 Found 239 hits with Last Name = 'stean' and Initial = 't'
Found 239 hits with Last Name = 'stean' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444496
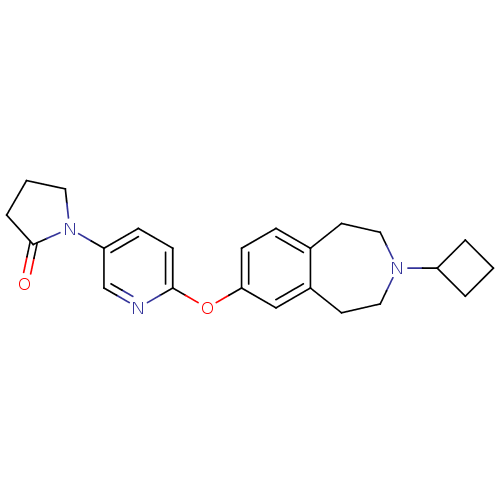
(CHEMBL3092650)Show SMILES O=C1CCCN1c1ccc(Oc2ccc3CCN(CCc3c2)C2CCC2)nc1 Show InChI InChI=1S/C23H27N3O2/c27-23-5-2-12-26(23)20-7-9-22(24-16-20)28-21-8-6-17-10-13-25(19-3-1-4-19)14-11-18(17)15-21/h6-9,15-16,19H,1-5,10-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from rat histamine H3 receptor expressed in HEK293 cells after 45 mins by liquid scintillation spectromet... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444491

(CHEMBL3092823)Show InChI InChI=1S/C22H25N3O2/c26-22-10-13-25(22)19-5-7-21(23-15-19)27-20-6-4-16-8-11-24(18-2-1-3-18)12-9-17(16)14-20/h4-7,14-15,18H,1-3,8-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50410424
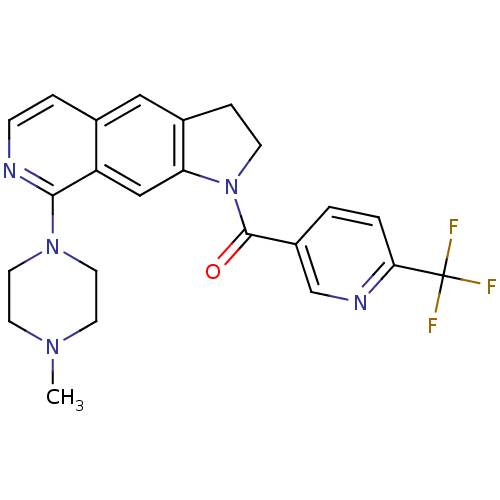
(CHEMBL199088)Show SMILES CN1CCN(CC1)c1nccc2cc3CCN(C(=O)c4ccc(nc4)C(F)(F)F)c3cc12 Show InChI InChI=1S/C23H22F3N5O/c1-29-8-10-30(11-9-29)21-18-13-19-16(12-15(18)4-6-27-21)5-7-31(19)22(32)17-2-3-20(28-14-17)23(24,25)26/h2-4,6,12-14H,5,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean binding affinity towards 5-hydroxytryptamine 1B receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand |
Bioorg Med Chem Lett 15: 4370-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.042
BindingDB Entry DOI: 10.7270/Q2319X3T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475465
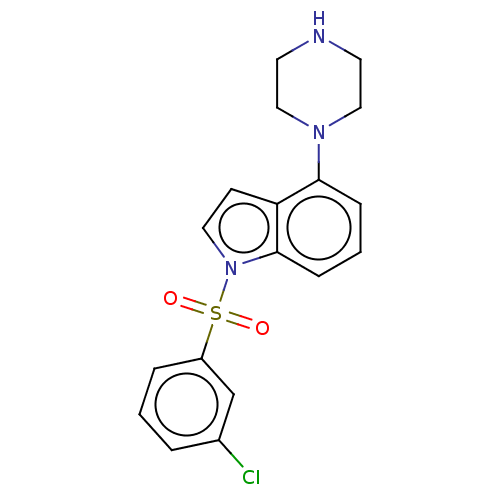
(CHEMBL196410)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C18H18ClN3O2S/c19-14-3-1-4-15(13-14)25(23,24)22-10-7-16-17(5-2-6-18(16)22)21-11-8-20-9-12-21/h1-7,10,13,20H,8-9,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50410426

(CHEMBL370852)Show SMILES CN1CCN(CC1)c1nccc2cc3CCN(C(=O)c4ccc(Cl)cc4)c3cc12 Show InChI InChI=1S/C23H23ClN4O/c1-26-10-12-27(13-11-26)22-20-15-21-18(14-17(20)6-8-25-22)7-9-28(21)23(29)16-2-4-19(24)5-3-16/h2-6,8,14-15H,7,9-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean binding affinity towards 5-hydroxytryptamine 1B receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand |
Bioorg Med Chem Lett 15: 4370-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.042
BindingDB Entry DOI: 10.7270/Q2319X3T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50410428

(CHEMBL380812)Show SMILES CN1CCN(CC1)c1nccc2cc3CCN(C(=O)c4cnn5ccccc45)c3cc12 Show InChI InChI=1S/C24H24N6O/c1-27-10-12-28(13-11-27)23-19-15-22-18(14-17(19)5-7-25-23)6-9-29(22)24(31)20-16-26-30-8-3-2-4-21(20)30/h2-5,7-8,14-16H,6,9-13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]-5-HT or [3H]8-OH-DPAT as the radioligand |
Bioorg Med Chem Lett 15: 4370-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.042
BindingDB Entry DOI: 10.7270/Q2319X3T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475467

