 Found 198 hits with Last Name = 'sundaram' and Initial = 's'
Found 198 hits with Last Name = 'sundaram' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251493

((2S,4S)-1-(2-(1-(4-cyano-3,5-difluorophenyl)piperi...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CNC1CCN(CC1)c1cc(F)c(C#N)c(F)c1 |r| Show InChI InChI=1S/C19H20F3N5O/c20-12-5-15(8-23)27(11-12)19(28)10-25-13-1-3-26(4-2-13)14-6-17(21)16(9-24)18(22)7-14/h6-7,12-13,15,25H,1-5,10-11H2/t12-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251494
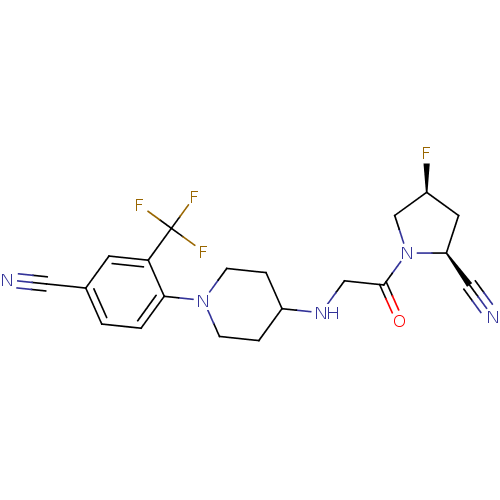
((2S,4S)-1-(2-(1-(4-cyano-2-(trifluoromethyl)phenyl...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CNC1CCN(CC1)c1ccc(cc1C(F)(F)F)C#N |r| Show InChI InChI=1S/C20H21F4N5O/c21-14-8-16(10-26)29(12-14)19(30)11-27-15-3-5-28(6-4-15)18-2-1-13(9-25)7-17(18)20(22,23)24/h1-2,7,14-16,27H,3-6,8,11-12H2/t14-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251495
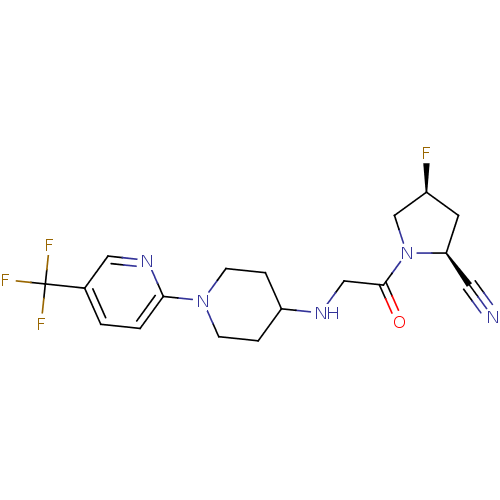
((2S,4S)-4-fluoro-1-(2-(1-(5-(trifluoromethyl)pyrid...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CNC1CCN(CC1)c1ccc(cn1)C(F)(F)F |r| Show InChI InChI=1S/C18H21F4N5O/c19-13-7-15(8-23)27(11-13)17(28)10-24-14-3-5-26(6-4-14)16-2-1-12(9-25-16)18(20,21)22/h1-2,9,13-15,24H,3-7,10-11H2/t13-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251517
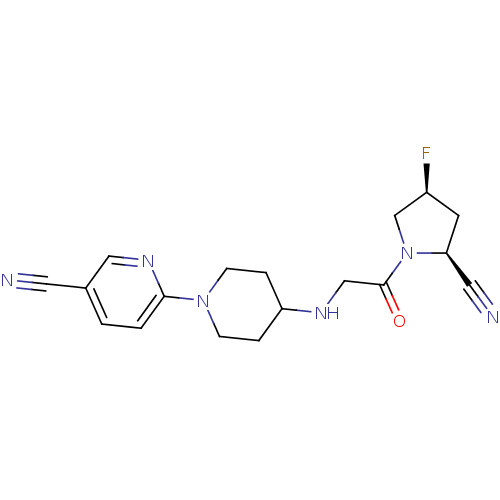
(6-(4-(2-((2S,4S)-2-cyano-4-fluoropyrrolidin-1-yl)-...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CNC1CCN(CC1)c1ccc(cn1)C#N |r| Show InChI InChI=1S/C18H21FN6O/c19-14-7-16(9-21)25(12-14)18(26)11-22-15-3-5-24(6-4-15)17-2-1-13(8-20)10-23-17/h1-2,10,14-16,22H,3-7,11-12H2/t14-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251518
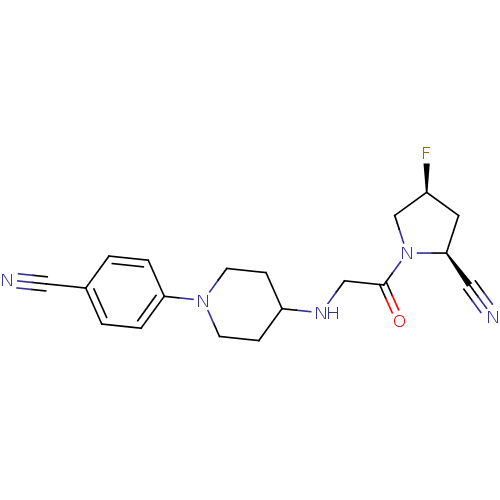
((2S,4S)-1-(2-(1-(4-cyanophenyl)piperidin-4-ylamino...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CNC1CCN(CC1)c1ccc(cc1)C#N |r| Show InChI InChI=1S/C19H22FN5O/c20-15-9-18(11-22)25(13-15)19(26)12-23-16-5-7-24(8-6-16)17-3-1-14(10-21)2-4-17/h1-4,15-16,18,23H,5-9,12-13H2/t15-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50608608
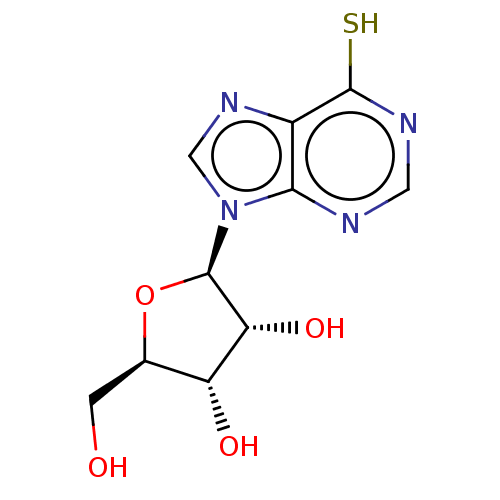
(THIOINOSINE | Thioinosine)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(S)ncnc12 |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50423778
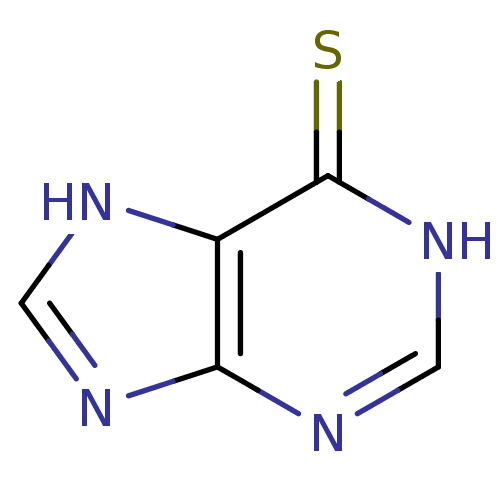
(Leukerin | MERCAPTOPURINE | Mercaleukin | Mercapto...)Show InChI InChI=1S/C5H4N4S/c10-5-3-4(7-1-6-3)8-2-9-5/h1-2H,(H2,6,7,8,9,10) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
UniChem
| | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50200099
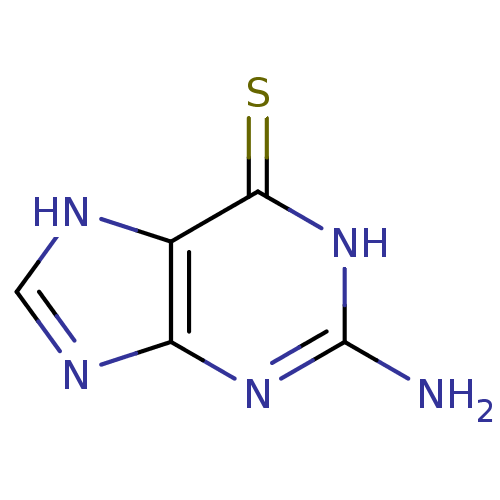
(2-Amino-1,7-dihydro-purine-6-thione | 2-Amino-1,9-...)Show InChI InChI=1S/C5H5N5S/c6-5-9-3-2(4(11)10-5)7-1-8-3/h1H,(H4,6,7,8,9,10,11) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
| | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50373919

(AZATHIOPRINE | Azasan)Show InChI InChI=1S/C9H7N7O2S/c1-15-4-14-7(16(17)18)9(15)19-8-5-6(11-2-10-5)12-3-13-8/h2-4H,1H3,(H,10,11,12,13) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
| | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM14774

(3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...)Show SMILES FC(F)Oc1ccc(cc1OCC1CC1)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PDE4B using [3H]cAMP as substrate incubated for 5 mins followed by substrate addition and measured after 10 mins by scintillation... |
Citation and Details
Article DOI: 10.1016/j.bmc.2016.09.011
BindingDB Entry DOI: 10.7270/Q2N58R28 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50558593
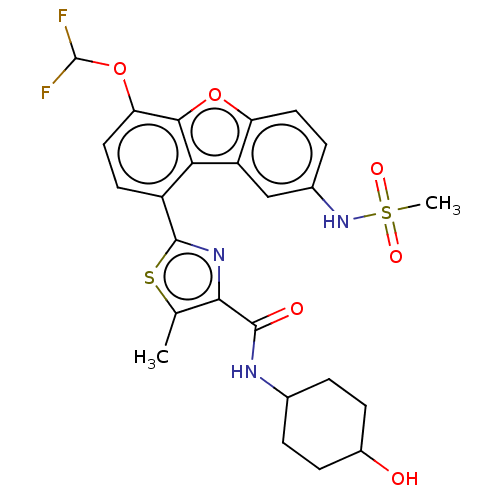
(CHEMBL4747406)Show SMILES Cc1sc(nc1C(=O)NC1CCC(O)CC1)-c1ccc(OC(F)F)c2oc3ccc(NS(C)(=O)=O)cc3c12 |(50.66,-6.46,;51.56,-7.7,;51.09,-9.17,;52.35,-10.07,;53.59,-9.16,;53.11,-7.69,;54.01,-6.45,;53.38,-5.04,;55.54,-6.61,;56.44,-5.36,;55.81,-3.96,;56.72,-2.71,;58.25,-2.87,;59.15,-1.62,;58.87,-4.27,;57.97,-5.52,;52.36,-11.61,;51.02,-12.39,;51.02,-13.94,;52.36,-14.71,;52.37,-16.26,;51.04,-17.03,;51.04,-18.57,;49.7,-16.26,;53.69,-13.93,;55.17,-14.41,;56.08,-13.15,;57.61,-12.99,;58.23,-11.58,;57.32,-10.33,;57.94,-8.92,;59.46,-8.75,;60.08,-7.35,;60.95,-9.15,;59.86,-10.24,;55.79,-10.5,;55.17,-11.91,;53.69,-12.38,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PDE4B using [3H]cAMP as substrate incubated for 5 mins followed by substrate addition and measured after 10 mins by scintillation... |
Citation and Details
Article DOI: 10.1016/j.bmc.2016.09.011
BindingDB Entry DOI: 10.7270/Q2N58R28 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251413
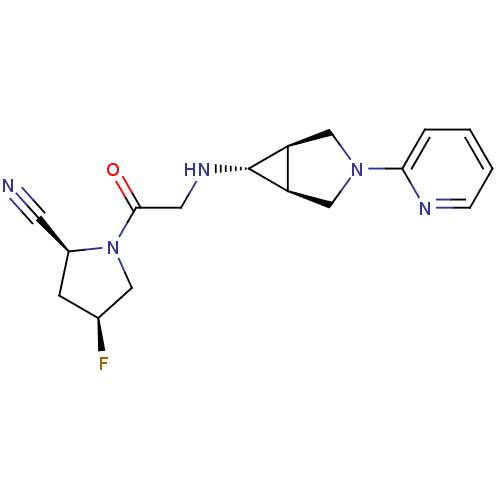
((2S,4S)-4-fluoro-1-(2-((1S,5R,6s)-3-(pyridin-2-yl)...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CN[C@@H]1[C@H]2CN(C[C@@H]12)c1ccccn1 |r| Show InChI InChI=1S/C17H20FN5O/c18-11-5-12(6-19)23(8-11)16(24)7-21-17-13-9-22(10-14(13)17)15-3-1-2-4-20-15/h1-4,11-14,17,21H,5,7-10H2/t11-,12-,13-,14+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251758

((2S,4S)-1-(2-((1S,5R,6s)-3-(3-chloro-2-cyanophenyl...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CN[C@@H]1[C@H]2CN(C[C@@H]12)c1cccc(Cl)c1C#N |r| Show InChI InChI=1S/C19H19ClFN5O/c20-16-2-1-3-17(13(16)6-23)25-9-14-15(10-25)19(14)24-7-18(27)26-8-11(21)4-12(26)5-22/h1-3,11-12,14-15,19,24H,4,7-10H2/t11-,12-,14-,15+,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50558594
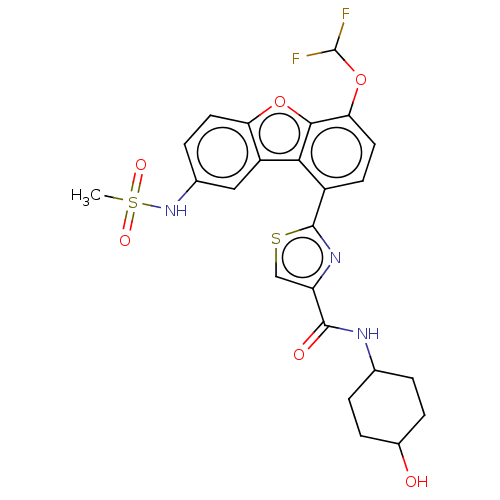
(CHEMBL4744412)Show SMILES CS(=O)(=O)Nc1ccc2oc3c(OC(F)F)ccc(-c4nc(cs4)C(=O)NC4CCC(O)CC4)c3c2c1 |(15.21,-7.74,;14.59,-9.14,;16.07,-9.54,;14.98,-10.62,;13.06,-9.31,;12.44,-10.72,;13.35,-11.96,;12.73,-13.37,;11.2,-13.54,;10.29,-14.79,;8.82,-14.32,;7.49,-15.1,;7.49,-16.64,;6.16,-17.41,;6.16,-18.95,;4.83,-16.65,;6.15,-14.33,;6.15,-12.78,;7.48,-12,;7.47,-10.46,;8.71,-9.55,;8.23,-8.08,;6.69,-8.09,;6.22,-9.56,;9.14,-6.84,;8.51,-5.43,;10.67,-6.99,;11.57,-5.75,;10.94,-4.34,;11.84,-3.1,;13.37,-3.25,;14.27,-2.01,;14,-4.66,;13.1,-5.9,;8.82,-12.77,;10.29,-12.29,;10.91,-10.89,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PDE4B using [3H]cAMP as substrate incubated for 5 mins followed by substrate addition and measured after 10 mins by scintillation... |
