 Found 576 hits with Last Name = 'steward' and Initial = 'or'
Found 576 hits with Last Name = 'steward' and Initial = 'or' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593696
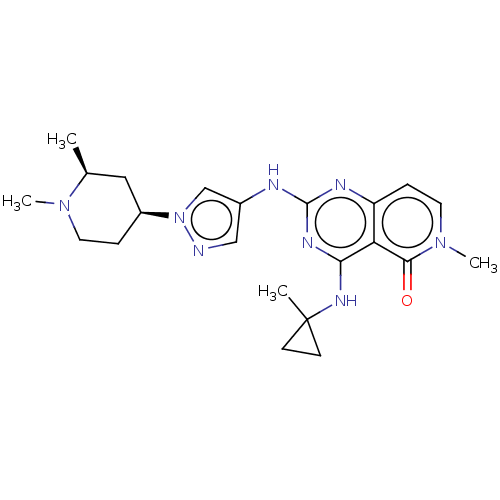
(CHEMBL5200601)Show SMILES C[C@H]1C[C@H](CCN1C)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593694
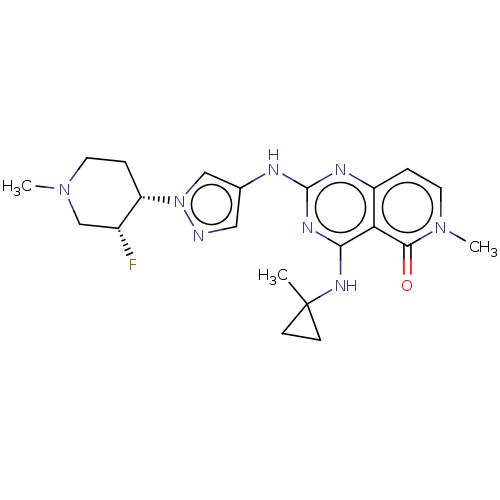
(CHEMBL5193253)Show SMILES CN1CC[C@@H]([C@H](F)C1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612201
(CHEMBL5270030) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612188

(CHEMBL5283628) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593693

(CHEMBL5201376)Show SMILES CN(C)C(=O)CN1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612193

(CHEMBL5290090) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612189

(CHEMBL5281595) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612187
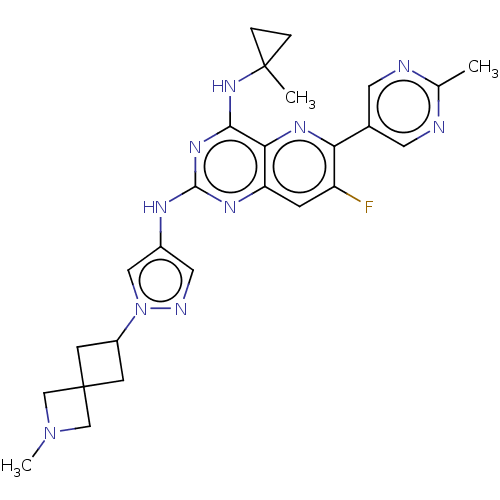
(CHEMBL5288696) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593689
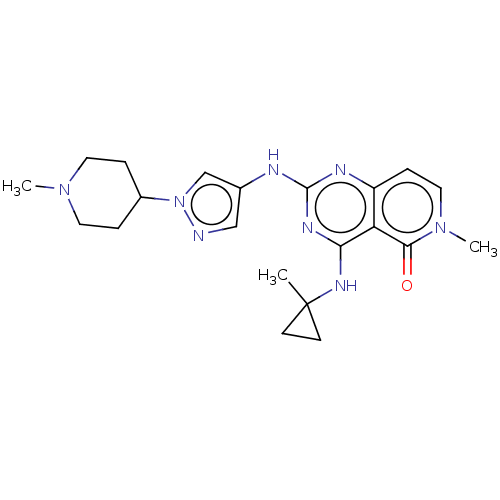
(CHEMBL5174529)Show SMILES CN1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593700

(CHEMBL5193024)Show SMILES CN1CC2(CC(C2)n2cc(Nc3nc(NC4(C)CC4)c4c(ccn(C)c4=O)n3)cn2)C1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593697