(CHEMBL425015)Show SMILES Clc1cccc(c1)S(=O)(=O)c1c[nH]c2c(cccc12)N1CCNCC1 Show InChI InChI=1S/C18H18ClN3O2S/c19-13-3-1-4-14(11-13)25(23,24)17-12-21-18-15(17)5-2-6-16(18)22-9-7-20-8-10-22/h1-6,11-12,20-21H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475480
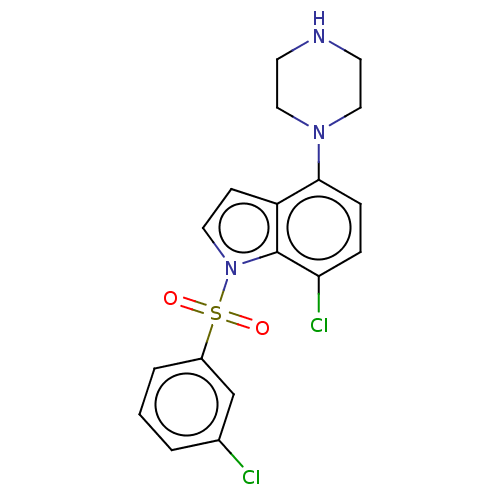
(CHEMBL193629)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(ccc(Cl)c12)N1CCNCC1 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-2-1-3-14(12-13)26(24,25)23-9-6-15-17(5-4-16(20)18(15)23)22-10-7-21-8-11-22/h1-6,9,12,21H,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50174269

(1-(phenylsulfonyl)-4-(piperazin-1-yl)-1H-indole | ...)Show InChI InChI=1S/C18H19N3O2S/c22-24(23,15-5-2-1-3-6-15)21-12-9-16-17(7-4-8-18(16)21)20-13-10-19-11-14-20/h1-9,12,19H,10-11,13-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475462
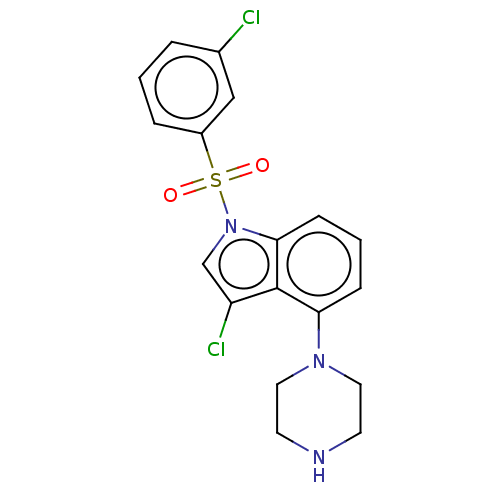
(CHEMBL371375)Show SMILES Clc1cn(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-13-3-1-4-14(11-13)26(24,25)23-12-15(20)18-16(5-2-6-17(18)23)22-9-7-21-8-10-22/h1-6,11-12,21H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444496

(CHEMBL3092650)Show SMILES O=C1CCCN1c1ccc(Oc2ccc3CCN(CCc3c2)C2CCC2)nc1 Show InChI InChI=1S/C23H27N3O2/c27-23-5-2-12-26(23)20-7-9-22(24-16-20)28-21-8-6-17-10-13-25(19-3-1-4-19)14-11-18(17)15-21/h6-9,15-16,19H,1-5,10-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.363 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475477
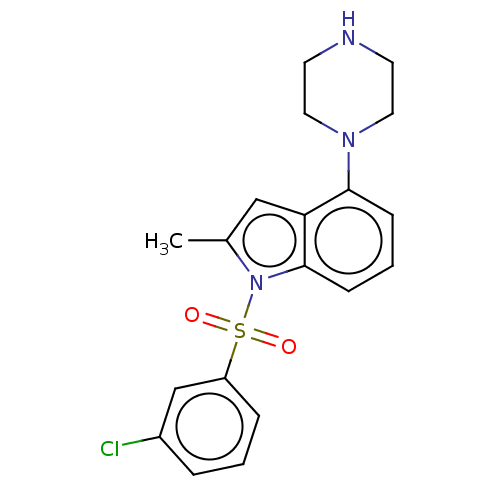
(CHEMBL372929)Show SMILES Cc1cc2c(cccc2n1S(=O)(=O)c1cccc(Cl)c1)N1CCNCC1 Show InChI InChI=1S/C19H20ClN3O2S/c1-14-12-17-18(22-10-8-21-9-11-22)6-3-7-19(17)23(14)26(24,25)16-5-2-4-15(20)13-16/h2-7,12-13,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475475
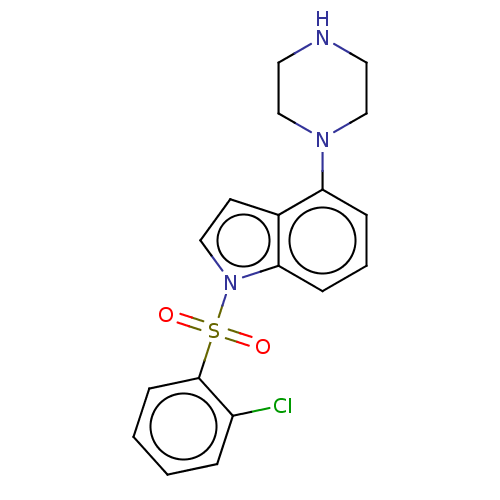
(CHEMBL372513)Show InChI InChI=1S/C18H18ClN3O2S/c19-15-4-1-2-7-18(15)25(23,24)22-11-8-14-16(5-3-6-17(14)22)21-12-9-20-10-13-21/h1-8,11,20H,9-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50410424

(CHEMBL199088)Show SMILES CN1CCN(CC1)c1nccc2cc3CCN(C(=O)c4ccc(nc4)C(F)(F)F)c3cc12 Show InChI InChI=1S/C23H22F3N5O/c1-29-8-10-30(11-9-29)21-18-13-19-16(12-15(18)4-6-27-21)5-7-31(19)22(32)17-2-3-20(28-14-17)23(24,25)26/h2-4,6,12-14H,5,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean binding affinity towards 5-hydroxytryptamine 1D receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand |
Bioorg Med Chem Lett 15: 4370-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.042
BindingDB Entry DOI: 10.7270/Q2319X3T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50410426