Citation and Details
Article DOI: 10.1016/j.bmc.2016.09.011
BindingDB Entry DOI: 10.7270/Q2N58R28 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251681
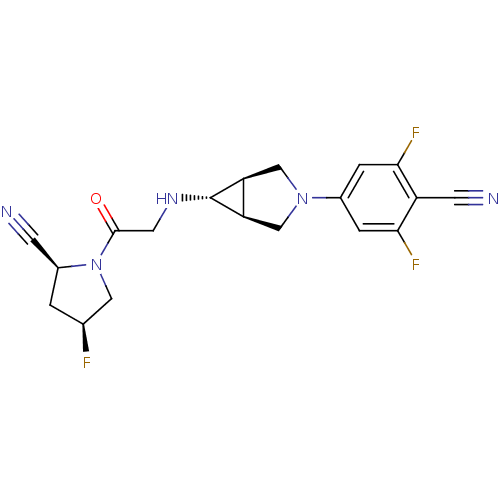
((2S,4S)-1-(2-((1S,5R,6s)-3-(4-cyano-3,5-difluoroph...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CN[C@@H]1[C@H]2CN(C[C@@H]12)c1cc(F)c(C#N)c(F)c1 |r| Show InChI InChI=1S/C19H18F3N5O/c20-10-1-12(4-23)27(7-10)18(28)6-25-19-14-8-26(9-15(14)19)11-2-16(21)13(5-24)17(22)3-11/h2-3,10,12,14-15,19,25H,1,6-9H2/t10-,12-,14-,15+,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50558595
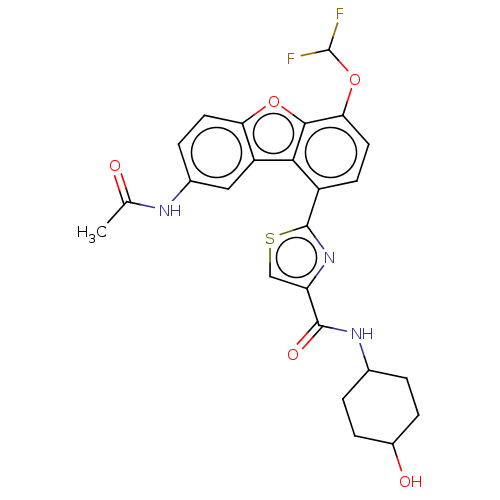
(CHEMBL4764245)Show SMILES CC(=O)Nc1ccc2oc3c(OC(F)F)ccc(-c4nc(cs4)C(=O)NC4CCC(O)CC4)c3c2c1 |(67.92,-47.27,;67.3,-48.68,;68.21,-49.92,;65.78,-48.84,;65.16,-50.25,;66.07,-51.5,;65.45,-52.91,;63.91,-53.07,;63,-54.33,;61.53,-53.85,;60.2,-54.63,;60.2,-56.17,;58.87,-56.95,;58.88,-58.49,;57.54,-56.18,;58.86,-53.86,;58.86,-52.31,;60.2,-51.53,;60.19,-49.99,;61.43,-49.08,;60.95,-47.61,;59.4,-47.62,;58.93,-49.09,;61.85,-46.37,;61.22,-44.96,;63.38,-46.53,;64.28,-45.28,;63.65,-43.88,;64.55,-42.63,;66.08,-42.79,;66.99,-41.54,;66.71,-44.19,;65.81,-45.44,;61.53,-52.3,;63,-51.83,;63.63,-50.42,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PDE4B using [3H]cAMP as substrate incubated for 5 mins followed by substrate addition and measured after 10 mins by scintillation... |
Citation and Details
Article DOI: 10.1016/j.bmc.2016.09.011
BindingDB Entry DOI: 10.7270/Q2N58R28 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251786
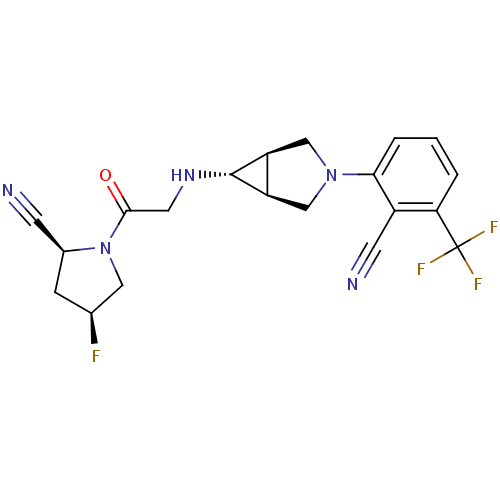
((2S,4S)-1-(2-((1S,5R,6s)-3-(2-cyano-3-(trifluorome...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CN[C@@H]1[C@H]2CN(C[C@@H]12)c1cccc(c1C#N)C(F)(F)F |r| Show InChI InChI=1S/C20H19F4N5O/c21-11-4-12(5-25)29(8-11)18(30)7-27-19-14-9-28(10-15(14)19)17-3-1-2-16(13(17)6-26)20(22,23)24/h1-3,11-12,14-15,19,27H,4,7-10H2/t11-,12-,14-,15+,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50558598
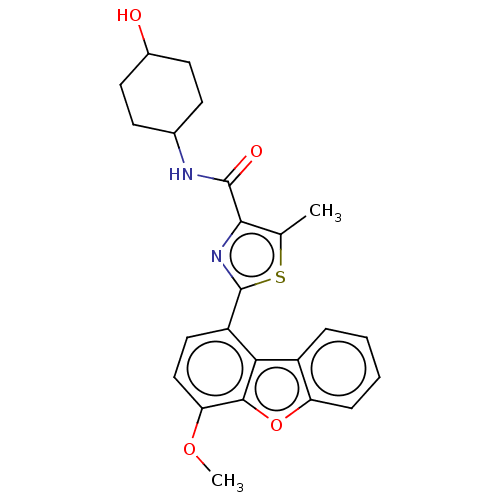
(CHEMBL4756900)Show SMILES COc1ccc(-c2nc(C(=O)NC3CCC(O)CC3)c(C)s2)c2c3ccccc3oc12 |(44.37,-16.57,;43.04,-15.79,;43.03,-14.25,;41.69,-13.48,;41.7,-11.93,;43.03,-11.15,;43.02,-9.61,;44.26,-8.7,;43.78,-7.24,;44.68,-5.99,;44.05,-4.59,;46.21,-6.15,;47.11,-4.9,;46.49,-3.5,;47.39,-2.25,;48.92,-2.41,;49.82,-1.16,;49.55,-3.81,;48.64,-5.06,;42.24,-7.24,;41.33,-6,;41.77,-8.71,;44.36,-11.92,;45.84,-11.45,;46.46,-10.04,;47.99,-9.87,;48.9,-11.12,;48.28,-12.53,;46.75,-12.69,;45.84,-13.95,;44.36,-13.47,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PDE4B using [3H]cAMP as substrate incubated for 5 mins followed by substrate addition and measured after 10 mins by scintillation... |
Citation and Details
Article DOI: 10.1016/j.bmc.2016.09.011
BindingDB Entry DOI: 10.7270/Q2N58R28 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50251852