(CHEMBL5196755)Show SMILES C[C@@H]1C[C@H](CCN1C)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593695
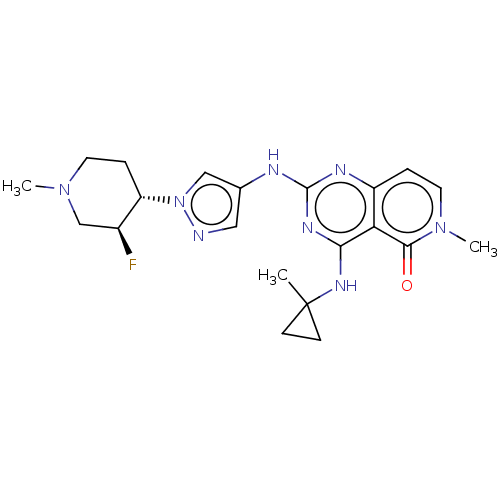
(CHEMBL5209502)Show SMILES CN1CC[C@@H]([C@@H](F)C1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593710
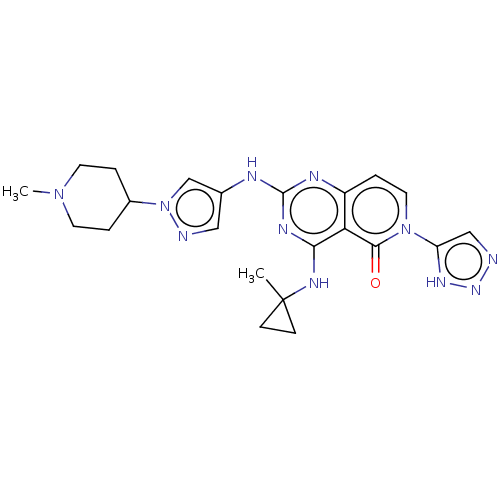
(CHEMBL5193053)Show SMILES CN1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(-c4cnn[nH]4)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593692

(CHEMBL5200432)Show SMILES CN(C)CC(=O)N1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612191

(CHEMBL5273100) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593716

(CHEMBL5207654)Show SMILES C[C@H](F)Cn1ccc2nc(Nc3cnn(c3)C3CC4(C3)CN(C)C4)nc(NC3(C)CC3)c2c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593685
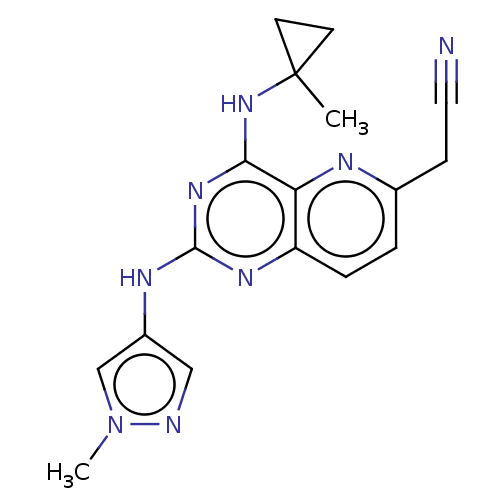
(CHEMBL5179237) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612181

(CHEMBL5281524) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593691
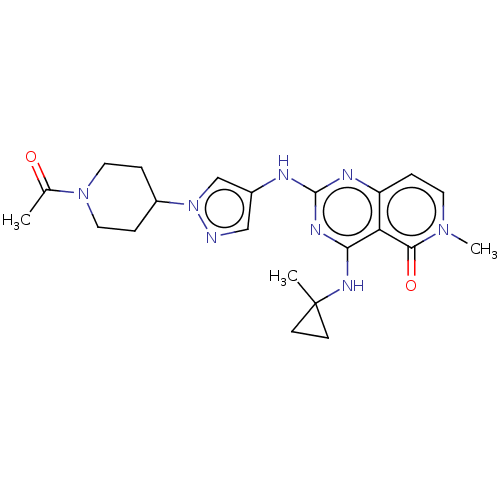
(CHEMBL5207332)Show SMILES CC(=O)N1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593706

(CHEMBL5190750)Show SMILES C[C@H](F)Cn1ccc2nc(Nc3cnn(c3)C3CCN(C)CC3)nc(NC3(C)CC3)c2c1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612202

(CHEMBL5274954) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 1
(Homo sapiens (Human)) | BDBM50612188

