

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50531739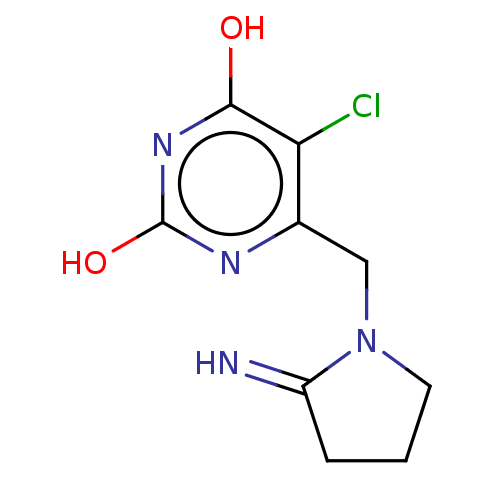 (MA-1 | TPI (freebase) | Tipiracil) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa Curated by ChEMBL | Assay Description Competitive inhibition of thymidine phosphorylase (unknown origin) | J Med Chem 62: 1231-1245 (2019) Article DOI: 10.1021/acs.jmedchem.8b01305 BindingDB Entry DOI: 10.7270/Q2FT8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50531739 (MA-1 | TPI (freebase) | Tipiracil) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa Curated by ChEMBL | Assay Description Inhibition of thymidine phosphorylase (unknown origin) | J Med Chem 62: 1231-1245 (2019) Article DOI: 10.1021/acs.jmedchem.8b01305 BindingDB Entry DOI: 10.7270/Q2FT8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50122770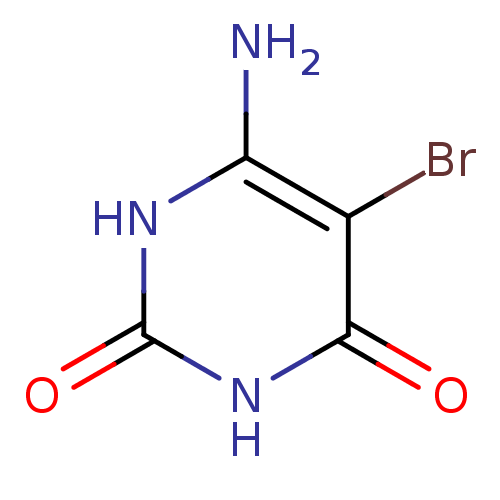 (6-Amino-5-bromo-1H-pyrimidine-2,4-dione | 6-Amino-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa Curated by ChEMBL | Assay Description Inhibition of thymidine phosphorylase (unknown origin) | J Med Chem 62: 1231-1245 (2019) Article DOI: 10.1021/acs.jmedchem.8b01305 BindingDB Entry DOI: 10.7270/Q2FT8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50531732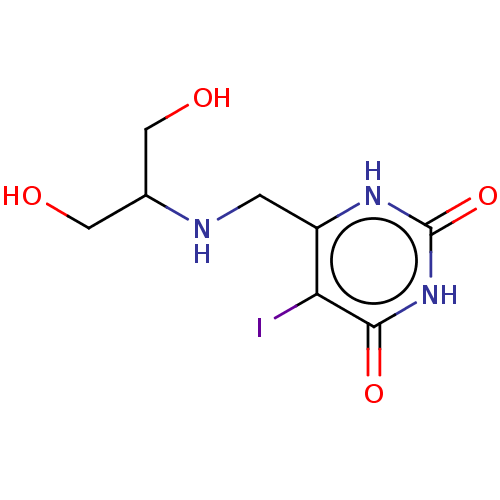 (CHEMBL4455140) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa Curated by ChEMBL | Assay Description Inhibition of recombinant human thymidine phosphorylase expressed in Escherichia coli Rosetta (DE3) cells using thymidine as substrate after 1 min by... | J Med Chem 62: 1231-1245 (2019) Article DOI: 10.1021/acs.jmedchem.8b01305 BindingDB Entry DOI: 10.7270/Q2FT8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50531729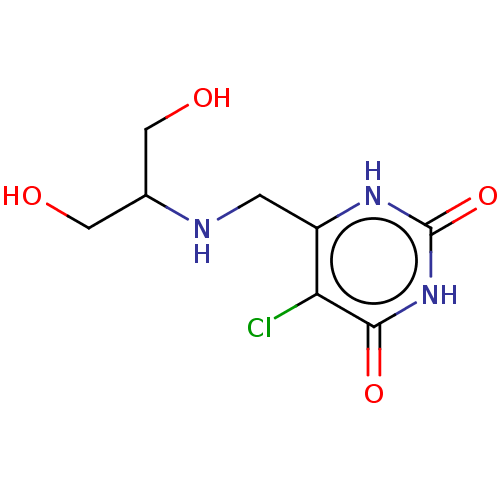 (CHEMBL4469338) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa Curated by ChEMBL | Assay Description Inhibition of recombinant human thymidine phosphorylase expressed in Escherichia coli Rosetta (DE3) cells using thymidine as substrate after 1 min by... | J Med Chem 62: 1231-1245 (2019) Article DOI: 10.1021/acs.jmedchem.8b01305 BindingDB Entry DOI: 10.7270/Q2FT8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50531734 (CHEMBL4468378) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa Curated by ChEMBL | Assay Description Inhibition of recombinant human thymidine phosphorylase expressed in Escherichia coli Rosetta (DE3) cells using thymidine as substrate after 1 min by... | J Med Chem 62: 1231-1245 (2019) Article DOI: 10.1021/acs.jmedchem.8b01305 BindingDB Entry DOI: 10.7270/Q2FT8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50531742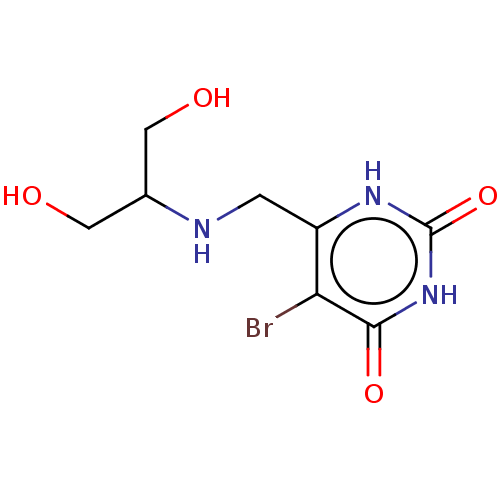 (CHEMBL4579188) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa Curated by ChEMBL | Assay Description Inhibition of recombinant human thymidine phosphorylase expressed in Escherichia coli Rosetta (DE3) cells using thymidine as substrate after 1 min by... | J Med Chem 62: 1231-1245 (2019) Article DOI: 10.1021/acs.jmedchem.8b01305 BindingDB Entry DOI: 10.7270/Q2FT8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50531736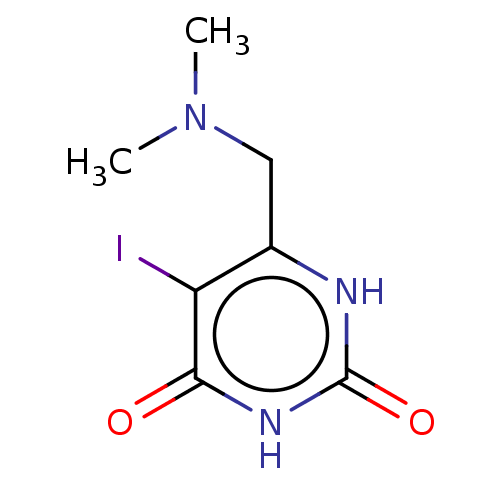 (CHEMBL4438928) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa Curated by ChEMBL | Assay Description Inhibition of recombinant human thymidine phosphorylase expressed in Escherichia coli Rosetta (DE3) cells using thymidine as substrate after 1 min by... | J Med Chem 62: 1231-1245 (2019) Article DOI: 10.1021/acs.jmedchem.8b01305 BindingDB Entry DOI: 10.7270/Q2FT8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50531740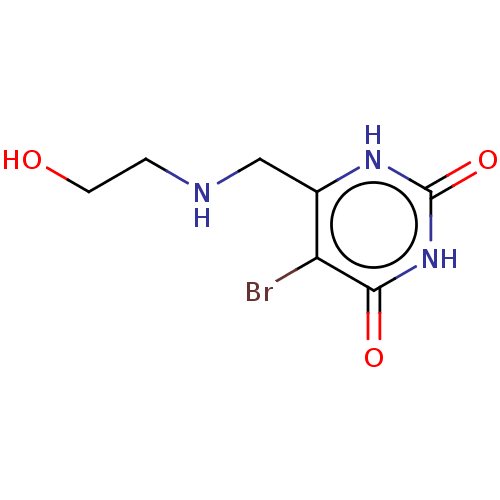 (CHEMBL4445259) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa Curated by ChEMBL | Assay Description Inhibition of recombinant human thymidine phosphorylase expressed in Escherichia coli Rosetta (DE3) cells using thymidine as substrate after 1 min by... | J Med Chem 62: 1231-1245 (2019) Article DOI: 10.1021/acs.jmedchem.8b01305 BindingDB Entry DOI: 10.7270/Q2FT8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50531737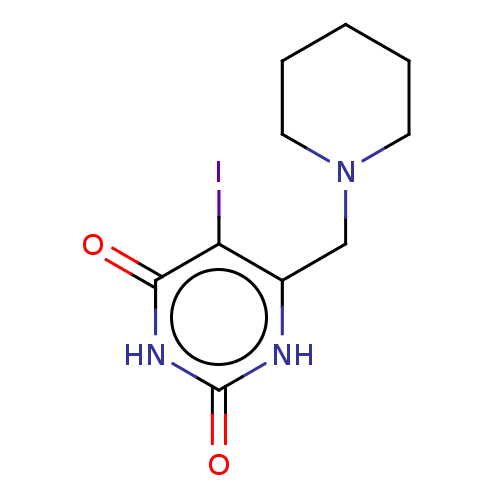 (CHEMBL4527207) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa Curated by ChEMBL | Assay Description Inhibition of recombinant human thymidine phosphorylase expressed in Escherichia coli Rosetta (DE3) cells using thymidine as substrate after 1 min by... | J Med Chem 62: 1231-1245 (2019) Article DOI: 10.1021/acs.jmedchem.8b01305 BindingDB Entry DOI: 10.7270/Q2FT8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50234734 (CHEMBL4094462) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pontif£cia Universidade Cat£lica do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes using S-(+)-mephenytoin as substrate by HPLC-MS/MS method | Eur J Med Chem 126: 491-501 (2017) Article DOI: 10.1016/j.ejmech.2016.11.048 BindingDB Entry DOI: 10.7270/Q2988972 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50234734 (CHEMBL4094462) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pontif£cia Universidade Cat£lica do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes using chlorzoxazone as substrate by HPLC-MS/MS method | Eur J Med Chem 126: 491-501 (2017) Article DOI: 10.1016/j.ejmech.2016.11.048 BindingDB Entry DOI: 10.7270/Q2988972 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50234734 (CHEMBL4094462) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pontif£cia Universidade Cat£lica do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate by HPLC-MS/MS method | Eur J Med Chem 126: 491-501 (2017) Article DOI: 10.1016/j.ejmech.2016.11.048 BindingDB Entry DOI: 10.7270/Q2988972 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50234734 (CHEMBL4094462) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pontif£cia Universidade Cat£lica do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using tolbutamide as substrate by HPLC-MS/MS method | Eur J Med Chem 126: 491-501 (2017) Article DOI: 10.1016/j.ejmech.2016.11.048 BindingDB Entry DOI: 10.7270/Q2988972 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50234734 (CHEMBL4094462) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pontif£cia