl)-(4-met...)Show SMILES CO[C@H]1CC[C@H](CC1)C(=O)c1ccc2nc3CCCc3cc2c1 |wU:5.8,2.1,(-8.28,-.89,;-6.95,-1.66,;-5.62,-.89,;-5.62,.65,;-4.31,1.42,;-2.96,.65,;-2.96,-.89,;-4.31,-1.66,;-1.63,1.42,;-1.65,2.96,;-.3,.67,;-.3,-.87,;1.03,-1.64,;2.36,-.85,;3.72,-1.62,;5.05,-.82,;6.52,-1.29,;7.43,-.03,;6.49,1.23,;5.02,.74,;3.67,1.49,;2.36,.69,;1.01,1.44,)| Show InChI InChI=1S/C20H23NO2/c1-23-17-8-5-13(6-9-17)20(22)15-7-10-19-16(12-15)11-14-3-2-4-18(14)21-19/h7,10-13,17H,2-6,8-9H2,1H3/t13-,17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat metabotropic glutamate receptor 1 |
J Med Chem 48: 2134-53 (2005)
Article DOI: 10.1021/jm049499o
BindingDB Entry DOI: 10.7270/Q2RR2017 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50163616
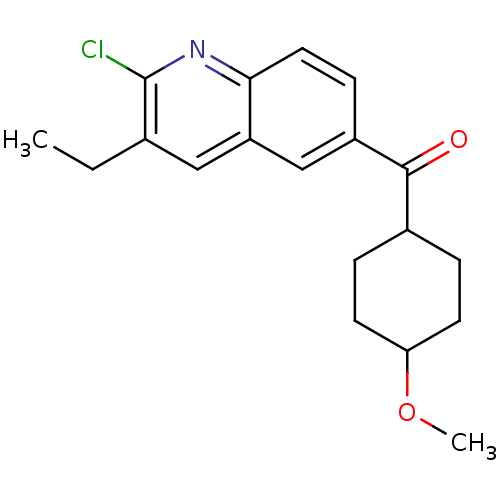
((2-Chloro-3-ethyl-quinolin-6-yl)-(4-methoxy-cycloh...)Show SMILES CCc1cc2cc(ccc2nc1Cl)C(=O)C1CCC(CC1)OC |(7.72,.65,;6.37,1.42,;5.03,.65,;3.7,1.42,;2.37,.65,;1.04,1.42,;-.3,.65,;-.3,-.9,;1.03,-1.67,;2.37,-.9,;3.7,-1.67,;5.05,-.9,;6.38,-1.67,;-1.63,1.42,;-1.61,2.96,;-2.97,.65,;-4.3,1.42,;-5.63,.65,;-5.63,-.9,;-4.3,-1.67,;-2.97,-.9,;-6.97,-1.67,;-8.3,-.9,)| Show InChI InChI=1S/C19H22ClNO2/c1-3-12-10-15-11-14(6-9-17(15)21-19(12)20)18(22)13-4-7-16(23-2)8-5-13/h6,9-11,13,16H,3-5,7-8H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human metabotropic glutamate receptor |
J Med Chem 48: 2134-53 (2005)
Article DOI: 10.1021/jm049499o
BindingDB Entry DOI: 10.7270/Q2RR2017 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598908

(CHEMBL5193091)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2cnccc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50163616

((2-Chloro-3-ethyl-quinolin-6-yl)-(4-methoxy-cycloh...)Show SMILES CCc1cc2cc(ccc2nc1Cl)C(=O)C1CCC(CC1)OC |(7.72,.65,;6.37,1.42,;5.03,.65,;3.7,1.42,;2.37,.65,;1.04,1.42,;-.3,.65,;-.3,-.9,;1.03,-1.67,;2.37,-.9,;3.7,-1.67,;5.05,-.9,;6.38,-1.67,;-1.63,1.42,;-1.61,2.96,;-2.97,.65,;-4.3,1.42,;-5.63,.65,;-5.63,-.9,;-4.3,-1.67,;-2.97,-.9,;-6.97,-1.67,;-8.3,-.9,)| Show InChI InChI=1S/C19H22ClNO2/c1-3-12-10-15-11-14(6-9-17(15)21-19(12)20)18(22)13-4-7-16(23-2)8-5-13/h6,9-11,13,16H,3-5,7-8H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat metabotropic glutamate receptor 1 |
J Med Chem 48: 2134-53 (2005)
Article DOI: 10.1021/jm049499o
BindingDB Entry DOI: 10.7270/Q2RR2017 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50163589