(CHEMBL5283628) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM321944

((E)-1-(4-((1-Methyl-1H-pyrazol-4-yl)methyl)piperaz...)Show SMILES Cc1nnn(Cc2cc(ccc2\C=C\C(=O)N2CCN(Cc3cnn(C)c3)CC2)C(F)(F)F)n1 Show InChI InChI=1S/C22H25F3N8O/c1-16-27-29-33(28-16)15-19-11-20(22(23,24)25)5-3-18(19)4-6-21(34)32-9-7-31(8-10-32)14-17-12-26-30(2)13-17/h3-6,11-13H,7-10,14-15H2,1-2H3/b6-4+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Reagents LPC (oleoyl (18:1)) was purchased from Avanti Polar Lipids (Alabaster, Ala.) and solubilized in methanol to 20 mM. Amplex Red was obtained f... |
US Patent US10183025 (2019)
BindingDB Entry DOI: 10.7270/Q2RN39XZ |
More data for this
Ligand-Target Pair | |
Ataxin-1
(Homo sapiens (Human)) | BDBM322065
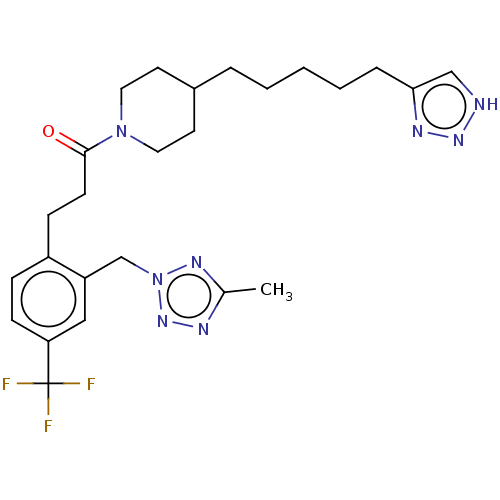
(1-(4-(5-(1H-1,2,3-Triazol-4-yl)pentyl)piperidin-1-...)Show SMILES Cc1nnn(Cc2cc(ccc2CCC(=O)N2CCC(CCCCCc3c[nH]nn3)CC2)C(F)(F)F)n1 Show InChI InChI=1S/C25H33F3N8O/c1-18-30-34-36(32-18)17-21-15-22(25(26,27)28)9-7-20(21)8-10-24(37)35-13-11-19(12-14-35)5-3-2-4-6-23-16-29-33-31-23/h7,9,15-16,19H,2-6,8,10-14,17H2,1H3,(H,29,31,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Method All experimental measurements were performed in black 384 well polystyrene (low volume, round bottom, Corning (3676)) plates. PerkinElmer EnVi... |
US Patent US9763957 (2017)
BindingDB Entry DOI: 10.7270/Q2QF8W0D |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM322065

(1-(4-(5-(1H-1,2,3-Triazol-4-yl)pentyl)piperidin-1-...)Show SMILES Cc1nnn(Cc2cc(ccc2CCC(=O)N2CCC(CCCCCc3c[nH]nn3)CC2)C(F)(F)F)n1 Show InChI InChI=1S/C25H33F3N8O/c1-18-30-34-36(32-18)17-21-15-22(25(26,27)28)9-7-20(21)8-10-24(37)35-13-11-19(12-14-35)5-3-2-4-6-23-16-29-33-31-23/h7,9,15-16,19H,2-6,8,10-14,17H2,1H3,(H,29,31,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Reagents LPC (oleoyl (18:1)) was purchased from Avanti Polar Lipids (Alabaster, Ala.) and solubilized in methanol to 20 mM. Amplex Red was obtained f... |
US Patent US10183025 (2019)
BindingDB Entry DOI: 10.7270/Q2RN39XZ |
More data for this
Ligand-Target Pair | |
Ataxin-1
(Homo sapiens (Human)) | BDBM321944

((E)-1-(4-((1-Methyl-1H-pyrazol-4-yl)methyl)piperaz...)Show SMILES Cc1nnn(Cc2cc(ccc2\C=C\C(=O)N2CCN(Cc3cnn(C)c3)CC2)C(F)(F)F)n1 Show InChI InChI=1S/C22H25F3N8O/c1-16-27-29-33(28-16)15-19-11-20(22(23,24)25)5-3-18(19)4-6-21(34)32-9-7-31(8-10-32)14-17-12-26-30(2)13-17/h3-6,11-13H,7-10,14-15H2,1-2H3/b6-4+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Method All experimental measurements were performed in black 384 well polystyrene (low volume, round bottom, Corning (3676)) plates. PerkinElmer EnVi... |
US Patent US9763957 (2017)
BindingDB Entry DOI: 10.7270/Q2QF8W0D |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593704
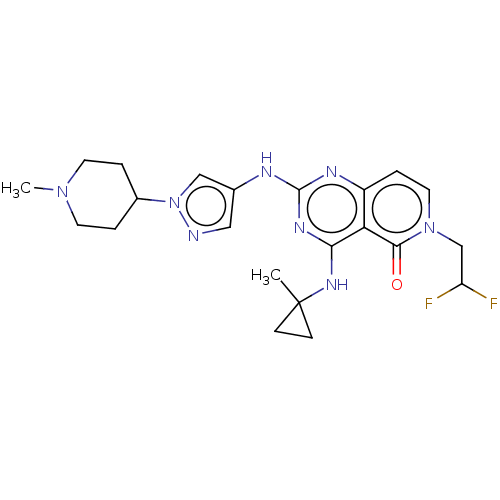
(CHEMBL5186874)Show SMILES CN1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(CC(F)F)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593685