(CHEMBL370852)Show SMILES CN1CCN(CC1)c1nccc2cc3CCN(C(=O)c4ccc(Cl)cc4)c3cc12 Show InChI InChI=1S/C23H23ClN4O/c1-26-10-12-27(13-11-26)22-20-15-21-18(14-17(20)6-8-25-22)7-9-28(21)23(29)16-2-4-19(24)5-3-16/h2-6,8,14-15H,7,9-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean binding affinity towards 5-hydroxytryptamine 1D receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand |
Bioorg Med Chem Lett 15: 4370-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.042
BindingDB Entry DOI: 10.7270/Q2319X3T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50410427
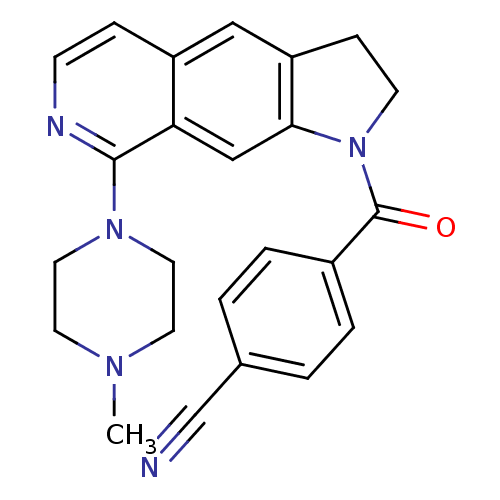
(CHEMBL194647)Show SMILES CN1CCN(CC1)c1nccc2cc3CCN(C(=O)c4ccc(cc4)C#N)c3cc12 Show InChI InChI=1S/C24H23N5O/c1-27-10-12-28(13-11-27)23-21-15-22-20(14-19(21)6-8-26-23)7-9-29(22)24(30)18-4-2-17(16-25)3-5-18/h2-6,8,14-15H,7,9-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean binding affinity towards 5-hydroxytryptamine 1B receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand |
Bioorg Med Chem Lett 15: 4370-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.042
BindingDB Entry DOI: 10.7270/Q2319X3T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444500
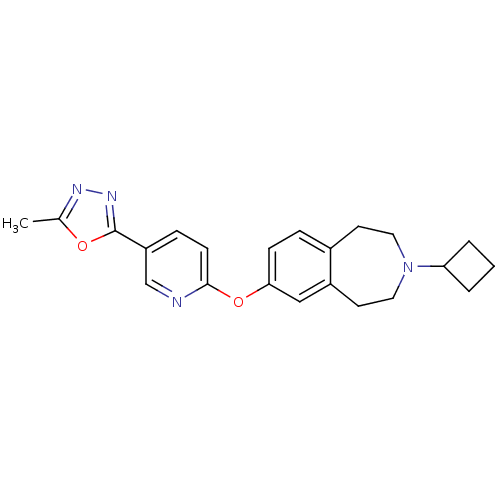
(CHEMBL3092834)Show SMILES Cc1nnc(o1)-c1ccc(Oc2ccc3CCN(CCc3c2)C2CCC2)nc1 Show InChI InChI=1S/C22H24N4O2/c1-15-24-25-22(27-15)18-6-8-21(23-14-18)28-20-7-5-16-9-11-26(19-3-2-4-19)12-10-17(16)13-20/h5-8,13-14,19H,2-4,9-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475463
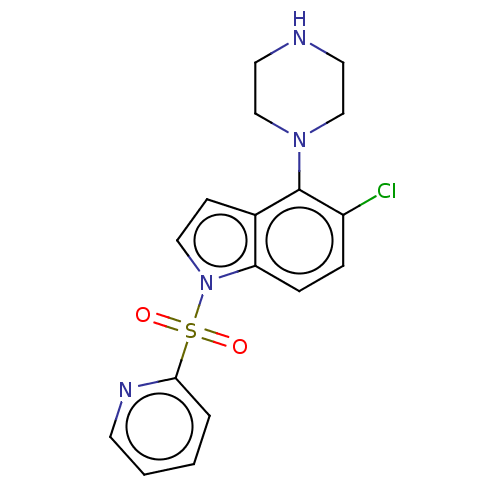
(CHEMBL194915)Show InChI InChI=1S/C17H17ClN4O2S/c18-14-4-5-15-13(17(14)21-11-8-19-9-12-21)6-10-22(15)25(23,24)16-3-1-2-7-20-16/h1-7,10,19H,8-9,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50217828