Universidade Cat£lica do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using testosterone as substrate by HPLC-MS/MS method | Eur J Med Chem 126: 491-501 (2017) Article DOI: 10.1016/j.ejmech.2016.11.048 BindingDB Entry DOI: 10.7270/Q2988972 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50234734 (CHEMBL4094462) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pontif£cia Universidade Cat£lica do Rio Grande do Sul Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate by HPLC-MS/MS method | Eur J Med Chem 126: 491-501 (2017) Article DOI: 10.1016/j.ejmech.2016.11.048 BindingDB Entry DOI: 10.7270/Q2988972 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50531738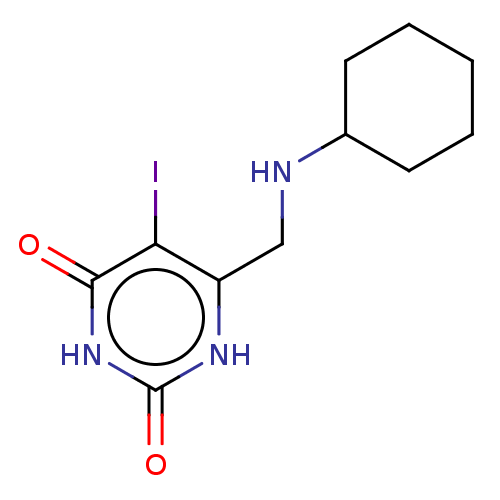 (CHEMBL4542724) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa Curated by ChEMBL | Assay Description Inhibition of recombinant human thymidine phosphorylase expressed in Escherichia coli Rosetta (DE3) cells using thymidine as substrate after 1 min by... | J Med Chem 62: 1231-1245 (2019) Article DOI: 10.1021/acs.jmedchem.8b01305 BindingDB Entry DOI: 10.7270/Q2FT8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50531735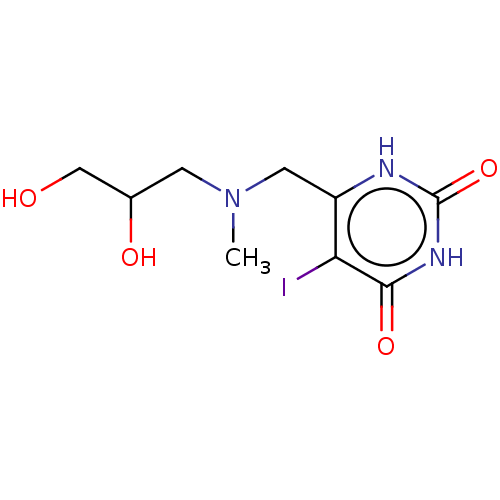 (CHEMBL4435521) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa Curated by ChEMBL | Assay Description Inhibition of recombinant human thymidine phosphorylase expressed in Escherichia coli Rosetta (DE3) cells using thymidine as substrate after 1 min by... | J Med Chem 62: 1231-1245 (2019) Article DOI: 10.1021/acs.jmedchem.8b01305 BindingDB Entry DOI: 10.7270/Q2FT8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50531731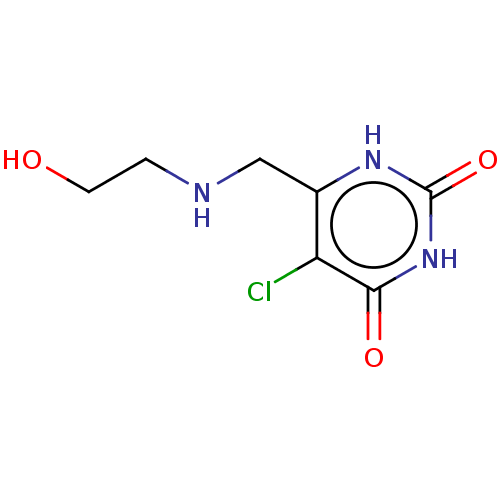 (CHEMBL4439474) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa Curated by ChEMBL | Assay Description Inhibition of recombinant human thymidine phosphorylase expressed in Escherichia coli Rosetta (DE3) cells using thymidine as substrate after 1 min by... | J Med Chem 62: 1231-1245 (2019) Article DOI: 10.1021/acs.jmedchem.8b01305 BindingDB Entry