

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26976 (2-benzoylquinazoline analogue, (1R, 2S)-17 | N-(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40 | -50.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26975 (2-benzoylquinazoline analogue, (rac)-17 | N-{4-[(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.10 | -49.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19459 (5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of binding of 17 beta-estradiol to human Estrogen receptor alpha | Bioorg Med Chem Lett 11: 1839-42 (2001) BindingDB Entry DOI: 10.7270/Q29P325H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26973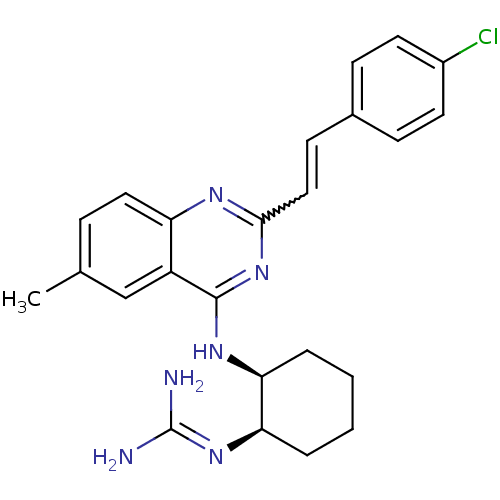 ((1R,2S)-2-({2-[(E)-2-(4-chlorophenyl)ethenyl]-6-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 43 | -42.0 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM26974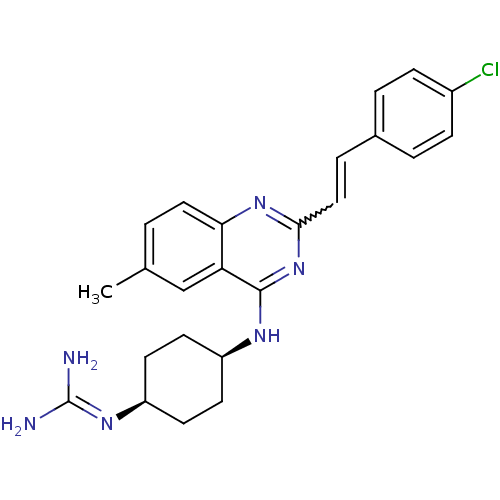 (4-({2-[(E)-2-(4-chlorophenyl)ethenyl]-6-methylquin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26971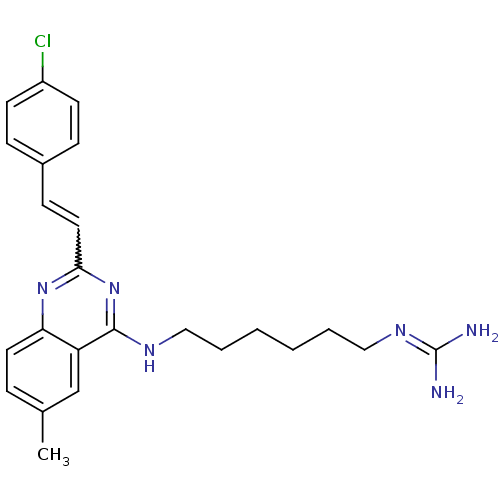 (3-[6-({2-[(E)-2-(4-chlorophenyl)ethenyl]-6-methylq...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 89 | -40.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM26960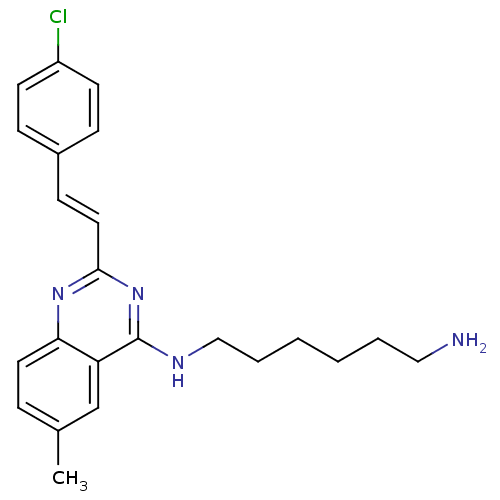 (4-aminoquinazoline derivative, 7c | N-(6-aminohexy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26956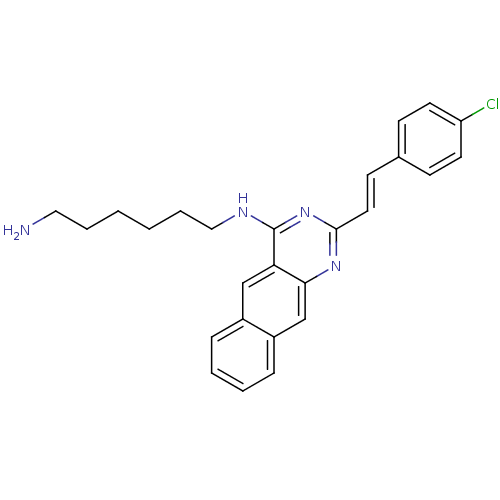 (N-(6-aminohexyl)-2-[(E)-2-(4-chlorophenyl)ethenyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 133 | -39.