(CHEMBL5179237) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ataxin-1
(Homo sapiens (Human)) | BDBM321962
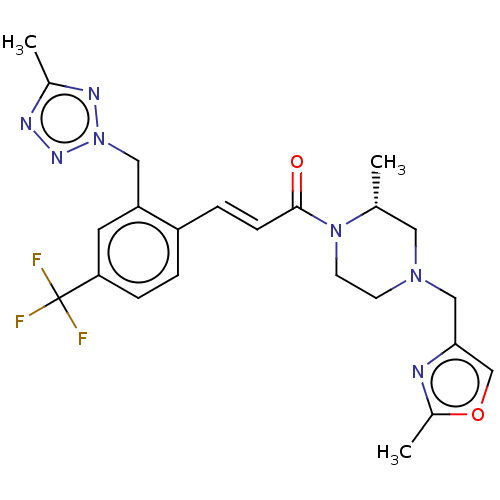
((R,E)-3-(2-((5-Methyl-2H-tetrazol-2-yl)methyl)-4-(...)Show SMILES C[C@@H]1CN(Cc2coc(C)n2)CCN1C(=O)\C=C\c1ccc(cc1Cn1nnc(C)n1)C(F)(F)F |r| Show InChI InChI=1S/C23H26F3N7O2/c1-15-11-31(13-21-14-35-17(3)27-21)8-9-32(15)22(34)7-5-18-4-6-20(23(24,25)26)10-19(18)12-33-29-16(2)28-30-33/h4-7,10,14-15H,8-9,11-13H2,1-3H3/b7-5+/t15-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Method All experimental measurements were performed in black 384 well polystyrene (low volume, round bottom, Corning (3676)) plates. PerkinElmer EnVi... |
US Patent US9763957 (2017)
BindingDB Entry DOI: 10.7270/Q2QF8W0D |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593690
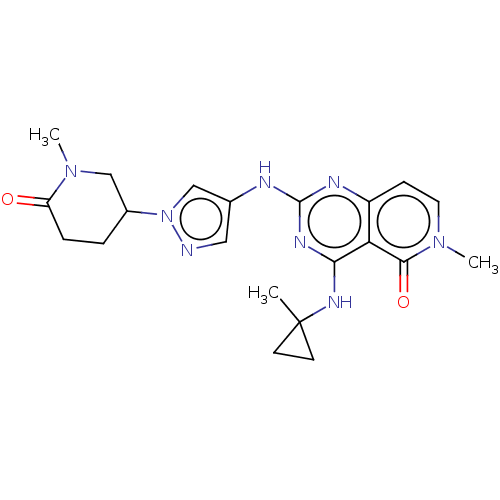
(CHEMBL5178970)Show SMILES CN1CC(CCC1=O)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM321962