(CHEMBL413707)Show SMILES Fc1ccc(OC2CCN(CC[C@H]3CCCN3S(=O)(=O)c3ccc4cc[nH]c4c3)CC2)cc1 Show InChI InChI=1S/C25H30FN3O3S/c26-20-4-6-22(7-5-20)32-23-11-16-28(17-12-23)15-10-21-2-1-14-29(21)33(30,31)24-8-3-19-9-13-27-25(19)18-24/h3-9,13,18,21,23,27H,1-2,10-12,14-17H2/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand |
Bioorg Med Chem Lett 12: 3341-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8P7C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50044607
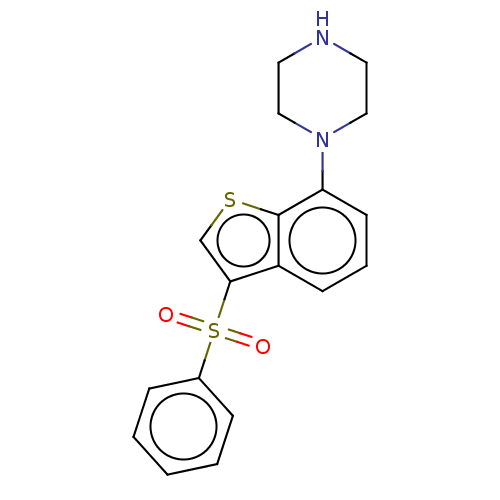
(CHEMBL372537)Show InChI InChI=1S/C18H18N2O2S2/c21-24(22,14-5-2-1-3-6-14)17-13-23-18-15(17)7-4-8-16(18)20-11-9-19-10-12-20/h1-8,13,19H,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475473
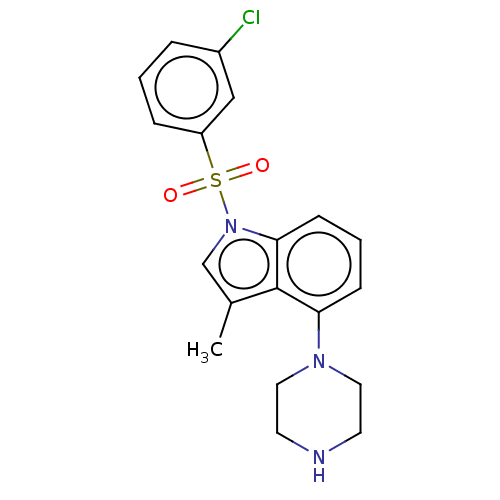
(CHEMBL194039)Show SMILES Cc1cn(c2cccc(N3CCNCC3)c12)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H20ClN3O2S/c1-14-13-23(26(24,25)16-5-2-4-15(20)12-16)18-7-3-6-17(19(14)18)22-10-8-21-9-11-22/h2-7,12-13,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444509
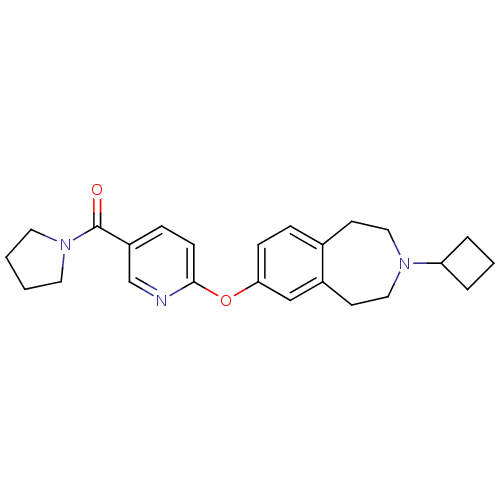
(CHEMBL3092840)Show SMILES O=C(N1CCCC1)c1ccc(Oc2ccc3CCN(CCc3c2)C2CCC2)nc1 Show InChI InChI=1S/C24H29N3O2/c28-24(27-12-1-2-13-27)20-7-9-23(25-17-20)29-22-8-6-18-10-14-26(21-4-3-5-21)15-11-19(18)16-22/h6-9,16-17,21H,1-5,10-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444507
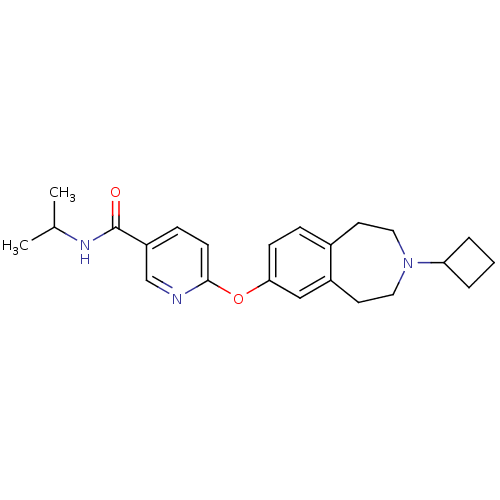
(CHEMBL3092826)Show SMILES CC(C)NC(=O)c1ccc(Oc2ccc3CCN(CCc3c2)C2CCC2)nc1 Show InChI InChI=1S/C23H29N3O2/c1-16(2)25-23(27)19-7-9-22(24-15-19)28-21-8-6-17-10-12-26(20-4-3-5-20)13-11-18(17)14-21/h6-9,14-16,20H,3-5,10-13H2,1-2H3,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444505
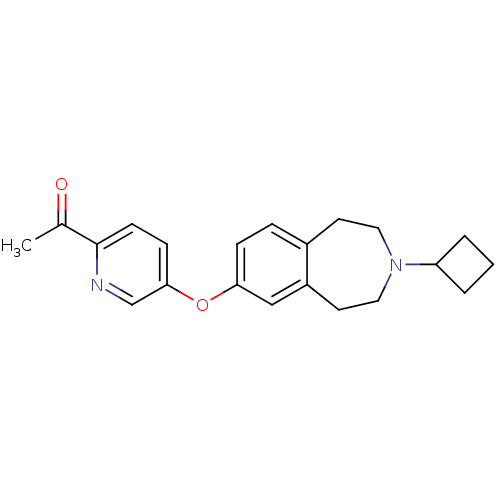
(CHEMBL3092828)Show InChI InChI=1S/C21H24N2O2/c1-15(24)21-8-7-20(14-22-21)25-19-6-5-16-9-11-23(18-3-2-4-18)12-10-17(16)13-19/h5-8,13-14,18H,2-4,9-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50410440
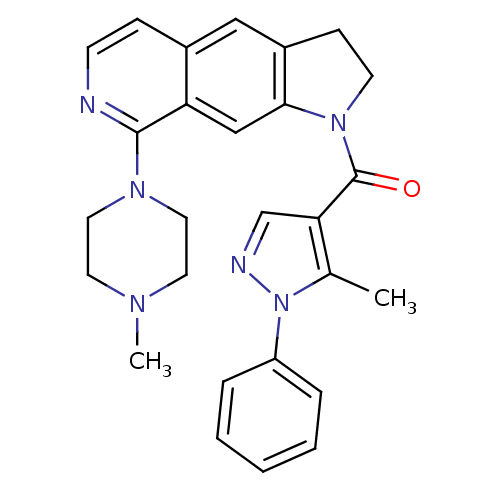
(CHEMBL198975)Show SMILES CN1CCN(CC1)c1nccc2cc3CCN(C(=O)c4cnn(c4C)-c4ccccc4)c3cc12 Show InChI InChI=1S/C27H28N6O/c1-19-24(18-29-33(19)22-6-4-3-5-7-22)27(34)32-11-9-21-16-20-8-10-28-26(23(20)17-25(21)32)31-14-12-30(2)13-15-31/h3-8,10,16-18H,9,11-15H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]-5-HT or [3H]8-OH-DPAT as the radioligand |
Bioorg Med Chem Lett 15: 4370-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.042
BindingDB Entry DOI: 10.7270/Q2319X3T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50410430
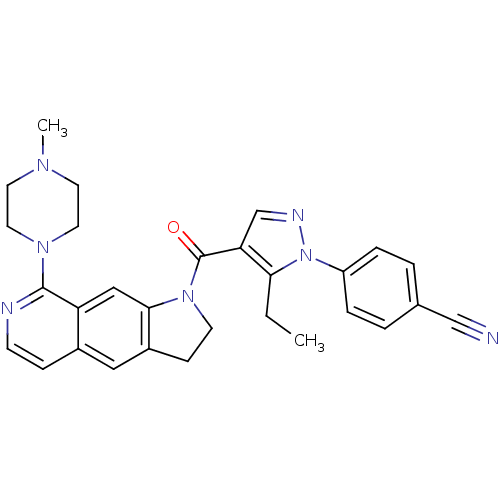
(CHEMBL372743)Show SMILES CCc1c(cnn1-c1ccc(cc1)C#N)C(=O)N1CCc2cc3ccnc(N4CCN(C)CC4)c3cc12 Show InChI InChI=1S/C29H29N7O/c1-3-26-25(19-32-36(26)23-6-4-20(18-30)5-7-23)29(37)35-11-9-22-16-21-8-10-31-28(24(21)17-27(22)35)34-14-12-33(2)13-15-34/h4-8,10,16-17,19H,3,9,11-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean binding affinity towards 5-hydroxytryptamine 1D receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand |
Bioorg Med Chem Lett 15: 4370-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.042
BindingDB Entry DOI: 10.7270/Q2319X3T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50410440

