 Found 14 hits with Last Name = 'sun' and Initial = 'qy'
Found 14 hits with Last Name = 'sun' and Initial = 'qy' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221070
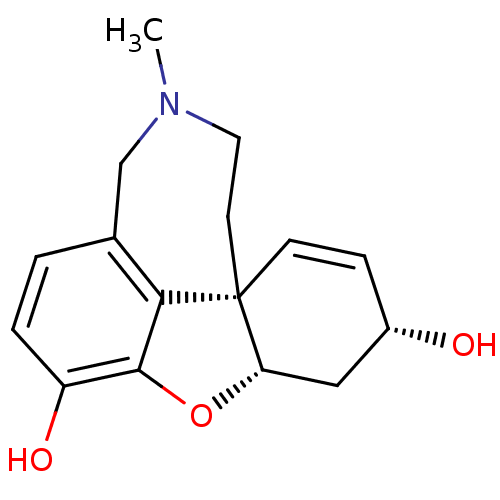
(CHEMBL1524 | Sanguinine)Show SMILES CN1CC[C@@]23C=C[C@H](O)C[C@@H]2Oc2c3c(C1)ccc2O |c:5| Show InChI InChI=1S/C16H19NO3/c1-17-7-6-16-5-4-11(18)8-13(16)20-15-12(19)3-2-10(9-17)14(15)16/h2-5,11,13,18-19H,6-9H2,1H3/t11-,13-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of AChE by Ellman method |
J Nat Prod 70: 1458-61 (2007)
Article DOI: 10.1021/np0702077
BindingDB Entry DOI: 10.7270/Q2MK6DQQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's assay |
J Nat Prod 72: 1151-4 (2009)
Article DOI: 10.1021/np9001515
BindingDB Entry DOI: 10.7270/Q22807ND |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of AChE by Ellman method |
J Nat Prod 70: 1458-61 (2007)
Article DOI: 10.1021/np0702077
BindingDB Entry DOI: 10.7270/Q2MK6DQQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221063

((1S,2S,12bS,12cS)-1,2-Diol-2,4,5,7, 12b,12c-hexahy...)Show SMILES CC(=O)O[C@@H]1[C@@H](O)C=C2CCN3Cc4cc5OCOc5cc4[C@H]1[C@@H]23 |r,t:7| Show InChI InChI=1S/C18H19NO5/c1-9(20)24-18-13(21)4-10-2-3-19-7-11-5-14-15(23-8-22-14)6-12(11)16(18)17(10)19/h4-6,13,16-18,21H,2-3,7-8H2,1H3/t13-,16-,17+,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of AChE by Ellman method |
J Nat Prod 70: 1458-61 (2007)
Article DOI: 10.1021/np0702077
BindingDB Entry DOI: 10.7270/Q2MK6DQQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of AChE by Ellman method |
J Nat Prod 70: 1458-61 (2007)
Article DOI: 10.1021/np0702077
BindingDB Entry DOI: 10.7270/Q2MK6DQQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221061
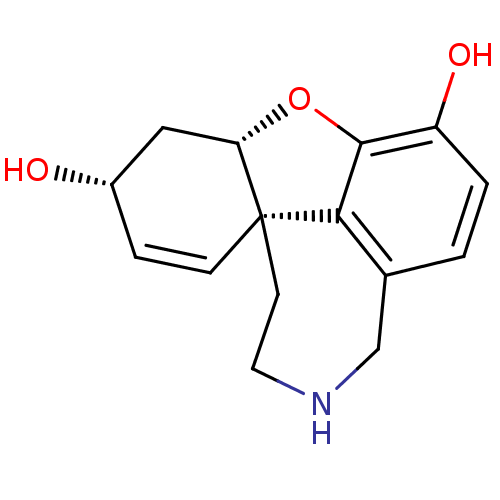
(CHEMBL1523 | norsanguinine)Show SMILES O[C@@H]1C[C@@H]2Oc3c4c(CNCC[C@@]24C=C1)ccc3O |c:15| Show InChI InChI=1S/C15H17NO3/c17-10-3-4-15-5-6-16-8-9-1-2-11(18)14(13(9)15)19-12(15)7-10/h1-4,10,12,16-18H,5-8H2/t10-,12-,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of AChE by Ellman method |
J Nat Prod 70: 1458-61 (2007)
Article DOI: 10.1021/np0702077