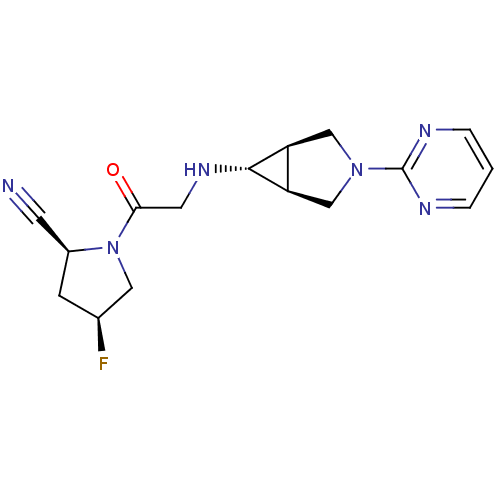
((2S,4S)-4-fluoro-1-(2-((1S,5R,6s)-3-(pyrimidin-2-y...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CN[C@@H]1[C@H]2CN(C[C@@H]12)c1ncccn1 |r| Show InChI InChI=1S/C16H19FN6O/c17-10-4-11(5-18)23(7-10)14(24)6-21-15-12-8-22(9-13(12)15)16-19-2-1-3-20-16/h1-3,10-13,15,21H,4,6-9H2/t10-,11-,12-,13+,15+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PPCE (unknown origin) |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50558600
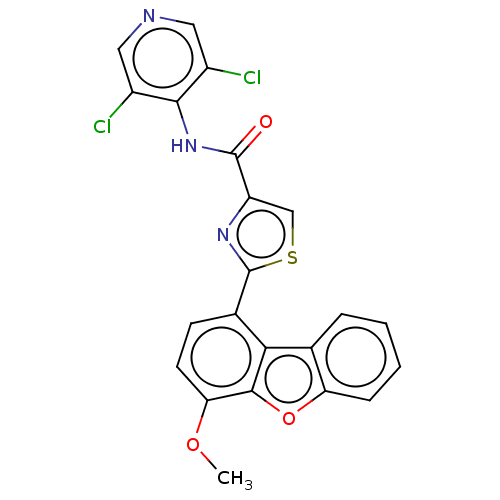
(CHEMBL4744894)Show SMILES COc1ccc(-c2nc(cs2)C(=O)Nc2c(Cl)cncc2Cl)c2c3ccccc3oc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PDE4B using [3H]cAMP as substrate incubated for 5 mins followed by substrate addition and measured after 10 mins by scintillation... |
Citation and Details
Article DOI: 10.1016/j.bmc.2016.09.011
BindingDB Entry DOI: 10.7270/Q2N58R28 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251414
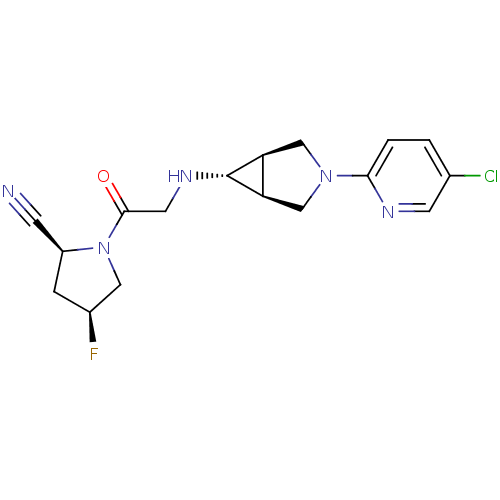
((2S,4S)-1-(2-((1S,5R,6s)-3-(5-chloropyridin-2-yl)-...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CN[C@@H]1[C@H]2CN(C[C@@H]12)c1ccc(Cl)cn1 |r| Show InChI InChI=1S/C17H19ClFN5O/c18-10-1-2-15(21-5-10)23-8-13-14(9-23)17(13)22-6-16(25)24-7-11(19)3-12(24)4-20/h1-2,5,11-14,17,22H,3,6-9H2/t11-,12-,13-,14+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50558599
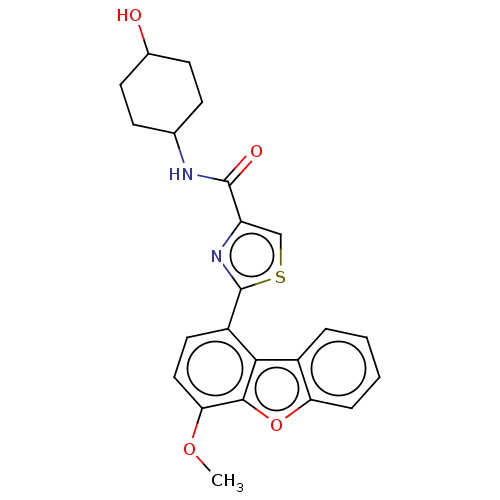
(CHEMBL4799096)Show SMILES COc1ccc(-c2nc(cs2)C(=O)NC2CCC(O)CC2)c2c3ccccc3oc12 |(61.11,-56.78,;59.77,-56.01,;59.77,-54.47,;58.43,-53.7,;58.43,-52.15,;59.77,-51.37,;59.76,-49.84,;61,-48.93,;60.52,-47.46,;58.97,-47.47,;58.5,-48.94,;61.42,-46.21,;60.79,-44.8,;62.96,-46.36,;63.86,-45.12,;63.23,-43.72,;64.13,-42.47,;65.66,-42.63,;66.56,-41.38,;66.29,-44.03,;65.39,-45.27,;61.1,-52.14,;62.57,-51.66,;63.19,-50.26,;64.72,-50.09,;65.63,-51.34,;65.01,-52.75,;63.48,-52.91,;62.57,-54.17,;61.1,-53.69,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PDE4B using [3H]cAMP as substrate incubated for 5 mins followed by substrate addition and measured after 10 mins by scintillation... |
Citation and Details
Article DOI: 10.1016/j.bmc.2016.09.011
BindingDB Entry DOI: 10.7270/Q2N58R28 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251757

((2S,4S)-1-(2-((1S,5R,6s)-3-(2-cyanophenyl)-3-aza-b...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CN[C@@H]1[C@H]2CN(C[C@@H]12)c1ccccc1C#N |r| Show InChI InChI=1S/C19H20FN5O/c20-13-5-14(7-22)25(9-13)18(26)8-23-19-15-10-24(11-16(15)19)17-4-2-1-3-12(17)6-21/h1-4,13-16,19,23H,5,8-11H2/t13-,14-,15-,16+,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251684
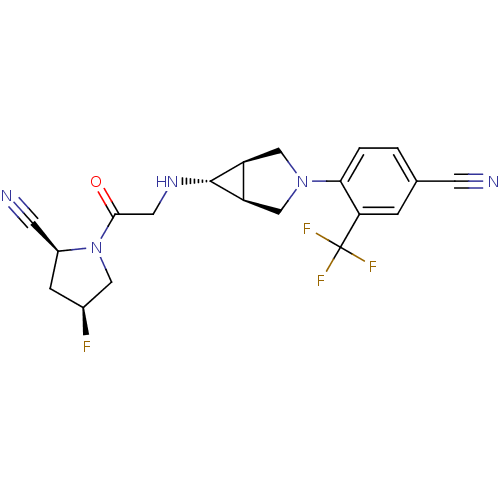
((2S,4S)-1-(2-((1S,5R,6s)-3-(4-cyano-2-(trifluorome...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CN[C@@H]1[C@H]2CN(C[C@@H]12)c1ccc(cc1C(F)(F)F)C#N |r| Show InChI InChI=1S/C20H19F4N5O/c21-12-4-13(6-26)29(8-12)18(30)7-27-19-14-9-28(10-15(14)19)17-2-1-11(5-25)3-16(17)20(22,23)24/h1-3,12-15,19,27H,4,7-10H2/t12-,13-,14-,15+,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251787