DOI: 10.7270/Q2FT8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50531743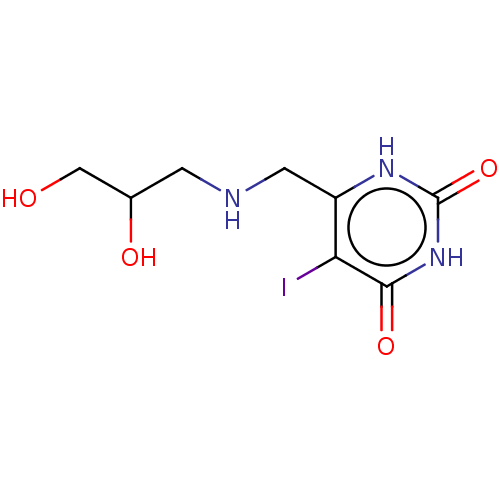 (CHEMBL4472112) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa Curated by ChEMBL | Assay Description Inhibition of recombinant human thymidine phosphorylase expressed in Escherichia coli Rosetta (DE3) cells using thymidine as substrate after 1 min by... | J Med Chem 62: 1231-1245 (2019) Article DOI: 10.1021/acs.jmedchem.8b01305 BindingDB Entry DOI: 10.7270/Q2FT8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50134399 (1,7-Dihydro-pyrrolo[2,3-d]pyrimidine-2,4-dione | 7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa Curated by ChEMBL | Assay Description Inhibition of thymidine phosphorylase (unknown origin) | J Med Chem 62: 1231-1245 (2019) Article DOI: 10.1021/acs.jmedchem.8b01305 BindingDB Entry DOI: 10.7270/Q2FT8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50531741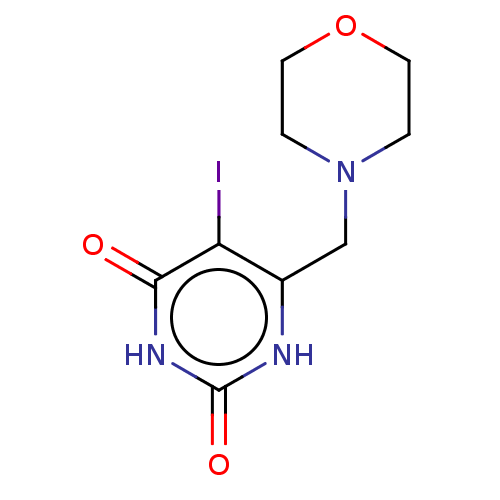 (CHEMBL4579033) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa Curated by ChEMBL | Assay Description Inhibition of recombinant human thymidine phosphorylase expressed in Escherichia coli Rosetta (DE3) cells using thymidine as substrate after 1 min by... | J Med Chem 62: 1231-1245 (2019) Article DOI: 10.1021/acs.jmedchem.8b01305 BindingDB Entry DOI: 10.7270/Q2FT8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50531732 (CHEMBL4455140) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes by HPLC-MS/MS analysis | J Med Chem 62: 1231-1245 (2019) Article DOI: 10.1021/acs.jmedchem.8b01305 BindingDB Entry DOI: 10.7270/Q2FT8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50531730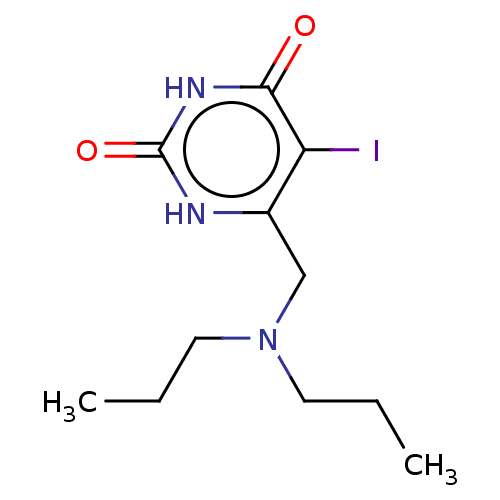 (CHEMBL4582134) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa Curated by ChEMBL | Assay Description Inhibition of recombinant human thymidine phosphorylase expressed in Escherichia coli Rosetta (DE3) cells using thymidine as substrate after 1 min by... | J Med Chem 62: 1231-1245 (2019) Article DOI: 10.1021/acs.jmedchem.8b01305 