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26977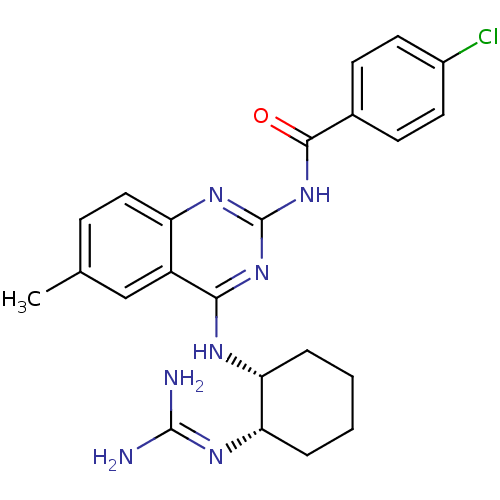 (2-benzoylquinazoline analogue, (1S, 2R)-17 | N-(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 140 | -39.1 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM26968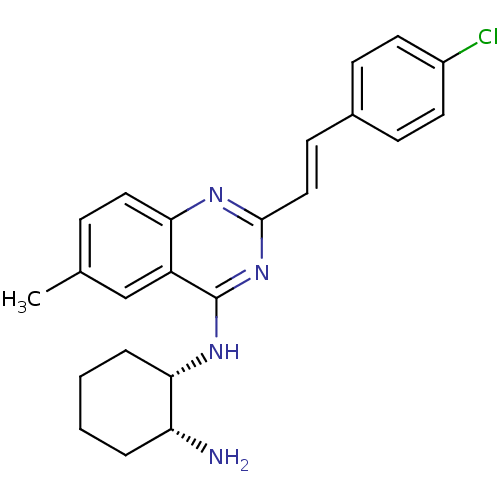 ((1S,2R)-1-N-{2-[(E)-2-(4-chlorophenyl)ethenyl]-6-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26974 (4-({2-[(E)-2-(4-chlorophenyl)ethenyl]-6-methylquin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 152 | -38.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26972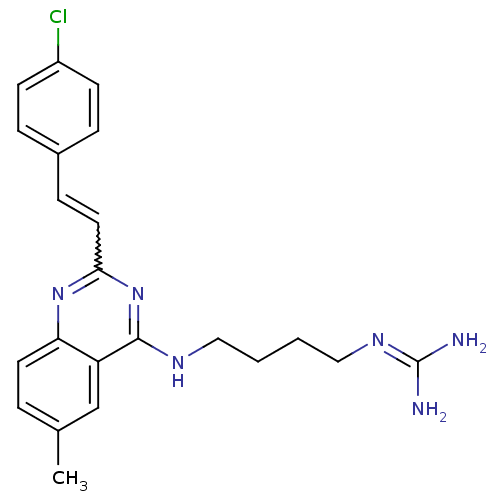 (3-[4-({2-[(E)-2-(4-chlorophenyl)ethenyl]-6-methylq...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 160 | -38.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26969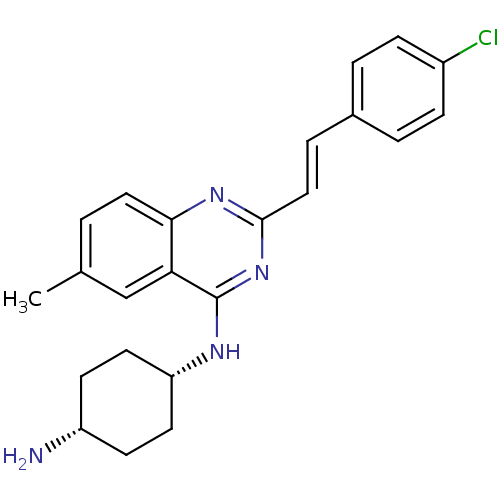 (1-N-{2-[(E)-2-(4-chlorophenyl)ethenyl]-6-methylqui...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 162 | -38.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM26959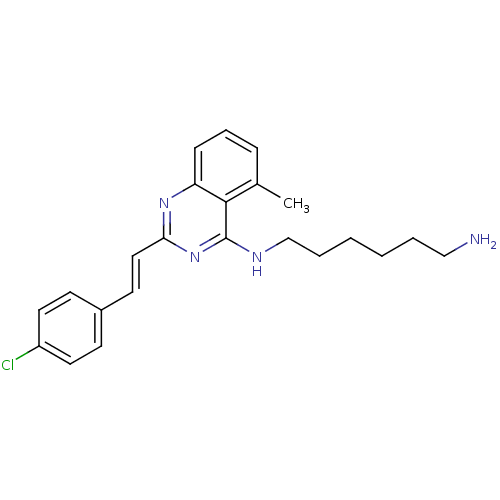 (4-aminoquinazoline derivative, 7b | N-(6-aminohexy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM26958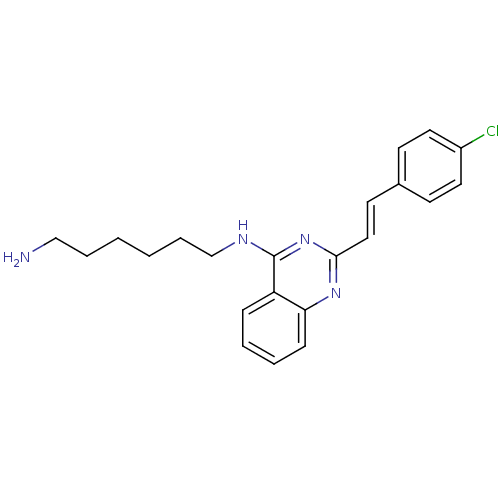 (4-aminoquinazoline derivative, 7a | N-(6-aminohexy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM26967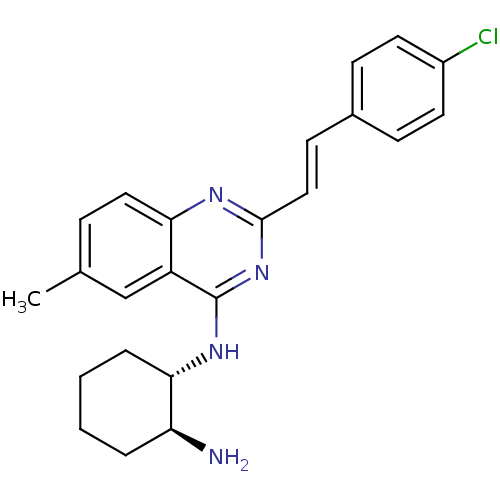 ((1S,2S)-1-N-{2-[(E)-2-(4-chlorophenyl)ethenyl]-6-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26965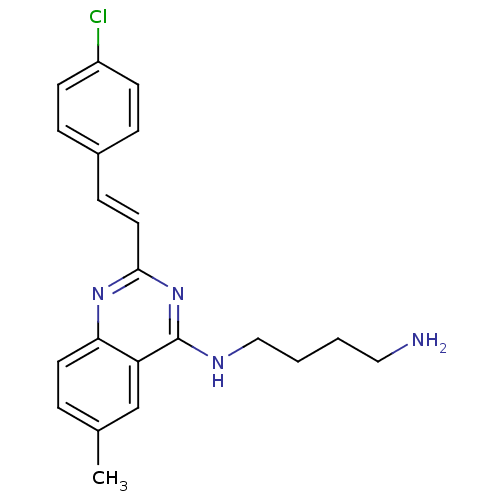 (4-aminoquinazoline derivative, 7h | N-(4-aminobuty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 194 | -38.