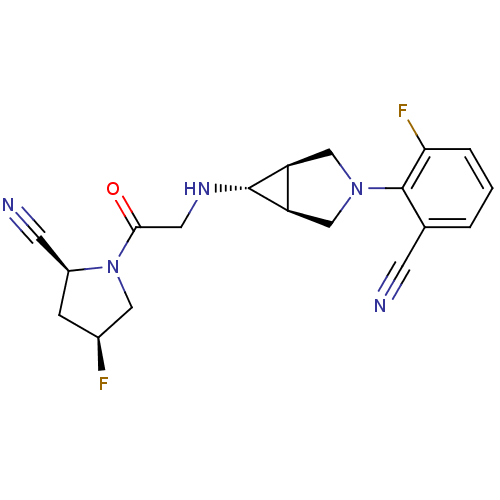
((2S,4S)-1-(2-((1S,5R,6s)-3-(2-cyano-6-fluorophenyl...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CN[C@@H]1[C@H]2CN(C[C@@H]12)c1c(F)cccc1C#N |r| Show InChI InChI=1S/C19H19F2N5O/c20-12-4-13(6-23)26(8-12)17(27)7-24-18-14-9-25(10-15(14)18)19-11(5-22)2-1-3-16(19)21/h1-3,12-15,18,24H,4,7-10H2/t12-,13-,14-,15+,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50558596
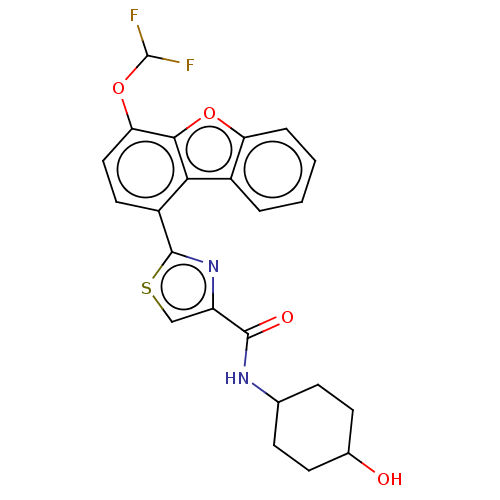
(CHEMBL4791975)Show SMILES OC1CCC(CC1)NC(=O)c1csc(n1)-c1ccc(OC(F)F)c2oc3ccccc3c12 |(67.36,-20.14,;66.46,-21.38,;64.93,-21.23,;64.03,-22.47,;64.66,-23.87,;66.19,-24.03,;67.09,-22.79,;63.76,-25.12,;62.23,-24.96,;61.6,-23.56,;61.33,-26.21,;59.78,-26.21,;59.31,-27.69,;60.57,-28.59,;61.81,-27.68,;60.58,-30.13,;59.24,-30.9,;59.24,-32.45,;60.58,-33.22,;60.58,-34.77,;59.25,-35.54,;59.26,-37.08,;57.92,-34.78,;61.91,-32.45,;63.38,-32.92,;64.29,-31.66,;65.82,-31.5,;66.45,-30.09,;65.54,-28.85,;64.01,-29.02,;63.38,-30.42,;61.91,-30.9,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PDE4B using [3H]cAMP as substrate incubated for 5 mins followed by substrate addition and measured after 10 mins by scintillation... |
Citation and Details
Article DOI: 10.1016/j.bmc.2016.09.011
BindingDB Entry DOI: 10.7270/Q2N58R28 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50251682
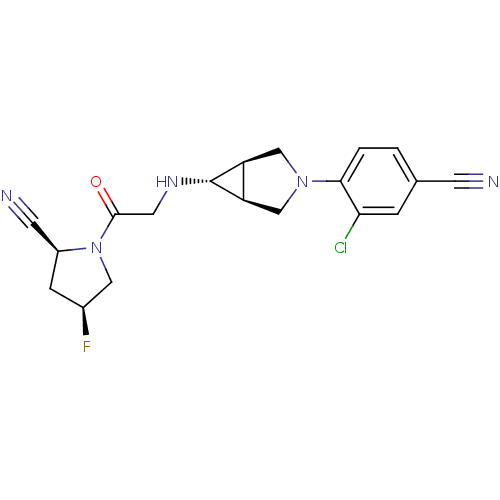
((2S,4S)-1-(2-((1S,5R,6s)-3-(2-chloro-4-cyanophenyl...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CN[C@@H]1[C@H]2CN(C[C@@H]12)c1ccc(cc1Cl)C#N |r| Show InChI InChI=1S/C19H19ClFN5O/c20-16-3-11(5-22)1-2-17(16)25-9-14-15(10-25)19(14)24-7-18(27)26-8-12(21)4-13(26)6-23/h1-3,12-15,19,24H,4,7-10H2/t12-,13-,14-,15+,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP9 (unknown origin) |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50248919

((S)-N-(2-(1-(3-ethoxy-4-methoxyphenyl)-2-(methylsu...)Show SMILES CCOc1cc(ccc1OC)[C@@H](CS(C)(=O)=O)N1C(=O)c2cccc(NC(C)=O)c2C1=O |r| Show InChI InChI=1S/C22H24N2O7S/c1-5-31-19-11-14(9-10-18(19)30-3)17(12-32(4,28)29)24-21(26)15-7-6-8-16(23-13(2)25)20(15)22(24)27/h6-11,17H,5,12H2,1-4H3,(H,23,25)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PDE4B using [3H]cAMP as substrate incubated for 5 mins followed by substrate addition and measured after 10 mins by scintillation... |
Citation and Details
Article DOI: 10.1016/j.bmc.2016.09.011
BindingDB Entry DOI: 10.7270/Q2N58R28 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251467
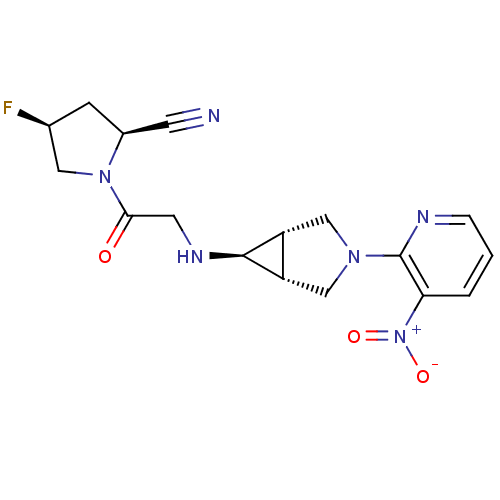
((2S,4S)-4-fluoro-1-(2-((1S,5R,6s)-3-(3-nitropyridi...)Show SMILES [O-][N+](=O)c1cccnc1N1C[C@H]2[C@@H](C1)[C@@H]2NCC(=O)N1C[C@@H](F)C[C@H]1C#N |r| Show InChI InChI=1S/C17H19FN6O3/c18-10-4-11(5-19)23(7-10)15(25)6-21-16-12-8-22(9-13(12)16)17-14(24(26)27)2-1-3-20-17/h1-3,10-13,16,21H,4,6-9H2/t10-,11-,12-,13+,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251852