((R,E)-3-(2-((5-Methyl-2H-tetrazol-2-yl)methyl)-4-(...)Show SMILES C[C@@H]1CN(Cc2coc(C)n2)CCN1C(=O)\C=C\c1ccc(cc1Cn1nnc(C)n1)C(F)(F)F |r| Show InChI InChI=1S/C23H26F3N7O2/c1-15-11-31(13-21-14-35-17(3)27-21)8-9-32(15)22(34)7-5-18-4-6-20(23(24,25)26)10-19(18)12-33-29-16(2)28-30-33/h4-7,10,14-15H,8-9,11-13H2,1-3H3/b7-5+/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Reagents LPC (oleoyl (18:1)) was purchased from Avanti Polar Lipids (Alabaster, Ala.) and solubilized in methanol to 20 mM. Amplex Red was obtained f... |
US Patent US10183025 (2019)
BindingDB Entry DOI: 10.7270/Q2RN39XZ |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM322066
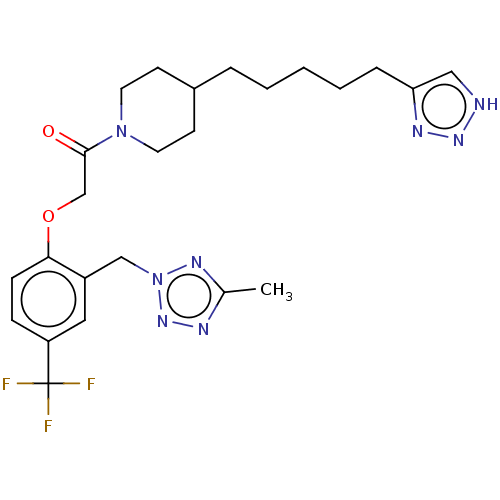
(1-(4-(5-(1H-1,2,3-Triazol-4-yl)pentyl)piperidin-1-...)Show SMILES Cc1nnn(Cc2cc(ccc2OCC(=O)N2CCC(CCCCCc3c[nH]nn3)CC2)C(F)(F)F)n1 Show InChI InChI=1S/C24H31F3N8O2/c1-17-29-33-35(31-17)15-19-13-20(24(25,26)27)7-8-22(19)37-16-23(36)34-11-9-18(10-12-34)5-3-2-4-6-21-14-28-32-30-21/h7-8,13-14,18H,2-6,9-12,15-16H2,1H3,(H,28,30,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Reagents LPC (oleoyl (18:1)) was purchased from Avanti Polar Lipids (Alabaster, Ala.) and solubilized in methanol to 20 mM. Amplex Red was obtained f... |
US Patent US10183025 (2019)
BindingDB Entry DOI: 10.7270/Q2RN39XZ |
More data for this
Ligand-Target Pair | |
Ataxin-1
(Homo sapiens (Human)) | BDBM322066

(1-(4-(5-(1H-1,2,3-Triazol-4-yl)pentyl)piperidin-1-...)Show SMILES Cc1nnn(Cc2cc(ccc2OCC(=O)N2CCC(CCCCCc3c[nH]nn3)CC2)C(F)(F)F)n1 Show InChI InChI=1S/C24H31F3N8O2/c1-17-29-33-35(31-17)15-19-13-20(24(25,26)27)7-8-22(19)37-16-23(36)34-11-9-18(10-12-34)5-3-2-4-6-21-14-28-32-30-21/h7-8,13-14,18H,2-6,9-12,15-16H2,1H3,(H,28,30,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Method All experimental measurements were performed in black 384 well polystyrene (low volume, round bottom, Corning (3676)) plates. PerkinElmer EnVi... |
US Patent US9763957 (2017)
BindingDB Entry DOI: 10.7270/Q2QF8W0D |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612200
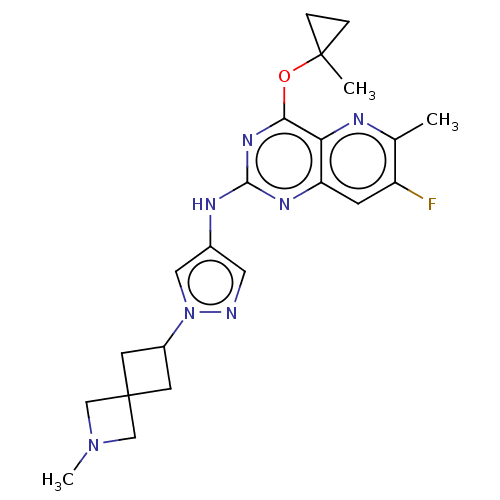
(CHEMBL5286869) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612199

(CHEMBL5285779) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612195

(CHEMBL5288931) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PDB
UniChem
| PDB
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612183
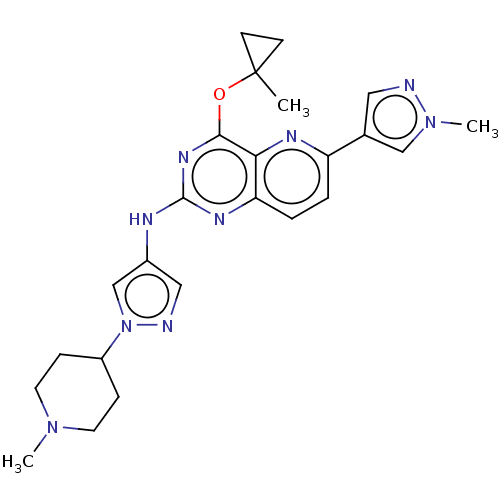
(CHEMBL5266181) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PDB
UniChem
| PDB
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612180