BindingDB Entry DOI: 10.7270/Q2FT8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50531732 (CHEMBL4455140) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes by HPLC-MS/MS analysis | J Med Chem 62: 1231-1245 (2019) Article DOI: 10.1021/acs.jmedchem.8b01305 BindingDB Entry DOI: 10.7270/Q2FT8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50531732 (CHEMBL4455140) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes by HPLC-MS/MS analysis | J Med Chem 62: 1231-1245 (2019) Article DOI: 10.1021/acs.jmedchem.8b01305 BindingDB Entry DOI: 10.7270/Q2FT8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50531732 (CHEMBL4455140) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa Curated by ChEMBL | Assay Description Inhibition of CYP2E1 in human liver microsomes by HPLC-MS/MS analysis | J Med Chem 62: 1231-1245 (2019) Article DOI: 10.1021/acs.jmedchem.8b01305 BindingDB Entry DOI: 10.7270/Q2FT8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50531733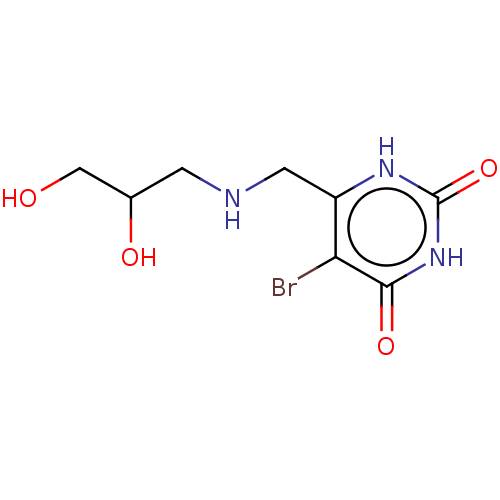 (CHEMBL4464628) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa Curated by ChEMBL | Assay Description Inhibition of recombinant human thymidine phosphorylase expressed in Escherichia coli Rosetta (DE3) cells using thymidine as substrate after 1 min by... | J Med Chem 62: 1231-1245 (2019) Article DOI: 10.1021/acs.jmedchem.8b01305 BindingDB Entry DOI: 10.7270/Q2FT8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50531732 (CHEMBL4455140) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes by HPLC-MS/MS analysis | J Med Chem 62: 1231-1245 (2019) Article DOI: 10.1021/acs.jmedchem.8b01305 BindingDB Entry DOI: 10.7270/Q2FT8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50531732 (CHEMBL4455140) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Pampa Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes by HPLC-MS/MS analysis | J Med Chem 62: 1231-1245 (2019) Article DOI: 10.1021/acs.jmedchem.8b01305 BindingDB Entry DOI: 10.7270/Q2FT8QHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||