((2S,4S)-4-fluoro-1-(2-((1S,5R,6s)-3-(pyrimidin-2-y...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CN[C@@H]1[C@H]2CN(C[C@@H]12)c1ncccn1 |r| Show InChI InChI=1S/C16H19FN6O/c17-10-4-11(5-18)23(7-10)14(24)6-21-15-12-8-22(9-13(12)15)16-19-2-1-3-20-16/h1-3,10-13,15,21H,4,6-9H2/t10-,11-,12-,13+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251427
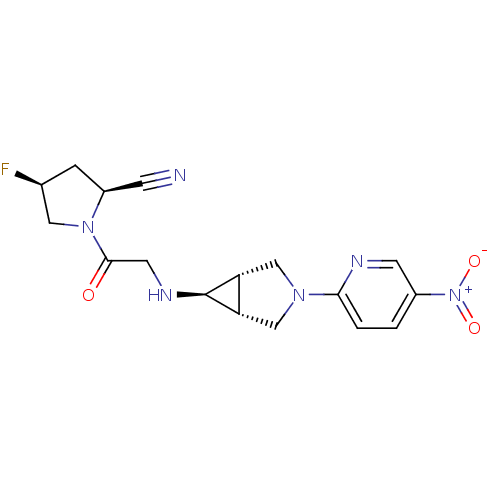
((2S,4S)-4-fluoro-1-(2-((1S,5R,6s)-3-(5-nitropyridi...)Show SMILES [O-][N+](=O)c1ccc(nc1)N1C[C@H]2[C@@H](C1)[C@@H]2NCC(=O)N1C[C@@H](F)C[C@H]1C#N |r| Show InChI InChI=1S/C17H19FN6O3/c18-10-3-12(4-19)23(7-10)16(25)6-21-17-13-8-22(9-14(13)17)15-2-1-11(5-20-15)24(26)27/h1-2,5,10,12-14,17,21H,3,6-9H2/t10-,12-,13-,14+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251468
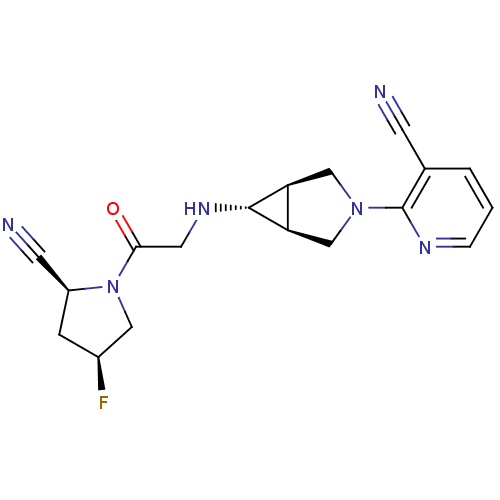
(2-((1S,5R,6s)-6-(2-((2S,4S)-2-cyano-4-fluoropyrrol...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CN[C@@H]1[C@H]2CN(C[C@@H]12)c1ncccc1C#N |r| Show InChI InChI=1S/C18H19FN6O/c19-12-4-13(6-21)25(8-12)16(26)7-23-17-14-9-24(10-15(14)17)18-11(5-20)2-1-3-22-18/h1-3,12-15,17,23H,4,7-10H2/t12-,13-,14-,15+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251788
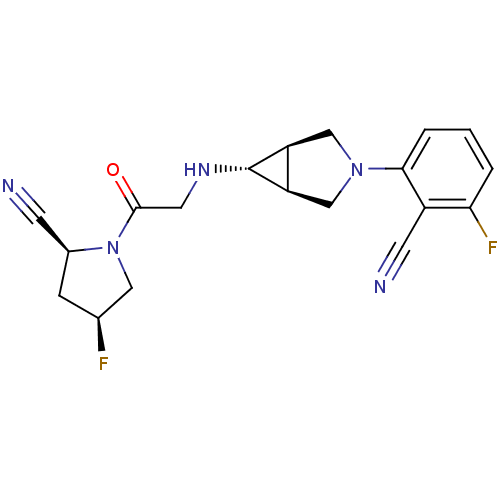
((2S,4S)-1-(2-((1S,5R,6s)-3-(2-cyano-3-fluorophenyl...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CN[C@@H]1[C@H]2CN(C[C@@H]12)c1cccc(F)c1C#N |r| Show InChI InChI=1S/C19H19F2N5O/c20-11-4-12(5-22)26(8-11)18(27)7-24-19-14-9-25(10-15(14)19)17-3-1-2-16(21)13(17)6-23/h1-3,11-12,14-15,19,24H,4,7-10H2/t11-,12-,14-,15+,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251491
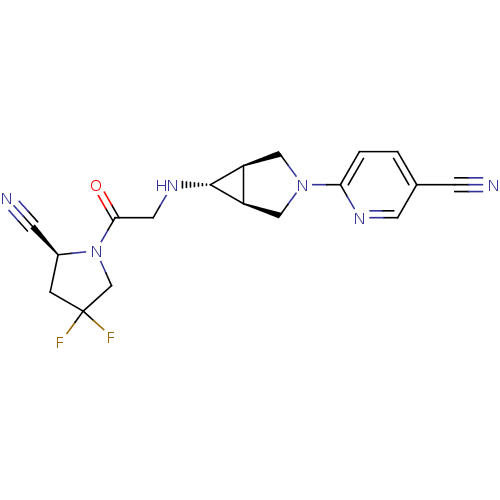
(6-((1S,5R,6s)-6-(2-((S)-2-cyano-4,4-difluoropyrrol...)Show SMILES FC1(F)C[C@@H](C#N)N(C1)C(=O)CN[C@@H]1[C@H]2CN(C[C@@H]12)c1ccc(cn1)C#N |r| Show InChI InChI=1S/C18H18F2N6O/c19-18(20)3-12(5-22)26(10-18)16(27)7-24-17-13-8-25(9-14(13)17)15-2-1-11(4-21)6-23-15/h1-2,6,12-14,17,24H,3,7-10H2/t12-,13-,14+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251682

((2S,4S)-1-(2-((1S,5R,6s)-3-(2-chloro-4-cyanophenyl...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CN[C@@H]1[C@H]2CN(C[C@@H]12)c1ccc(cc1Cl)C#N |r| Show InChI InChI=1S/C19H19ClFN5O/c20-16-3-11(5-22)1-2-17(16)25-9-14-15(10-25)19(14)24-7-18(27)26-8-12(21)4-13(26)6-23/h1-3,12-15,19,24H,4,7-10H2/t12-,13-,14-,15+,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251721
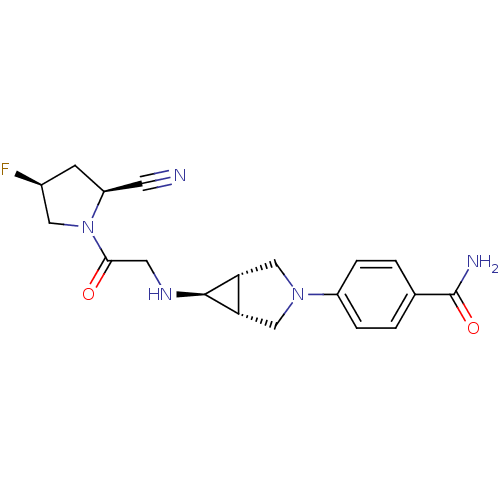
(4-((1S,5R,6s)-6-(2-((2S,4S)-2-cyano-4-fluoropyrrol...)Show SMILES NC(=O)c1ccc(cc1)N1C[C@H]2[C@@H](C1)[C@@H]2NCC(=O)N1C[C@@H](F)C[C@H]1C#N |r| Show InChI InChI=1S/C19H22FN5O2/c20-12-5-14(6-21)25(8-12)17(26)7-23-18-15-9-24(10-16(15)18)13-3-1-11(2-4-13)19(22)27/h1-4,12,14-16,18,23H,5,7-10H2,(H2,22,27)/t12-,14-,15-,16+,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50251758

((2S,4S)-1-(2-((1S,5R,6s)-3-(3-chloro-2-cyanophenyl...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CN[C@@H]1[C@H]2CN(C[C@@H]12)c1cccc(Cl)c1C#N |r| Show InChI InChI=1S/C19H19ClFN5O/c20-16-2-1-3-17(13(16)6-23)25-9-14-15(10-25)19(14)24-7-18(27)26-8-11(21)4-12(26)5-22/h1-3,11-12,14-15,19,24H,4,7-10H2/t11-,12-,14-,15+,19+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP2 (unknown origin) |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251719