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26968 ((1S,2R)-1-N-{2-[(E)-2-(4-chlorophenyl)ethenyl]-6-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 200 | -38.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM26961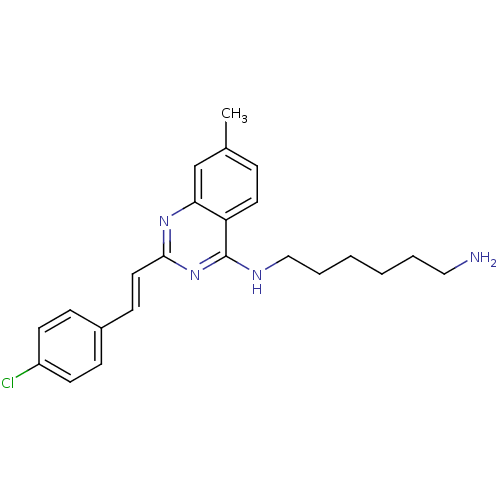 (4-aminoquinazoline derivative, 7d | N-(6-aminohexy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM26973 ((1R,2S)-2-({2-[(E)-2-(4-chlorophenyl)ethenyl]-6-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26960 (4-aminoquinazoline derivative, 7c | N-(6-aminohexy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 232 | -37.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM26966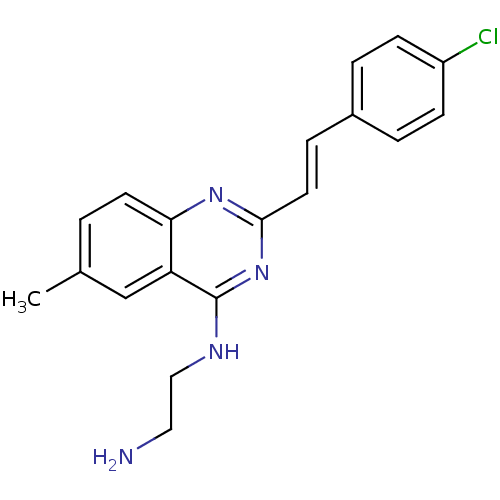 (4-aminoquinazoline derivative, 7i | N-(2-aminoethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM26965 (4-aminoquinazoline derivative, 7h | N-(4-aminobuty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM26977 (2-benzoylquinazoline analogue, (1S, 2R)-17 | N-(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26963 (4-aminoquinazoline derivative, 7f | N-(6-aminohexy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 292 | -37.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26964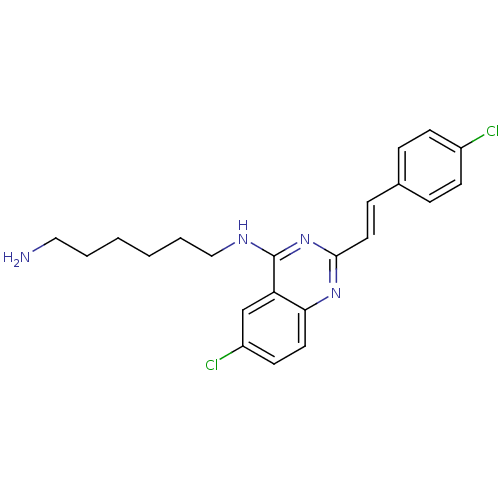 (4-aminoquinazoline derivative, 7g | N-(6-aminohexy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 294 | -37.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM26970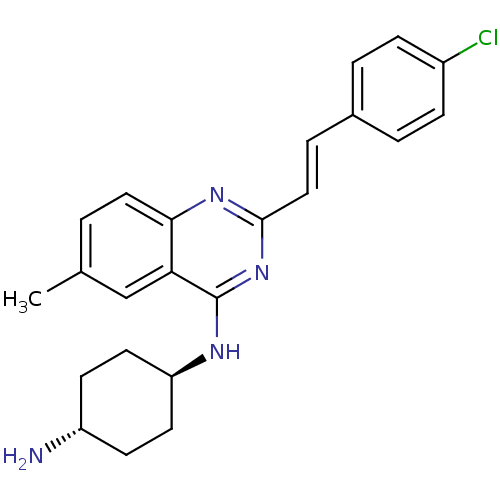 (1-N-{2-[(E)-2-(4-chlorophenyl)ethenyl]-6-methylqui...