(CHEMBL198975)Show SMILES CN1CCN(CC1)c1nccc2cc3CCN(C(=O)c4cnn(c4C)-c4ccccc4)c3cc12 Show InChI InChI=1S/C27H28N6O/c1-19-24(18-29-33(19)22-6-4-3-5-7-22)27(34)32-11-9-21-16-20-8-10-28-26(23(20)17-25(21)32)31-14-12-30(2)13-15-31/h3-8,10,16-18H,9,11-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean binding affinity towards 5-hydroxytryptamine 1D receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand |
Bioorg Med Chem Lett 15: 4370-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.042
BindingDB Entry DOI: 10.7270/Q2319X3T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50410440

(CHEMBL198975)Show SMILES CN1CCN(CC1)c1nccc2cc3CCN(C(=O)c4cnn(c4C)-c4ccccc4)c3cc12 Show InChI InChI=1S/C27H28N6O/c1-19-24(18-29-33(19)22-6-4-3-5-7-22)27(34)32-11-9-21-16-20-8-10-28-26(23(20)17-25(21)32)31-14-12-30(2)13-15-31/h3-8,10,16-18H,9,11-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean binding affinity towards 5-hydroxytryptamine 1B receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand |
Bioorg Med Chem Lett 15: 4370-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.042
BindingDB Entry DOI: 10.7270/Q2319X3T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50247054
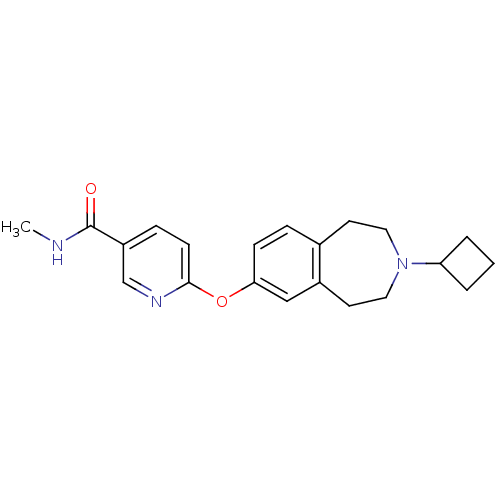
(6-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...)Show InChI InChI=1S/C21H25N3O2/c1-22-21(25)17-6-8-20(23-14-17)26-19-7-5-15-9-11-24(18-3-2-4-18)12-10-16(15)13-19/h5-8,13-14,18H,2-4,9-12H2,1H3,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from rat histamine H3 receptor expressed in HEK293 cells after 45 mins by liquid scintillation spectromet... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50217831

(CHEMBL430706)Show SMILES O=S(=O)(N1CCC[C@@H]1CCN1CCC(CC1)c1c[nH]c2ccccc12)c1ccc2cc[nH]c2c1 |r| Show InChI InChI=1S/C27H32N4O2S/c32-34(33,23-8-7-21-9-13-28-27(21)18-23)31-14-3-4-22(31)12-17-30-15-10-20(11-16-30)25-19-29-26-6-2-1-5-24(25)26/h1-2,5-9,13,18-20,22,28-29H,3-4,10-12,14-17H2/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand |
Bioorg Med Chem Lett 12: 3341-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8P7C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50217829
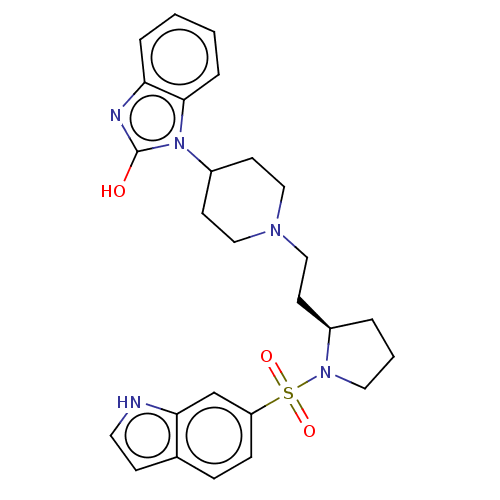
(CHEMBL115262)Show SMILES Oc1nc2ccccc2n1C1CCN(CC[C@H]2CCCN2S(=O)(=O)c2ccc3cc[nH]c3c2)CC1 Show InChI InChI=1S/C26H31N5O3S/c32-26-28-23-5-1-2-6-25(23)31(26)21-11-16-29(17-12-21)15-10-20-4-3-14-30(20)35(33,34)22-8-7-19-9-13-27-24(19)18-22/h1-2,5-9,13,18,20-21,27H,3-4,10-12,14-17H2,(H,28,32)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand |
Bioorg Med Chem Lett 12: 3341-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8P7C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50410426