((4-Methoxy-cyclohexyl)-(2-propyl-quinolin-6-yl)-me...)Show SMILES CCCc1ccc2cc(ccc2n1)C(=O)[C@@H]1CC[C@@H](CC1)OC |wU:15.16,18.23,(8.94,-1.38,;7.59,-.61,;6.26,-1.41,;4.92,-.66,;4.91,.9,;3.55,1.65,;2.24,.86,;.89,1.61,;-.41,.83,;-.41,-.71,;.92,-1.48,;2.25,-.68,;3.6,-1.45,;-1.74,1.58,;-1.77,3.12,;-3.07,.81,;-4.43,1.58,;-5.73,.81,;-5.73,-.73,;-4.43,-1.5,;-3.07,-.73,;-7.06,-1.5,;-8.4,-.73,)| Show InChI InChI=1S/C20H25NO2/c1-3-4-17-9-5-15-13-16(8-12-19(15)21-17)20(22)14-6-10-18(23-2)11-7-14/h5,8-9,12-14,18H,3-4,6-7,10-11H2,1-2H3/t14-,18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat metabotropic glutamate receptor 1 |
J Med Chem 48: 2134-53 (2005)
Article DOI: 10.1021/jm049499o
BindingDB Entry DOI: 10.7270/Q2RR2017 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50163616

((2-Chloro-3-ethyl-quinolin-6-yl)-(4-methoxy-cycloh...)Show SMILES CCc1cc2cc(ccc2nc1Cl)C(=O)C1CCC(CC1)OC |(7.72,.65,;6.37,1.42,;5.03,.65,;3.7,1.42,;2.37,.65,;1.04,1.42,;-.3,.65,;-.3,-.9,;1.03,-1.67,;2.37,-.9,;3.7,-1.67,;5.05,-.9,;6.38,-1.67,;-1.63,1.42,;-1.61,2.96,;-2.97,.65,;-4.3,1.42,;-5.63,.65,;-5.63,-.9,;-4.3,-1.67,;-2.97,-.9,;-6.97,-1.67,;-8.3,-.9,)| Show InChI InChI=1S/C19H22ClNO2/c1-3-12-10-15-11-14(6-9-17(15)21-19(12)20)18(22)13-4-7-16(23-2)8-5-13/h6,9-11,13,16H,3-5,7-8H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat metabotropic glutamate receptor 1 |
J Med Chem 48: 2134-53 (2005)
Article DOI: 10.1021/jm049499o
BindingDB Entry DOI: 10.7270/Q2RR2017 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598907
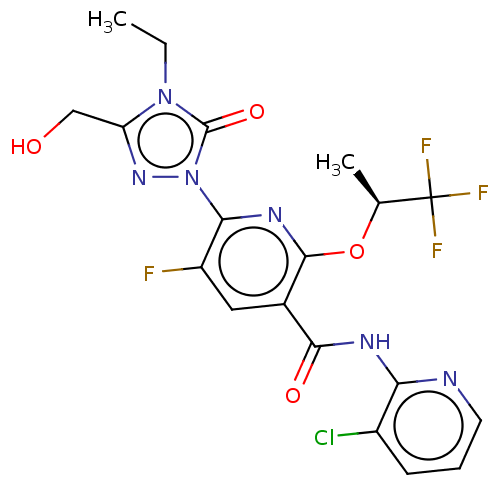
(CHEMBL5178023)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2ncccc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598912

(CHEMBL5170313)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2cc(C)cnc2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50163606