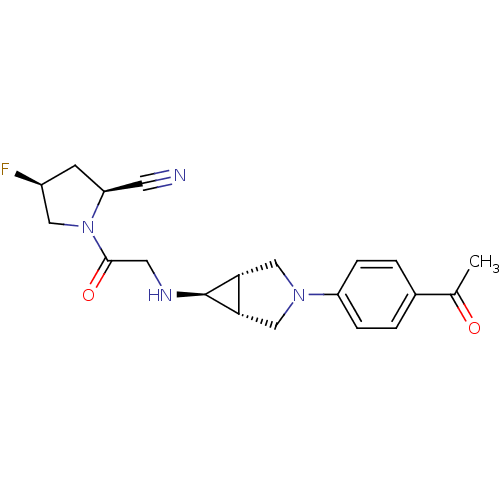
((2S,4S)-1-(2-((1S,5R,6s)-3-(4-acetylphenyl)-3-aza-...)Show SMILES CC(=O)c1ccc(cc1)N1C[C@H]2[C@@H](C1)[C@@H]2NCC(=O)N1C[C@@H](F)C[C@H]1C#N |r| Show InChI InChI=1S/C20H23FN4O2/c1-12(26)13-2-4-15(5-3-13)24-10-17-18(11-24)20(17)23-8-19(27)25-9-14(21)6-16(25)7-22/h2-5,14,16-18,20,23H,6,8-11H2,1H3/t14-,16-,17-,18+,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251643

((2S,4S)-1-(2-((1S,5R,6s)-3-(4-cyano-3-fluorophenyl...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CN[C@@H]1[C@H]2CN(C[C@@H]12)c1ccc(C#N)c(F)c1 |r| Show InChI InChI=1S/C19H19F2N5O/c20-12-3-14(6-23)26(8-12)18(27)7-24-19-15-9-25(10-16(15)19)13-2-1-11(5-22)17(21)4-13/h1-2,4,12,14-16,19,24H,3,7-10H2/t12-,14-,15-,16+,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251819
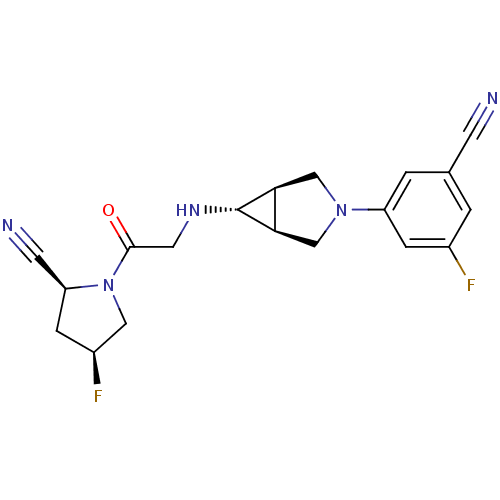
((2S,4S)-1-(2-((1S,5R,6s)-3-(3-cyano-5-fluorophenyl...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CN[C@@H]1[C@H]2CN(C[C@@H]12)c1cc(F)cc(c1)C#N |r| Show InChI InChI=1S/C19H19F2N5O/c20-12-1-11(5-22)2-14(3-12)25-9-16-17(10-25)19(16)24-7-18(27)26-8-13(21)4-15(26)6-23/h1-3,13,15-17,19,24H,4,7-10H2/t13-,15-,16-,17+,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50251758

((2S,4S)-1-(2-((1S,5R,6s)-3-(3-chloro-2-cyanophenyl...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CN[C@@H]1[C@H]2CN(C[C@@H]12)c1cccc(Cl)c1C#N |r| Show InChI InChI=1S/C19H19ClFN5O/c20-16-2-1-3-17(13(16)6-23)25-9-14-15(10-25)19(14)24-7-18(27)26-8-11(21)4-12(26)5-22/h1-3,11-12,14-15,19,24H,4,7-10H2/t11-,12-,14-,15+,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP9 (unknown origin) |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251465
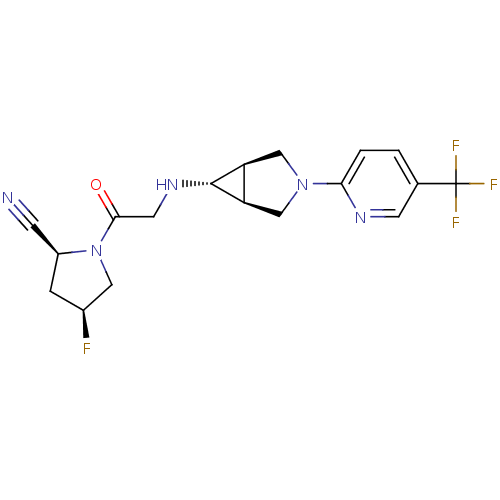
((2S,4S)-4-fluoro-1-(2-((1S,5R,6s)-3-(5-(trifluorom...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CN[C@@H]1[C@H]2CN(C[C@@H]12)c1ccc(cn1)C(F)(F)F |r| Show InChI InChI=1S/C18H19F4N5O/c19-11-3-12(4-23)27(7-11)16(28)6-25-17-13-8-26(9-14(13)17)15-2-1-10(5-24-15)18(20,21)22/h1-2,5,11-14,17,25H,3,6-9H2/t11-,12-,13-,14+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50558597

(CHEMBL4787884)Show SMILES FC(F)Oc1ccc(-c2nc(cs2)C(=O)Nc2c(Cl)cncc2Cl)c2c3ccccc3oc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PDE4B using [3H]cAMP as substrate incubated for 5 mins followed by substrate addition and measured after 10 mins by scintillation... |
Citation and Details
Article DOI: 10.1016/j.bmc.2016.09.011
BindingDB Entry DOI: 10.7270/Q2N58R28 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50251684

((2S,4S)-1-(2-((1S,5R,6s)-3-(4-cyano-2-(trifluorome...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CN[C@@H]1[C@H]2CN(C[C@@H]12)c1ccc(cc1C(F)(F)F)C#N |r| Show InChI InChI=1S/C20H19F4N5O/c21-12-4-13(6-26)29(8-12)18(30)7-27-19-14-9-28(10-15(14)19)17-2-1-11(5-25)3-16(17)20(22,23)24/h1-3,12-15,19,27H,4,7-10H2/t12-,13-,14-,15+,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP9 (unknown origin) |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50251642