(CHEMBL370852)Show SMILES CN1CCN(CC1)c1nccc2cc3CCN(C(=O)c4ccc(Cl)cc4)c3cc12 Show InChI InChI=1S/C23H23ClN4O/c1-26-10-12-27(13-11-26)22-20-15-21-18(14-17(20)6-8-25-22)7-9-28(21)23(29)16-2-4-19(24)5-3-16/h2-6,8,14-15H,7,9-13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]-5-HT or [3H]8-OH-DPAT as the radioligand |
Bioorg Med Chem Lett 15: 4370-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.042
BindingDB Entry DOI: 10.7270/Q2319X3T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50410437
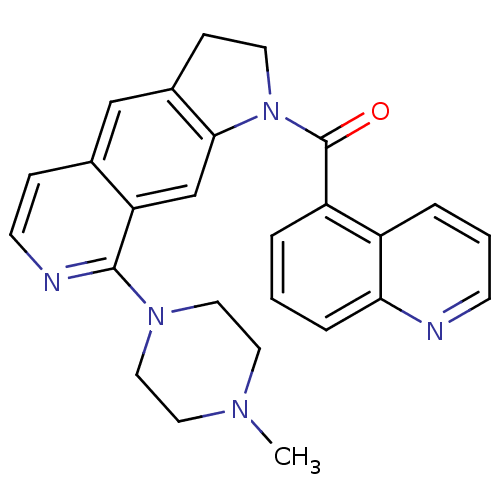
(CHEMBL370625)Show SMILES CN1CCN(CC1)c1nccc2cc3CCN(C(=O)c4cccc5ncccc45)c3cc12 Show InChI InChI=1S/C26H25N5O/c1-29-12-14-30(15-13-29)25-22-17-24-19(16-18(22)7-10-28-25)8-11-31(24)26(32)21-4-2-6-23-20(21)5-3-9-27-23/h2-7,9-10,16-17H,8,11-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean binding affinity towards 5-hydroxytryptamine 1B receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand |
Bioorg Med Chem Lett 15: 4370-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.042
BindingDB Entry DOI: 10.7270/Q2319X3T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444489
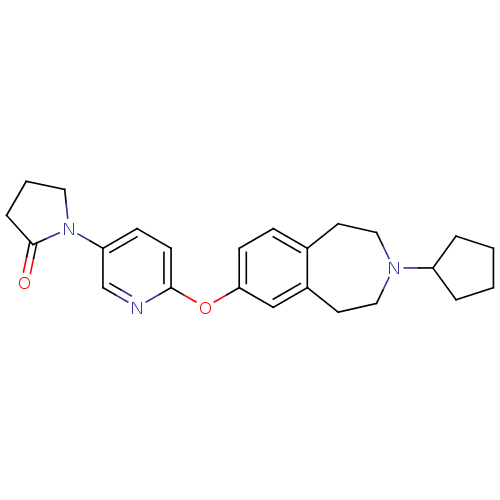
(CHEMBL3092825)Show SMILES O=C1CCCN1c1ccc(Oc2ccc3CCN(CCc3c2)C2CCCC2)nc1 Show InChI InChI=1S/C24H29N3O2/c28-24-6-3-13-27(24)21-8-10-23(25-17-21)29-22-9-7-18-11-14-26(15-12-19(18)16-22)20-4-1-2-5-20/h7-10,16-17,20H,1-6,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444495
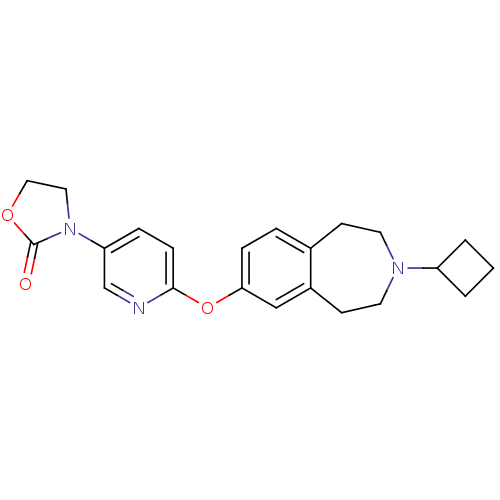
(CHEMBL3092651)Show SMILES O=C1OCCN1c1ccc(Oc2ccc3CCN(CCc3c2)C2CCC2)nc1 Show InChI InChI=1S/C22H25N3O3/c26-22-25(12-13-27-22)19-5-7-21(23-15-19)28-20-6-4-16-8-10-24(18-2-1-3-18)11-9-17(16)14-20/h4-7,14-15,18H,1-3,8-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50410427

(CHEMBL194647)Show SMILES CN1CCN(CC1)c1nccc2cc3CCN(C(=O)c4ccc(cc4)C#N)c3cc12 Show InChI InChI=1S/C24H23N5O/c1-27-10-12-28(13-11-27)23-21-15-22-20(14-19(21)6-8-26-23)7-9-29(22)24(30)18-4-2-17(16-25)3-5-18/h2-6,8,14-15H,7,9-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean binding affinity towards 5-hydroxytryptamine 1D receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand |
Bioorg Med Chem Lett 15: 4370-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.042
BindingDB Entry DOI: 10.7270/Q2319X3T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50217832
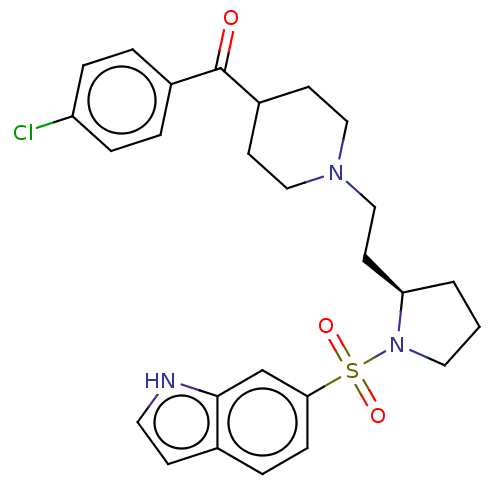
(CHEMBL116292)Show SMILES Clc1ccc(cc1)C(=O)C1CCN(CC[C@H]2CCCN2S(=O)(=O)c2ccc3cc[nH]c3c2)CC1 Show InChI InChI=1S/C26H30ClN3O3S/c27-22-6-3-20(4-7-22)26(31)21-10-15-29(16-11-21)17-12-23-2-1-14-30(23)34(32,33)24-8-5-19-9-13-28-25(19)18-24/h3-9,13,18,21,23,28H,1-2,10-12,14-17H2/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand |
Bioorg Med Chem Lett 12: 3341-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8P7C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50217835
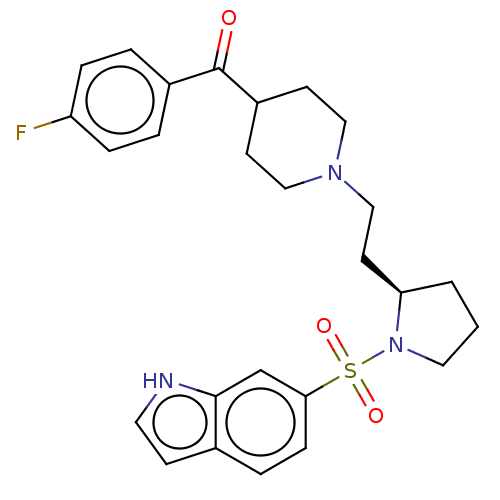
(CHEMBL114345)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CC[C@H]2CCCN2S(=O)(=O)c2ccc3cc[nH]c3c2)CC1 Show InChI InChI=1S/C26H30FN3O3S/c27-22-6-3-20(4-7-22)26(31)21-10-15-29(16-11-21)17-12-23-2-1-14-30(23)34(32,33)24-8-5-19-9-13-28-25(19)18-24/h3-9,13,18,21,23,28H,1-2,10-12,14-17H2/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.832 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand |
Bioorg Med Chem Lett 12: 3341-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8P7C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50346209