(CHEMBL5274369) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612179

(CHEMBL5272567) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50505743

(CHEMBL4582624)Show SMILES COC(=O)N1CCN(CC1)[C@H]1CC[C@@H](CC1)Nc1nc(Nc2cnn(C)c2)nc2ccc(CC#N)nc12 |r,wU:13.17,wD:10.10,(21.97,-21.58,;21.98,-20.04,;20.66,-19.26,;20.68,-17.72,;19.32,-20.02,;19.31,-21.56,;17.97,-22.32,;16.64,-21.54,;16.65,-19.99,;17.99,-19.23,;15.3,-22.29,;13.96,-21.52,;12.62,-22.28,;12.62,-23.82,;13.95,-24.6,;15.29,-23.83,;11.28,-24.59,;11.28,-26.13,;12.62,-26.91,;12.62,-28.45,;13.95,-29.22,;15.28,-28.46,;15.43,-26.92,;16.94,-26.61,;17.7,-27.94,;19.24,-27.93,;16.67,-29.08,;11.28,-29.22,;9.95,-28.45,;8.63,-29.24,;7.29,-28.48,;7.27,-26.93,;5.93,-26.19,;5.91,-24.65,;5.88,-23.11,;8.6,-26.15,;9.94,-26.91,)| Show InChI InChI=1S/C25H32N10O2/c1-33-16-19(15-27-33)30-24-31-21-8-5-18(9-10-26)28-22(21)23(32-24)29-17-3-6-20(7-4-17)34-11-13-35(14-12-34)25(36)37-2/h5,8,15-17,20H,3-4,6-7,9,11-14H2,1-2H3,(H2,29,30,31,32)/t17-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593715

(CHEMBL5177080)Show SMILES [H][C@@]12CN(C)[C@@]([H])(C[C@@H]1n1cc(Nc3nc(NC4(C)CC4)c4c(ccn(C[C@H](C)F)c4=O)n3)cn1)C2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593703
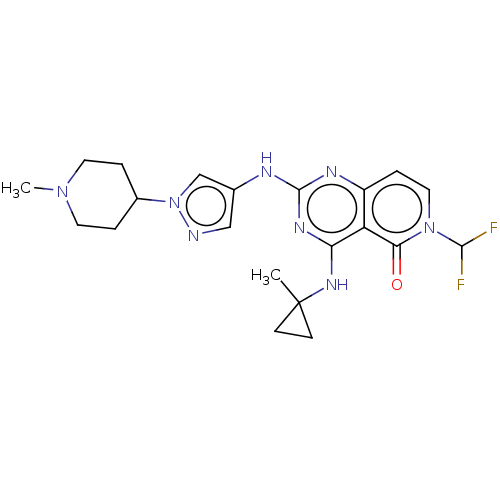
(CHEMBL5189582)Show SMILES CN1CCC(CC1)n1cc(Nc2nc(NC3(C)CC3)c3c(ccn(C(F)F)c3=O)n2)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50593698
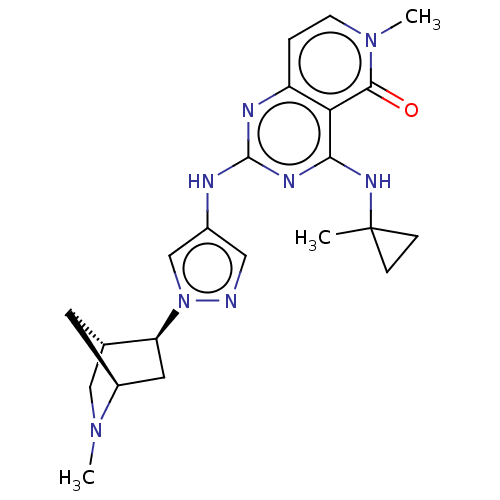
(CHEMBL5187604)Show SMILES [H][C@]12CN(C)[C@]([H])(C[C@@H]1n1cc(Nc3nc(NC4(C)CC4)c4c(ccn(C)c4=O)n3)cn1)C2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116729
BindingDB Entry DOI: 10.7270/Q22V2M4V |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50612198
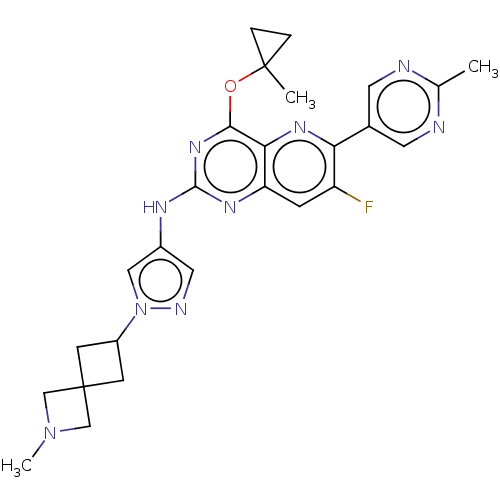
(CHEMBL5270903) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PDB
UniChem
| PDB
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM322063