(1-(3,4-Dihydro-2H-1-oxa-9-aza-anthracen-6-yl)-2-ph...)Show InChI InChI=1S/C20H17NO2/c22-19(11-14-5-2-1-3-6-14)15-8-9-18-17(12-15)13-16-7-4-10-23-20(16)21-18/h1-3,5-6,8-9,12-13H,4,7,10-11H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat metabotropic glutamate receptor 1 |
J Med Chem 48: 2134-53 (2005)
Article DOI: 10.1021/jm049499o
BindingDB Entry DOI: 10.7270/Q2RR2017 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50163609

((4-Methoxy-cyclohexyl)-(2-methyl-3-propyl-quinolin...)Show SMILES CCCc1cc2cc(ccc2nc1C)C(=O)[C@@H]1CC[C@@H](CC1)OC |wU:16.17,19.24,(8.65,1.46,;7.33,.67,;5.99,1.42,;4.66,.65,;3.3,1.39,;1.98,.6,;.64,1.35,;-.67,.56,;-.67,-.97,;.66,-1.74,;1.99,-.94,;3.35,-1.71,;4.67,-.92,;6.01,-1.68,;-2.01,1.31,;-2.02,2.85,;-3.33,.55,;-4.69,1.31,;-5.99,.55,;-5.99,-1,;-4.69,-1.76,;-3.33,-1,;-7.33,-1.76,;-8.68,-1,)| Show InChI InChI=1S/C21H27NO2/c1-4-5-16-12-18-13-17(8-11-20(18)22-14(16)2)21(23)15-6-9-19(24-3)10-7-15/h8,11-13,15,19H,4-7,9-10H2,1-3H3/t15-,19+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human metabotropic glutamate receptor |
J Med Chem 48: 2134-53 (2005)
Article DOI: 10.1021/jm049499o
BindingDB Entry DOI: 10.7270/Q2RR2017 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598906

(CHEMBL5181951)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2ccccc2F)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50163601

((2,3-Dihydro-benzo[1,4]dioxin-2-yl)-(3-ethyl-2-met...)Show InChI InChI=1S/C21H19NO3/c1-3-14-10-16-11-15(8-9-17(16)22-13(14)2)21(23)20-12-24-18-6-4-5-7-19(18)25-20/h4-11,20H,3,12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human metabotropic glutamate receptor |
J Med Chem 48: 2134-53 (2005)
Article DOI: 10.1021/jm049499o
BindingDB Entry DOI: 10.7270/Q2RR2017 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50163609

((4-Methoxy-cyclohexyl)-(2-methyl-3-propyl-quinolin...)Show SMILES CCCc1cc2cc(ccc2nc1C)C(=O)[C@@H]1CC[C@@H](CC1)OC |wU:16.17,19.24,(8.65,1.46,;7.33,.67,;5.99,1.42,;4.66,.65,;3.3,1.39,;1.98,.6,;.64,1.35,;-.67,.56,;-.67,-.97,;.66,-1.74,;1.99,-.94,;3.35,-1.71,;4.67,-.92,;6.01,-1.68,;-2.01,1.31,;-2.02,2.85,;-3.33,.55,;-4.69,1.31,;-5.99,.55,;-5.99,-1,;-4.69,-1.76,;-3.33,-1,;-7.33,-1.76,;-8.68,-1,)| Show InChI InChI=1S/C21H27NO2/c1-4-5-16-12-18-13-17(8-11-20(18)22-14(16)2)21(23)15-6-9-19(24-3)10-7-15/h8,11-13,15,19H,4-7,9-10H2,1-3H3/t15-,19+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat metabotropic glutamate receptor 1 |
J Med Chem 48: 2134-53 (2005)
Article DOI: 10.1021/jm049499o
BindingDB Entry DOI: 10.7270/Q2RR2017 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50163611
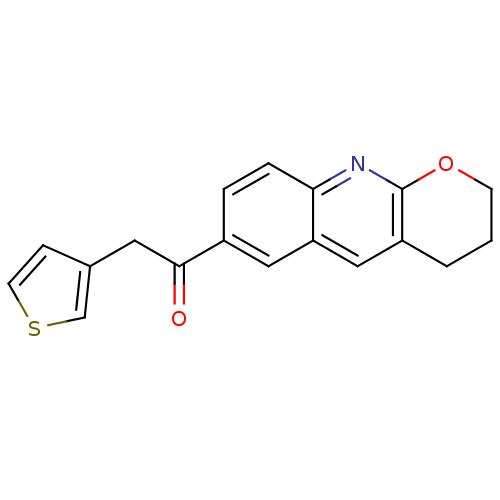
(1-(3,4-Dihydro-2H-1-oxa-9-aza-anthracen-6-yl)-2-th...)Show InChI InChI=1S/C18H15NO2S/c20-17(8-12-5-7-22-11-12)13-3-4-16-15(9-13)10-14-2-1-6-21-18(14)19-16/h3-5,7,9-11H,1-2,6,8H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat metabotropic glutamate receptor 1 |
J Med Chem 48: 2134-53 (2005)
Article DOI: 10.1021/jm049499o
BindingDB Entry DOI: 10.7270/Q2RR2017 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50163590