(5-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...)Show InChI InChI=1S/C20H24N4O2/c1-21-20(25)18-12-23-19(13-22-18)26-17-6-5-14-7-9-24(16-3-2-4-16)10-8-15(14)11-17/h5-6,11-13,16H,2-4,7-10H2,1H3,(H,21,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.832 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50410432
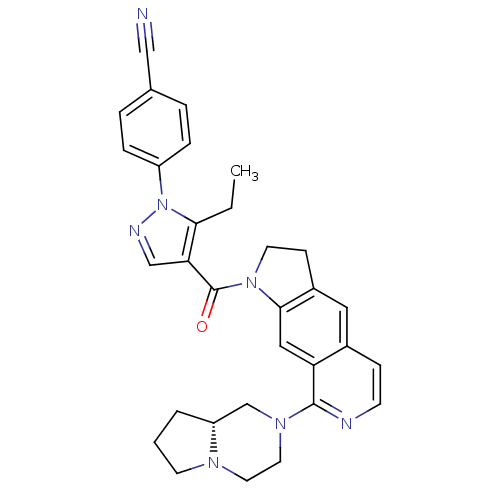
(CHEMBL197947)Show SMILES CCc1c(cnn1-c1ccc(cc1)C#N)C(=O)N1CCc2cc3ccnc(N4CCN5CCC[C@@H]5C4)c3cc12 Show InChI InChI=1S/C31H31N7O/c1-2-28-27(19-34-38(28)24-7-5-21(18-32)6-8-24)31(39)37-13-10-23-16-22-9-11-33-30(26(22)17-29(23)37)36-15-14-35-12-3-4-25(35)20-36/h5-9,11,16-17,19,25H,2-4,10,12-15,20H2,1H3/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean binding affinity towards 5-hydroxytryptamine 1D receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand |
Bioorg Med Chem Lett 15: 4370-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.042
BindingDB Entry DOI: 10.7270/Q2319X3T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475479
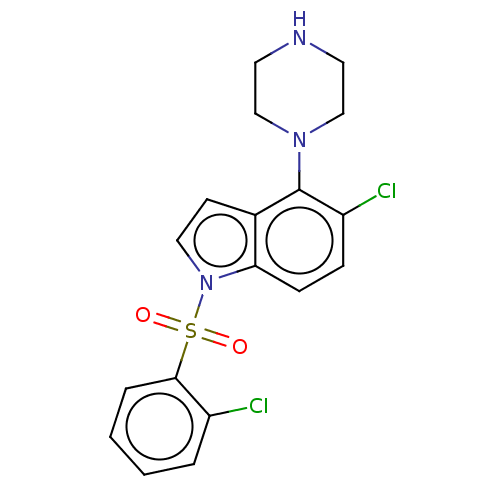
(CHEMBL371176)Show SMILES Clc1ccccc1S(=O)(=O)n1ccc2c(N3CCNCC3)c(Cl)ccc12 Show InChI InChI=1S/C18H17Cl2N3O2S/c19-14-3-1-2-4-17(14)26(24,25)23-10-7-13-16(23)6-5-15(20)18(13)22-11-8-21-9-12-22/h1-7,10,21H,8-9,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444506
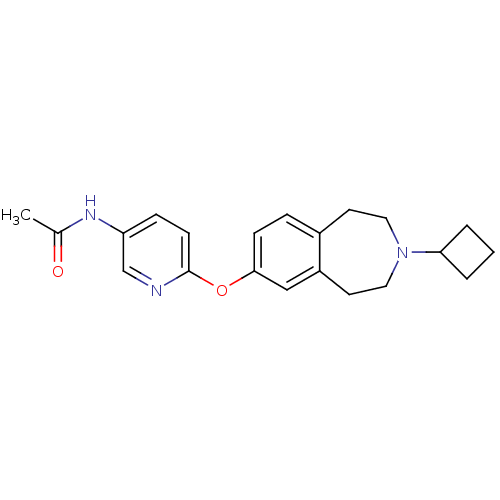
(CHEMBL3092827)Show InChI InChI=1S/C21H25N3O2/c1-15(25)23-18-6-8-21(22-14-18)26-20-7-5-16-9-11-24(19-3-2-4-19)12-10-17(16)13-20/h5-8,13-14,19H,2-4,9-12H2,1H3,(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50410430

(CHEMBL372743)Show SMILES CCc1c(cnn1-c1ccc(cc1)C#N)C(=O)N1CCc2cc3ccnc(N4CCN(C)CC4)c3cc12 Show InChI InChI=1S/C29H29N7O/c1-3-26-25(19-32-36(26)23-6-4-20(18-30)5-7-23)29(37)35-11-9-22-16-21-8-10-31-28(24(21)17-27(22)35)34-14-12-33(2)13-15-34/h4-8,10,16-17,19H,3,9,11-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean binding affinity towards 5-hydroxytryptamine 1B receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand |
Bioorg Med Chem Lett 15: 4370-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.042
BindingDB Entry DOI: 10.7270/Q2319X3T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50475470