BindingDB Entry DOI: 10.7270/Q2MK6DQQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221068
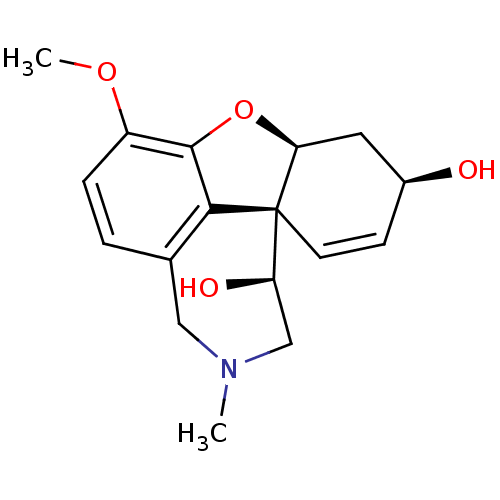
(11-Hydroxygalantamine | CHEMBL409610)Show SMILES COc1ccc2CN(C)C[C@@H](O)[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |c:13| Show InChI InChI=1S/C17H21NO4/c1-18-8-10-3-4-12(21-2)16-15(10)17(13(20)9-18)6-5-11(19)7-14(17)22-16/h3-6,11,13-14,19-20H,7-9H2,1-2H3/t11-,13+,14-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of AChE by Ellman method |
J Nat Prod 70: 1458-61 (2007)
Article DOI: 10.1021/np0702077
BindingDB Entry DOI: 10.7270/Q2MK6DQQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221065

(8-demethoxy-10-O-methylhostasine | CHEMBL252723)Show SMILES COc1cc2C(=O)O[C@@]3([C@@H]4N(C)CCC4=C[C@H](O)[C@H]3O)c2cc1OC |c:15| Show InChI InChI=1S/C18H21NO6/c1-19-5-4-9-6-12(20)16(21)18(15(9)19)11-8-14(24-3)13(23-2)7-10(11)17(22)25-18/h6-8,12,15-16,20-21H,4-5H2,1-3H3/t12-,15+,16+,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of AChE by Ellman method |
J Nat Prod 70: 1458-61 (2007)
Article DOI: 10.1021/np0702077
BindingDB Entry DOI: 10.7270/Q2MK6DQQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50358566
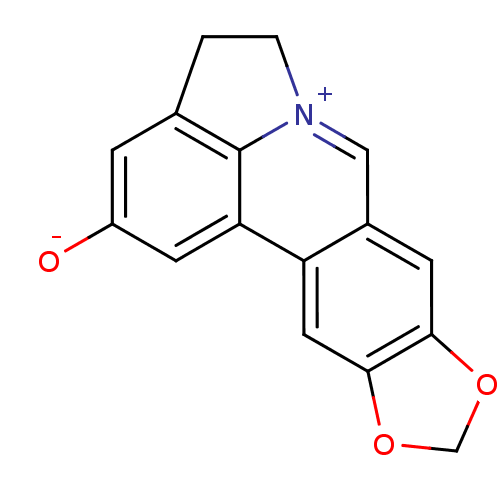
(UNGEREMINE)Show InChI InChI=1S/C16H11NO3/c18-11-3-9-1-2-17-7-10-4-14-15(20-8-19-14)6-12(10)13(5-11)16(9)17/h3-7H,1-2,8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of AChE by Ellman method |
J Nat Prod 70: 1458-61 (2007)
Article DOI: 10.1021/np0702077
BindingDB Entry DOI: 10.7270/Q2MK6DQQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221062
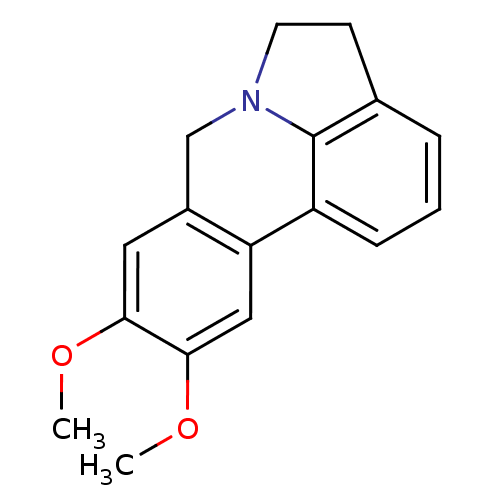
(9,10-dimethoxy-5,7-dihydro-4H-pyrrolo[3,2,1-de]phe...)Show InChI InChI=1S/C17H17NO2/c1-19-15-8-12-10-18-7-6-11-4-3-5-13(17(11)18)14(12)9-16(15)20-2/h3-5,8-9H,6-7,10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of AChE by Ellman method |
J Nat Prod 70: 1458-61 (2007)
Article DOI: 10.1021/np0702077
BindingDB Entry DOI: 10.7270/Q2MK6DQQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221072
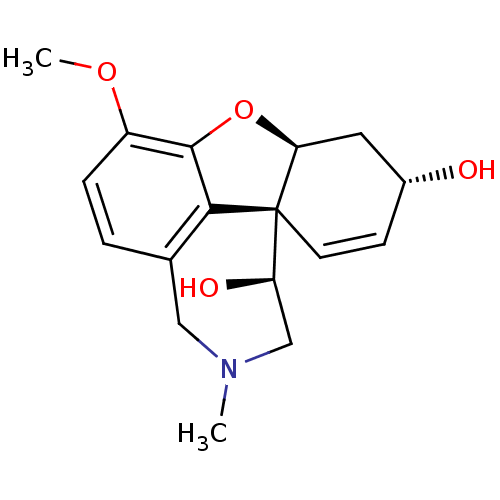