((2,3-Diethyl-quinolin-6-yl)-(4-methoxy-cyclohexyl)...)Show SMILES CCc1cc2cc(ccc2nc1CC)C(=O)C1CCC(CC1)OC |(7.38,.69,;6.04,1.46,;4.7,.68,;3.38,1.45,;2.05,.68,;.71,1.45,;-.61,.68,;-.63,-.86,;.71,-1.63,;2.05,-.86,;3.38,-1.63,;4.72,-.86,;6.04,-1.63,;7.38,-.86,;-1.95,1.45,;-1.94,2.99,;-3.29,.68,;-3.29,-.86,;-4.61,-1.62,;-5.94,-.86,;-5.94,.68,;-4.61,1.46,;-7.28,-1.63,;-8.6,-.86,)| Show InChI InChI=1S/C21H27NO2/c1-4-14-12-17-13-16(8-11-20(17)22-19(14)5-2)21(23)15-6-9-18(24-3)10-7-15/h8,11-13,15,18H,4-7,9-10H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human metabotropic glutamate receptor |
J Med Chem 48: 2134-53 (2005)
Article DOI: 10.1021/jm049499o
BindingDB Entry DOI: 10.7270/Q2RR2017 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50163615
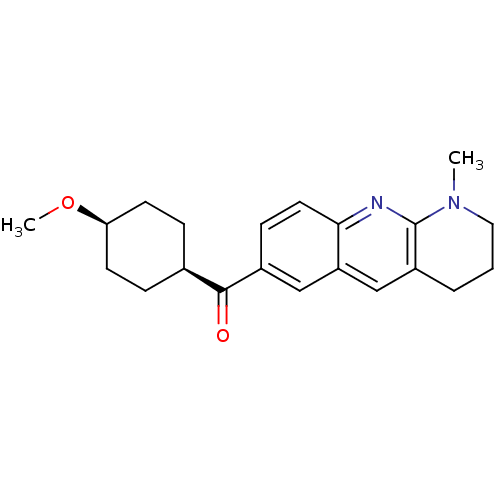
((4-Methoxy-cyclohexyl)-(1-methyl-1,2,3,4-tetrahydr...)Show SMILES CO[C@H]1CC[C@H](CC1)C(=O)c1ccc2nc3N(C)CCCc3cc2c1 |wU:5.8,2.1,(-8.84,-.78,;-7.51,-1.55,;-6.18,-.78,;-6.18,.76,;-4.85,1.53,;-3.52,.76,;-3.52,-.78,;-4.85,-1.55,;-2.19,1.53,;-2.22,3.07,;-.86,.79,;-.86,-.75,;.47,-1.52,;1.8,-.73,;3.15,-1.49,;4.48,-.7,;5.82,-1.45,;5.83,-2.99,;7.15,-.66,;7.12,.88,;5.79,1.65,;4.44,.84,;3.11,1.61,;1.8,.82,;.45,1.58,)| Show InChI InChI=1S/C21H26N2O2/c1-23-11-3-4-16-13-17-12-15(7-10-19(17)22-21(16)23)20(24)14-5-8-18(25-2)9-6-14/h7,10,12-14,18H,3-6,8-9,11H2,1-2H3/t14-,18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat metabotropic glutamate receptor 1 |
J Med Chem 48: 2134-53 (2005)
Article DOI: 10.1021/jm049499o
BindingDB Entry DOI: 10.7270/Q2RR2017 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50163623
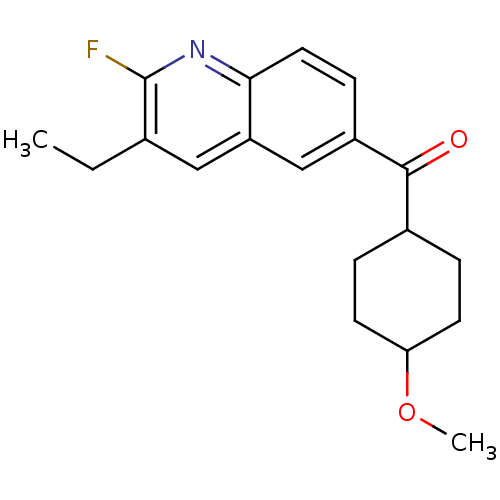
((3-Ethyl-2-fluoro-quinolin-6-yl)-(4-methoxy-cycloh...)Show SMILES CCc1cc2cc(ccc2nc1F)C(=O)C1CCC(CC1)OC |(7.72,.65,;6.37,1.42,;5.03,.65,;3.7,1.42,;2.37,.65,;1.04,1.42,;-.3,.65,;-.3,-.9,;1.03,-1.67,;2.37,-.9,;3.7,-1.67,;5.05,-.9,;6.38,-1.67,;-1.63,1.42,;-1.61,2.96,;-2.97,.65,;-4.3,1.42,;-5.63,.65,;-5.63,-.9,;-4.3,-1.67,;-2.97,-.9,;-6.97,-1.67,;-8.3,-.9,)| Show InChI InChI=1S/C19H22FNO2/c1-3-12-10-15-11-14(6-9-17(15)21-19(12)20)18(22)13-4-7-16(23-2)8-5-13/h6,9-11,13,16H,3-5,7-8H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human metabotropic glutamate receptor |
J Med Chem 48: 2134-53 (2005)
Article DOI: 10.1021/jm049499o
BindingDB Entry DOI: 10.7270/Q2RR2017 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Mus musculus) | BDBM50598923