(CHEMBL370209)Show InChI InChI=1S/C17H18N4O2S/c22-24(23,17-6-1-2-8-19-17)21-11-7-14-15(4-3-5-16(14)21)20-12-9-18-10-13-20/h1-8,11,18H,9-10,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-hydroxytryptamine 6 receptor expressed in HeLa cells |
Bioorg Med Chem Lett 15: 4867-71 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.107
BindingDB Entry DOI: 10.7270/Q2028V9Q |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50410427

(CHEMBL194647)Show SMILES CN1CCN(CC1)c1nccc2cc3CCN(C(=O)c4ccc(cc4)C#N)c3cc12 Show InChI InChI=1S/C24H23N5O/c1-27-10-12-28(13-11-27)23-21-15-22-20(14-19(21)6-8-26-23)7-9-29(22)24(30)18-4-2-17(16-25)3-5-18/h2-6,8,14-15H,7,9-13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean binding affinity towards 5-hydroxytryptamine 1A receptor using [3H]-5-HT or [3H]8-OH-DPAT as the radioligand |
Bioorg Med Chem Lett 15: 4370-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.042
BindingDB Entry DOI: 10.7270/Q2319X3T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85097
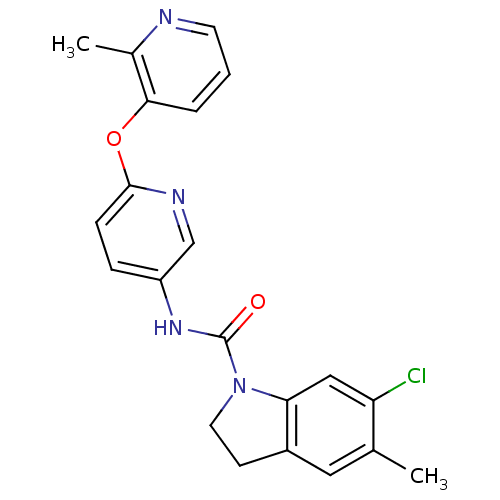
(CAS_181632-25-7 | CHEMBL14563 | SB 242084)Show SMILES Cc1cc2CCN(C(=O)Nc3ccc(Oc4cccnc4C)nc3)c2cc1Cl Show InChI InChI=1S/C21H19ClN4O2/c1-13-10-15-7-9-26(18(15)11-17(13)22)21(27)25-16-5-6-20(24-12-16)28-19-4-3-8-23-14(19)2/h3-6,8,10-12H,7,9H2,1-2H3,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 609-20 (1997)
Article DOI: 10.1016/s0028-3908(97)00038-5
BindingDB Entry DOI: 10.7270/Q2SN07GG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50410428

(CHEMBL380812)Show SMILES CN1CCN(CC1)c1nccc2cc3CCN(C(=O)c4cnn5ccccc45)c3cc12 Show InChI InChI=1S/C24H24N6O/c1-27-10-12-28(13-11-27)23-19-15-22-18(14-17(19)5-7-25-23)6-9-29(22)24(31)20-16-26-30-8-3-2-4-21(20)30/h2-5,7-8,14-16H,6,9-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean binding affinity towards 5-hydroxytryptamine 1B receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand |
Bioorg Med Chem Lett 15: 4370-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.042
BindingDB Entry DOI: 10.7270/Q2319X3T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50444494
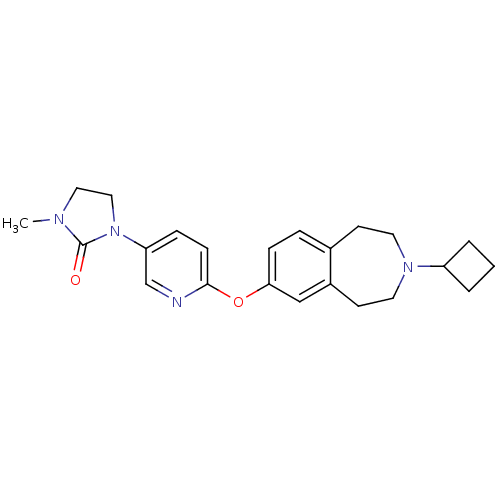
(CHEMBL3092820)Show SMILES CN1CCN(C1=O)c1ccc(Oc2ccc3CCN(CCc3c2)C2CCC2)nc1 Show InChI InChI=1S/C23H28N4O2/c1-25-13-14-27(23(25)28)20-6-8-22(24-16-20)29-21-7-5-17-9-11-26(19-3-2-4-19)12-10-18(17)15-21/h5-8,15-16,19H,2-4,9-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from histamine H3 receptor in rat cerebral cortical tissue membranes after 45 mins by liquid scintillatio... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50410432

(CHEMBL197947)Show SMILES CCc1c(cnn1-c1ccc(cc1)C#N)C(=O)N1CCc2cc3ccnc(N4CCN5CCC[C@@H]5C4)c3cc12 Show InChI InChI=1S/C31H31N7O/c1-2-28-27(19-34-38(28)24-7-5-21(18-32)6-8-24)31(39)37-13-10-23-16-22-9-11-33-30(26(22)17-29(23)37)36-15-14-35-12-3-4-25(35)20-36/h5-9,11,16-17,19,25H,2-4,10,12-15,20H2,1H3/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Mean binding affinity towards 5-hydroxytryptamine 1B receptor using [3H]5-HT or [3H]-8-OH-DPAT as the radioligand |
Bioorg Med Chem Lett 15: 4370-4 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.042
BindingDB Entry DOI: 10.7270/Q2319X3T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50346209

(5-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...)Show InChI InChI=1S/C20H24N4O2/c1-21-20(25)18-12-23-19(13-22-18)26-17-6-5-14-7-9-24(16-3-2-4-16)10-8-15(14)11-17/h5-6,11-13,16H,2-4,7-10H2,1H3,(H,21,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Displacement of [3H]-R-alpha-ethylhistamine from rat histamine H3 receptor expressed in HEK293 cells after 45 mins by liquid scintillation spectromet... |
Bioorg Med Chem Lett 23: 6890-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.090
BindingDB Entry DOI: 10.7270/Q2PZ5B83 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data