(CHEMBL251076 | Epinorgalantamine)Show SMILES COc1ccc2CN(C)C[C@@H](O)[C@@]34C=C[C@@H](O)C[C@@H]3Oc1c24 |c:13| Show InChI InChI=1S/C17H21NO4/c1-18-8-10-3-4-12(21-2)16-15(10)17(13(20)9-18)6-5-11(19)7-14(17)22-16/h3-6,11,13-14,19-20H,7-9H2,1-2H3/t11-,13-,14+,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of AChE by Ellman method |
J Nat Prod 70: 1458-61 (2007)
Article DOI: 10.1021/np0702077
BindingDB Entry DOI: 10.7270/Q2MK6DQQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221067
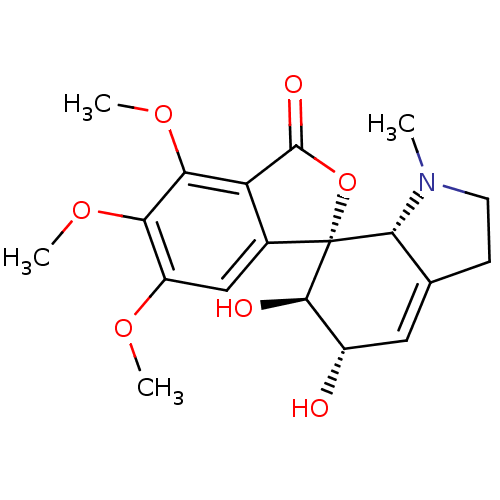
(10-O-methylhostasine | CHEMBL250688)Show SMILES COc1cc2c(C(=O)O[C@@]22[C@@H]3N(C)CCC3=C[C@H](O)[C@H]2O)c(OC)c1OC |c:17| Show InChI InChI=1S/C19H23NO7/c1-20-6-5-9-7-11(21)17(22)19(16(9)20)10-8-12(24-2)14(25-3)15(26-4)13(10)18(23)27-19/h7-8,11,16-17,21-22H,5-6H2,1-4H3/t11-,16+,17+,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.13E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of AChE by Ellman method |
J Nat Prod 70: 1458-61 (2007)
Article DOI: 10.1021/np0702077
BindingDB Entry DOI: 10.7270/Q2MK6DQQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221066
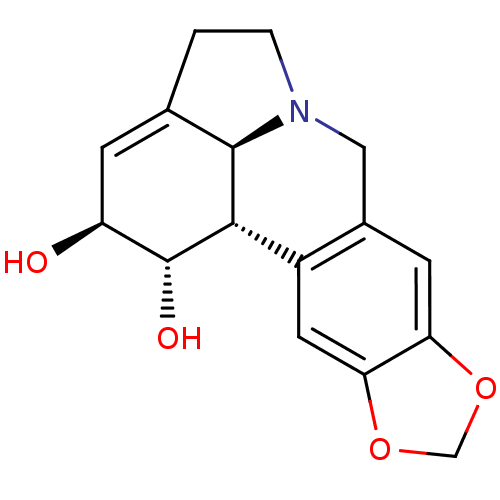
((-)-lycorine | 9,10-(methylenedioxy)-3,12-didehydr...)Show SMILES O[C@H]1C=C2CCN3Cc4cc5OCOc5cc4[C@@H]([C@@H]23)[C@@H]1O |r,t:2| Show InChI InChI=1S/C16H17NO4/c18-11-3-8-1-2-17-6-9-4-12-13(21-7-20-12)5-10(9)14(15(8)17)16(11)19/h3-5,11,14-16,18-19H,1-2,6-7H2/t11-,14-,15+,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.55E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of AChE by Ellman method |
J Nat Prod 70: 1458-61 (2007)
Article DOI: 10.1021/np0702077
BindingDB Entry DOI: 10.7270/Q2MK6DQQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50221069
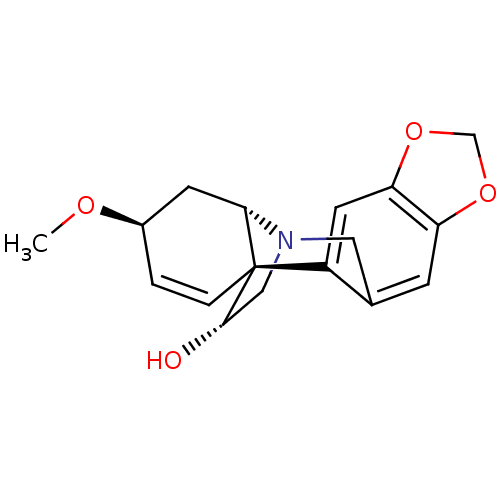
((+)-haemanthamine | CHEMBL401114)Show SMILES CO[C@H]1C[C@@H]2N3C[C@H](O)[C@@]2(C=C1)c1cc2OCOc2cc1C3 |c:11| Show InChI InChI=1S/C17H19NO4/c1-20-11-2-3-17-12-6-14-13(21-9-22-14)4-10(12)7-18(8-16(17)19)15(17)5-11/h2-4,6,11,15-16,19H,5,7-9H2,1H3/t11-,15+,16+,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.52E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of AChE by Ellman method |
J Nat Prod 70: 1458-61 (2007)
Article DOI: 10.1021/np0702077
BindingDB Entry DOI: 10.7270/Q2MK6DQQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data