(CHEMBL5193821)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2c(C)cnc(OC)c2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598924
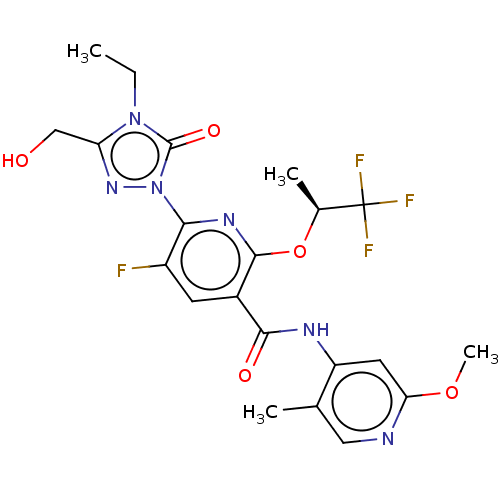
(CHEMBL5201357)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2cc(OC)ncc2C)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50163604
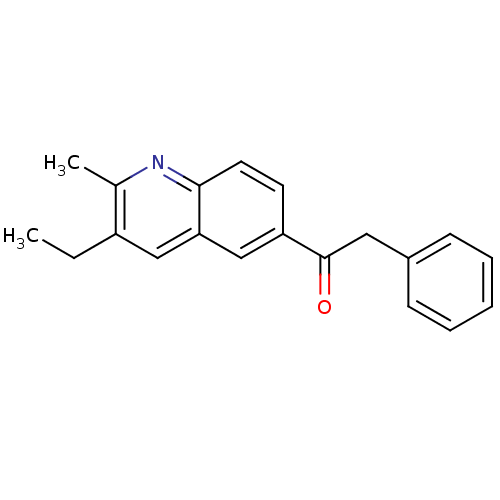
(1-(3-Ethyl-2-methyl-quinolin-6-yl)-2-phenyl-ethano...)Show InChI InChI=1S/C20H19NO/c1-3-16-12-18-13-17(9-10-19(18)21-14(16)2)20(22)11-15-7-5-4-6-8-15/h4-10,12-13H,3,11H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat metabotropic glutamate receptor 1 |
J Med Chem 48: 2134-53 (2005)
Article DOI: 10.1021/jm049499o
BindingDB Entry DOI: 10.7270/Q2RR2017 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50598925