((E)-N-(1-(3-(4-Chloro-2-((5-methyl-2H-tetrazol-2-y...)Show SMILES Cc1nnn(Cc2cc(Cl)ccc2\C=C\C(=O)N2CCC(CC2)NC(=O)CCCc2c[nH]nn2)n1 Show InChI InChI=1S/C23H28ClN9O2/c1-16-27-31-33(29-16)15-18-13-19(24)7-5-17(18)6-8-23(35)32-11-9-20(10-12-32)26-22(34)4-2-3-21-14-25-30-28-21/h5-8,13-14,20H,2-4,9-12,15H2,1H3,(H,26,34)(H,25,28,30)/b8-6+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Reagents LPC (oleoyl (18:1)) was purchased from Avanti Polar Lipids (Alabaster, Ala.) and solubilized in methanol to 20 mM. Amplex Red was obtained f... |
US Patent US10183025 (2019)
BindingDB Entry DOI: 10.7270/Q2RN39XZ |
More data for this
Ligand-Target Pair | |
Ataxin-1
(Homo sapiens (Human)) | BDBM322063

((E)-N-(1-(3-(4-Chloro-2-((5-methyl-2H-tetrazol-2-y...)Show SMILES Cc1nnn(Cc2cc(Cl)ccc2\C=C\C(=O)N2CCC(CC2)NC(=O)CCCc2c[nH]nn2)n1 Show InChI InChI=1S/C23H28ClN9O2/c1-16-27-31-33(29-16)15-18-13-19(24)7-5-17(18)6-8-23(35)32-11-9-20(10-12-32)26-22(34)4-2-3-21-14-25-30-28-21/h5-8,13-14,20H,2-4,9-12,15H2,1H3,(H,26,34)(H,25,28,30)/b8-6+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Method All experimental measurements were performed in black 384 well polystyrene (low volume, round bottom, Corning (3676)) plates. PerkinElmer EnVi... |
US Patent US9763957 (2017)
BindingDB Entry DOI: 10.7270/Q2QF8W0D |
More data for this
Ligand-Target Pair | |
Ataxin-1
(Homo sapiens (Human)) | BDBM321991