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM23420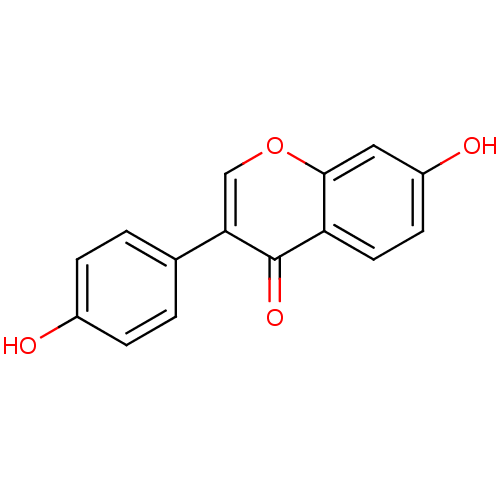 (7,4′-Dihydroxy-isoflavone (3a) | 7-hydroxy-3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of binding of 17 beta-estradiol to human Estrogen receptor alpha | Bioorg Med Chem Lett 11: 1839-42 (2001) BindingDB Entry DOI: 10.7270/Q29P325H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM26971 (3-[6-({2-[(E)-2-(4-chlorophenyl)ethenyl]-6-methylq...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50101979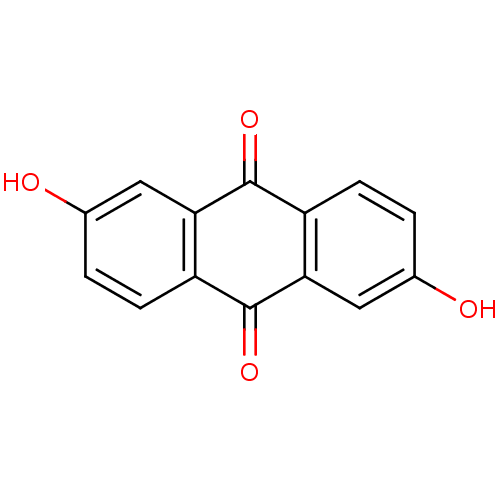 (2,6-Dihydroxyanthraquinone | 2,6-dihydroxy-9,10-an...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of binding of 17 beta-estradiol to human Estrogen receptor alpha | Bioorg Med Chem Lett 11: 1839-42 (2001) BindingDB Entry DOI: 10.7270/Q29P325H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM26975 (2-benzoylquinazoline analogue, (rac)-17 | N-{4-[(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM26976 (2-benzoylquinazoline analogue, (1R, 2S)-17 | N-(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19459 (5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of binding of 17 beta-estradiol to human Estrogen receptor alpha | Bioorg Med Chem Lett 11: 1839-42 (2001) BindingDB Entry DOI: 10.7270/Q29P325H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM26964 (4-aminoquinazoline derivative, 7g | N-(6-aminohexy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM26963 (4-aminoquinazoline derivative, 7f | N-(6-aminohexy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26958 (4-aminoquinazoline derivative, 7a | N-(6-aminohexy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 383 | -36.