(CHEMBL5197655)Show SMILES CCn1c(CO)nn(-c2nc(O[C@@H](C)C(F)(F)F)c(cc2F)C(=O)Nc2ccnc(OC)c2Cl)c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00788
BindingDB Entry DOI: 10.7270/Q26M3BWM |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50163584
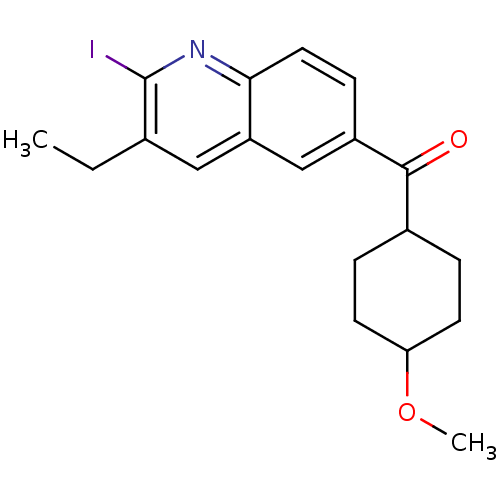
((3-Ethyl-2-iodo-quinolin-6-yl)-(4-methoxy-cyclohex...)Show SMILES CCc1cc2cc(ccc2nc1I)C(=O)C1CCC(CC1)OC |(7.72,.65,;6.37,1.42,;5.03,.65,;3.7,1.42,;2.37,.65,;1.04,1.42,;-.3,.65,;-.3,-.9,;1.03,-1.67,;2.37,-.9,;3.7,-1.67,;5.05,-.9,;6.38,-1.67,;-1.63,1.42,;-1.61,2.96,;-2.97,.65,;-4.3,1.42,;-5.63,.65,;-5.63,-.9,;-4.3,-1.67,;-2.97,-.9,;-6.97,-1.67,;-8.3,-.9,)| Show InChI InChI=1S/C19H22INO2/c1-3-12-10-15-11-14(6-9-17(15)21-19(12)20)18(22)13-4-7-16(23-2)8-5-13/h6,9-11,13,16H,3-5,7-8H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat metabotropic glutamate receptor 1 |
J Med Chem 48: 2134-53 (2005)
Article DOI: 10.1021/jm049499o
BindingDB Entry DOI: 10.7270/Q2RR2017 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50163599

(Bicyclo[2.2.1]hept-2-yl-(3-ethyl-2-methyl-quinolin...)Show InChI InChI=1S/C20H23NO/c1-3-14-10-17-11-16(6-7-19(17)21-12(14)2)20(22)18-9-13-4-5-15(18)8-13/h6-7,10-11,13,15,18H,3-5,8-9H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat metabotropic glutamate receptor 1 |
J Med Chem 48: 2134-53 (2005)
Article DOI: 10.1021/jm049499o
BindingDB Entry DOI: 10.7270/Q2RR2017 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data