((E)-1-(4-((1-Methyl-1H-pyrazol-3-yl)methyl)piperaz...)Show SMILES Cc1nnn(Cc2cc(ccc2\C=C\C(=O)N2CCN(Cc3ccn(C)n3)CC2)C(F)(F)F)n1 Show InChI InChI=1S/C22H25F3N8O/c1-16-26-29-33(27-16)14-18-13-19(22(23,24)25)5-3-17(18)4-6-21(34)32-11-9-31(10-12-32)15-20-7-8-30(2)28-20/h3-8,13H,9-12,14-15H2,1-2H3/b6-4+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Method All experimental measurements were performed in black 384 well polystyrene (low volume, round bottom, Corning (3676)) plates. PerkinElmer EnVi... |
US Patent US9763957 (2017)
BindingDB Entry DOI: 10.7270/Q2QF8W0D |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM322027
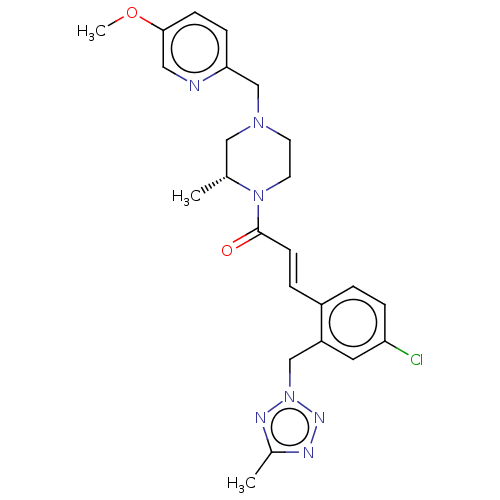
((R,E)-3-(4-Chloro-2-((5-methyl-2H-tetrazol-2-yl)me...)Show SMILES COc1ccc(CN2CCN([C@H](C)C2)C(=O)\C=C\c2ccc(Cl)cc2Cn2nnc(C)n2)nc1 |r| Show InChI InChI=1S/C24H28ClN7O2/c1-17-14-30(16-22-7-8-23(34-3)13-26-22)10-11-31(17)24(33)9-5-19-4-6-21(25)12-20(19)15-32-28-18(2)27-29-32/h4-9,12-13,17H,10-11,14-16H2,1-3H3/b9-5+/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Reagents LPC (oleoyl (18:1)) was purchased from Avanti Polar Lipids (Alabaster, Ala.) and solubilized in methanol to 20 mM. Amplex Red was obtained f... |
US Patent US10183025 (2019)
BindingDB Entry DOI: 10.7270/Q2RN39XZ |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM321868
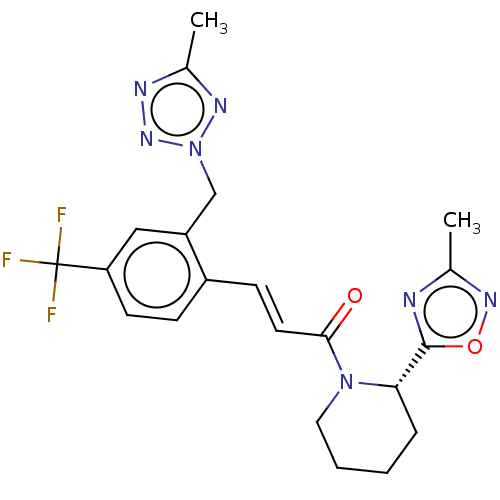
(US10183025, Example 5a | US9763957, Example 5b)Show SMILES Cc1noc(n1)[C@@H]1CCCCN1C(=O)\C=C\c1ccc(cc1Cn1nnc(C)n1)C(F)(F)F |r| Show InChI InChI=1S/C21H22F3N7O2/c1-13-25-20(33-28-13)18-5-3-4-10-30(18)19(32)9-7-15-6-8-17(21(22,23)24)11-16(15)12-31-27-14(2)26-29-31/h6-9,11,18H,3-5,10,12H2,1-2H3/b9-7+/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Reagents LPC (oleoyl (18:1)) was purchased from Avanti Polar Lipids (Alabaster, Ala.) and solubilized in methanol to 20 mM. Amplex Red was obtained f... |
US Patent US10183025 (2019)
BindingDB Entry DOI: 10.7270/Q2RN39XZ |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM321899
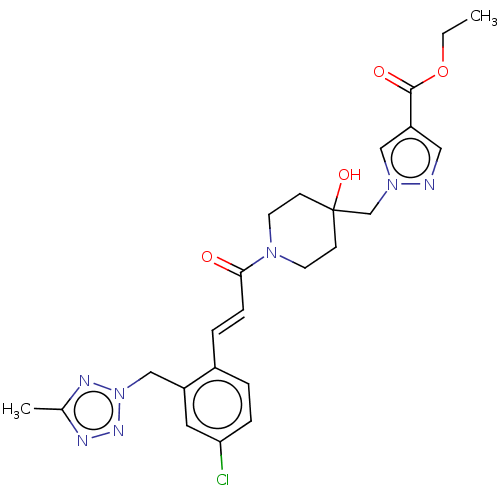
((E)-Ethyl 1-((1-(3-(4-chloro-2-((5-methyl-2H-tetra...)Show SMILES CCOC(=O)c1cnn(CC2(O)CCN(CC2)C(=O)\C=C\c2ccc(Cl)cc2Cn2nnc(C)n2)c1 Show InChI InChI=1S/C24H28ClN7O4/c1-3-36-23(34)20-13-26-31(14-20)16-24(35)8-10-30(11-9-24)22(33)7-5-18-4-6-21(25)12-19(18)15-32-28-17(2)27-29-32/h4-7,12-14,35H,3,8-11,15-16H2,1-2H3/b7-5+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG
US Patent
| Assay Description
Reagents LPC (oleoyl (18:1)) was purchased from Avanti Polar Lipids (Alabaster, Ala.) and solubilized in methanol to 20 mM. Amplex Red was obtained f... |
US Patent US10183025 (2019)
BindingDB Entry DOI: 10.7270/Q2RN39XZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data