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM26969 (1-N-{2-[(E)-2-(4-chlorophenyl)ethenyl]-6-methylqui...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM26956 (N-(6-aminohexyl)-2-[(E)-2-(4-chlorophenyl)ethenyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26961 (4-aminoquinazoline derivative, 7d | N-(6-aminohexy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 420 | -36.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM26976 (2-benzoylquinazoline analogue, (1R, 2S)-17 | N-(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26967 ((1S,2S)-1-N-{2-[(E)-2-(4-chlorophenyl)ethenyl]-6-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 538 | -35.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26962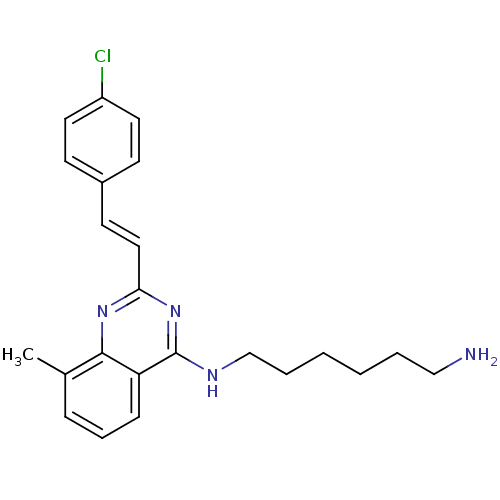 (4-aminoquinazoline derivative, 7e | N-(6-aminohexy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 653 | -35.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50101979 (2,6-Dihydroxyanthraquinone | 2,6-dihydroxy-9,10-an...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of binding of 17 beta-estradiol to human Estrogen receptor beta | Bioorg Med Chem Lett 11: 1839-42 (2001) BindingDB Entry DOI: 10.7270/Q29P325H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM11318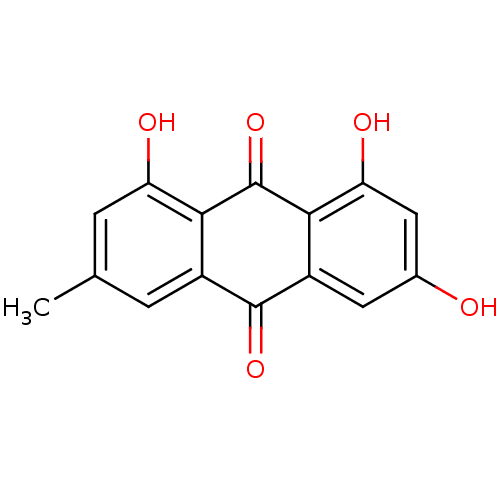 (1,3,8-trihydroxy-6-methyl-9,10-dihydroanthracene-9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of binding of 17 beta-estradiol to human Estrogen receptor alpha | Bioorg Med Chem Lett 11: 1839-42 (2001) BindingDB Entry DOI: 10.7270/Q29P325H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26970 (1-N-{2-[(E)-2-(4-chlorophenyl)ethenyl]-6-methylqui...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 795 | -34.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26959 (4-aminoquinazoline derivative, 7b | N-(6-aminohexy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 808 | -34.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM26962 (4-aminoquinazoline derivative, 7e | N-(6-aminohexy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM26966 (4-aminoquinazoline derivative, 7i | N-(2-aminoethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 972 | -34.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM26977 (2-benzoylquinazoline analogue, (1S, 2R)-17 | N-(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co. | Assay Description Competitive binding displacement analysis was performed with membranes prepared from CHO-K1 cells stably expressing receptors. After incubation, samp... | Bioorg Med Chem 17: 119-32 (2009) Article DOI: 10.1016/j.bmc.2008.11.012 BindingDB Entry DOI: 10.7270/Q2Q52MX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM11318 (1,3,8-trihydroxy-6-methyl-9,10-dihydroanthracene-9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of binding of 17 beta-estradiol to human Estrogen receptor beta | Bioorg Med Chem Lett 11: 1839-42 (2001) BindingDB Entry DOI: 10.7270/Q29P325H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 987 total ) | Next | Last >> |