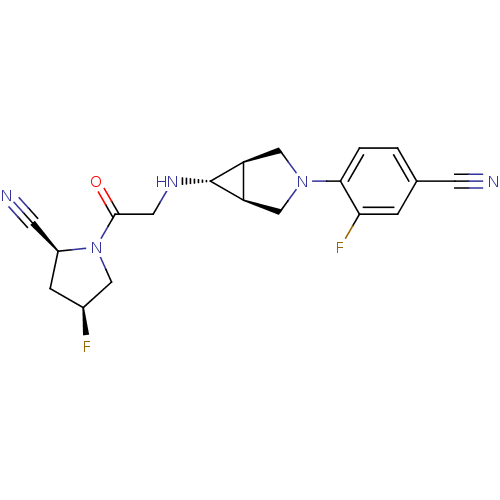
((2S,4S)-1-(2-((1S,5R,6s)-3-(4-cyano-2-fluorophenyl...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CN[C@@H]1[C@H]2CN(C[C@@H]12)c1ccc(cc1F)C#N |r| Show InChI InChI=1S/C19H19F2N5O/c20-12-4-13(6-23)26(8-12)18(27)7-24-19-14-9-25(10-15(14)19)17-2-1-11(5-22)3-16(17)21/h1-3,12-15,19,24H,4,7-10H2/t12-,13-,14-,15+,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP9 (unknown origin) |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251789
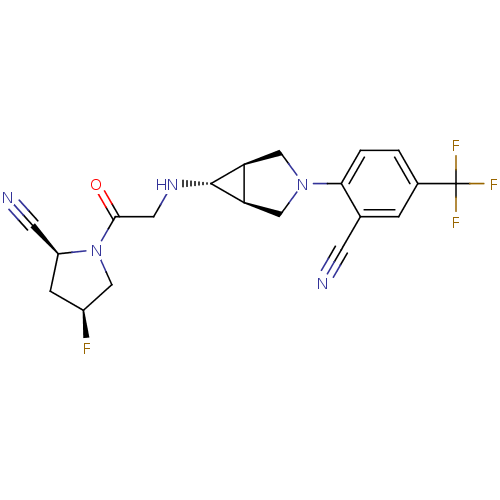
((2S,4S)-1-(2-((1S,5R,6s)-3-(2-cyano-4-(trifluorome...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CN[C@@H]1[C@H]2CN(C[C@@H]12)c1ccc(cc1C#N)C(F)(F)F |r| Show InChI InChI=1S/C20H19F4N5O/c21-13-4-14(6-26)29(8-13)18(30)7-27-19-15-9-28(10-16(15)19)17-2-1-12(20(22,23)24)3-11(17)5-25/h1-3,13-16,19,27H,4,7-10H2/t13-,14-,15-,16+,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251854

((2S,4S)-4-fluoro-1-(2-((1S,5R,6s)-3-(thiazol-2-yl)...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CN[C@@H]1[C@H]2CN(C[C@@H]12)c1nccs1 |r| Show InChI InChI=1S/C15H18FN5OS/c16-9-3-10(4-17)21(6-9)13(22)5-19-14-11-7-20(8-12(11)14)15-18-1-2-23-15/h1-2,9-12,14,19H,3,5-8H2/t9-,10-,11-,12+,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251466
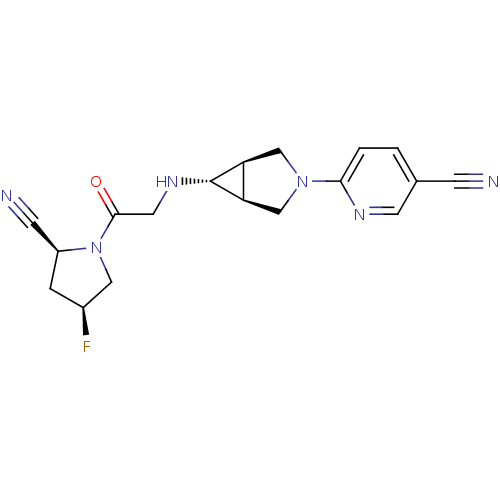
(6-((1S,5R,6s)-6-(2-((2S,4S)-2-cyano-4-fluoropyrrol...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CN[C@@H]1[C@H]2CN(C[C@@H]12)c1ccc(cn1)C#N |r| Show InChI InChI=1S/C18H19FN6O/c19-12-3-13(5-21)25(8-12)17(26)7-23-18-14-9-24(10-15(14)18)16-2-1-11(4-20)6-22-16/h1-2,6,12-15,18,23H,3,7-10H2/t12-,13-,14-,15+,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 147 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50251851
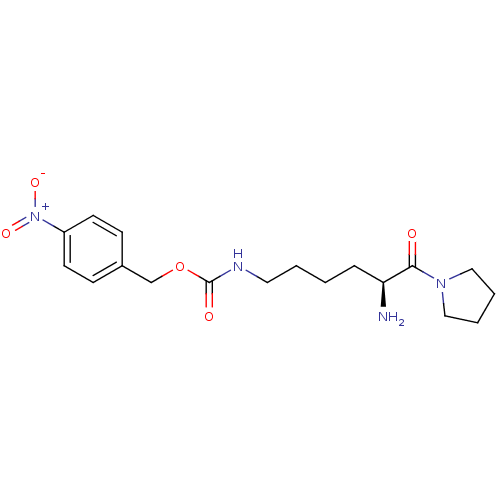
(CHEMBL505629 | Lys[Z(NO2)]pyrrolidide)Show SMILES N[C@@H](CCCCNC(=O)OCc1ccc(cc1)[N+]([O-])=O)C(=O)N1CCCC1 |r| Show InChI InChI=1S/C18H26N4O5/c19-16(17(23)21-11-3-4-12-21)5-1-2-10-20-18(24)27-13-14-6-8-15(9-7-14)22(25)26/h6-9,16H,1-5,10-13,19H2,(H,20,24)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 147 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP8 (unknown origin) |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50251683
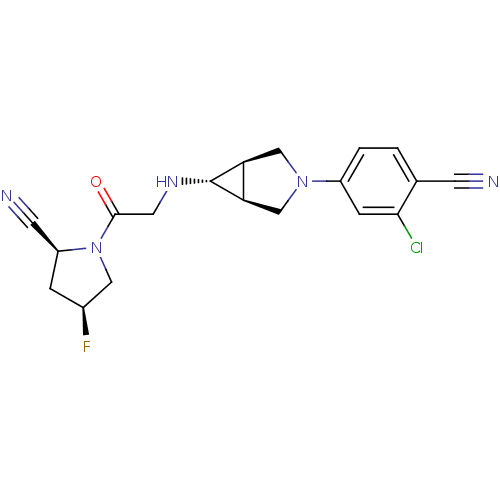
((2S,4S)-1-(2-((1S,5R,6s)-3-(3-chloro-4-cyanophenyl...)Show SMILES F[C@H]1C[C@@H](C#N)N(C1)C(=O)CN[C@@H]1[C@H]2CN(C[C@@H]12)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C19H19ClFN5O/c20-17-4-13(2-1-11(17)5-22)25-9-15-16(10-25)19(15)24-7-18(27)26-8-12(21)3-14(26)6-23/h1-2,4,12,14-16,19,24H,3,7-10H2/t12-,14-,15-,16+,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 18: 4087-91 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.101
BindingDB Entry DOI: 10.7270/Q2T153FC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data