 Found 57 hits with Last Name = 'sutherell' and Initial = 'cl'
Found 57 hits with Last Name = 'sutherell' and Initial = 'cl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539993
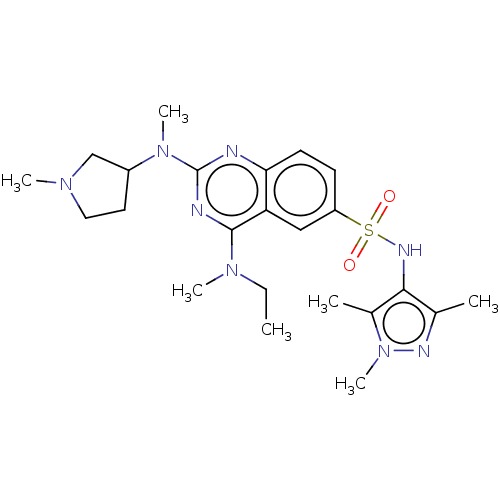
(CHEMBL4647500)Show SMILES CCN(C)c1nc(nc2ccc(cc12)S(=O)(=O)Nc1c(C)nn(C)c1C)N(C)C1CCN(C)C1 Show InChI InChI=1S/C23H34N8O2S/c1-8-29(5)22-19-13-18(34(32,33)27-21-15(2)26-31(7)16(21)3)9-10-20(19)24-23(25-22)30(6)17-11-12-28(4)14-17/h9-10,13,17,27H,8,11-12,14H2,1-7H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A
(Escherichia coli) | BDBM21690

(1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...)Show InChI InChI=1S/C17H18FN3O3/c18-13-7-11-14(8-15(13)20-5-3-19-4-6-20)21(10-1-2-10)9-12(16(11)22)17(23)24/h7-10,19H,1-6H2,(H,23,24) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli DNA gyrase (A2B2 tetramer) using relaxed pBR322 as substrate preincubated for 30 mins followed by enzyme addition by S... |
Bioorg Med Chem 27: (2019)
Article DOI: 10.1016/j.bmc.2019.06.025
BindingDB Entry DOI: 10.7270/Q2JM2F2T |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539980
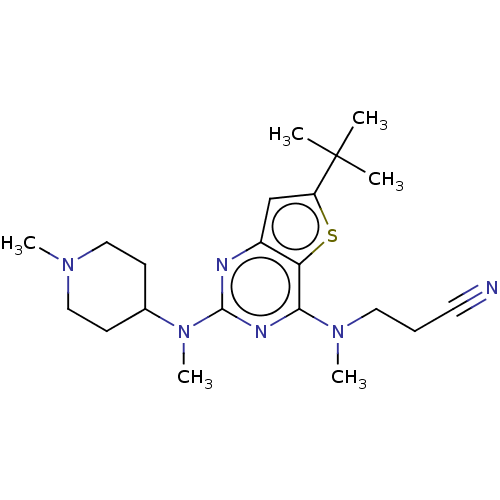
(CHEMBL4644304)Show SMILES CN(CCC#N)c1nc(nc2cc(sc12)C(C)(C)C)N(C)C1CCN(C)CC1 Show InChI InChI=1S/C21H32N6S/c1-21(2,3)17-14-16-18(28-17)19(26(5)11-7-10-22)24-20(23-16)27(6)15-8-12-25(4)13-9-15/h14-15H,7-9,11-13H2,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539992
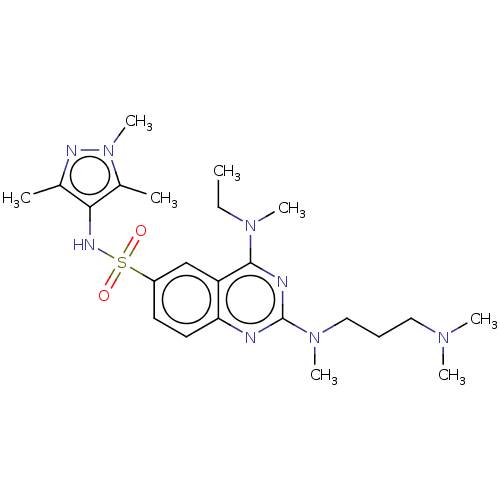
(CHEMBL4632916)Show SMILES CCN(C)c1nc(nc2ccc(cc12)S(=O)(=O)Nc1c(C)nn(C)c1C)N(C)CCCN(C)C Show InChI InChI=1S/C23H36N8O2S/c1-9-29(6)22-19-15-18(34(32,33)27-21-16(2)26-31(8)17(21)3)11-12-20(19)24-23(25-22)30(7)14-10-13-28(4)5/h11-12,15,27H,9-10,13-14H2,1-8H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539975
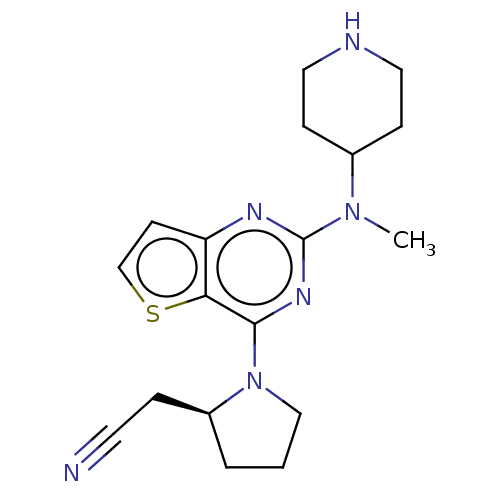
(CHEMBL4633418)Show SMILES CN(C1CCNCC1)c1nc(N2CCC[C@H]2CC#N)c2sccc2n1 |r| Show InChI InChI=1S/C18H24N6S/c1-23(13-5-9-20-10-6-13)18-21-15-7-12-25-16(15)17(22-18)24-11-2-3-14(24)4-8-19/h7,12-14,20H,2-6,9-11H2,1H3/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539991
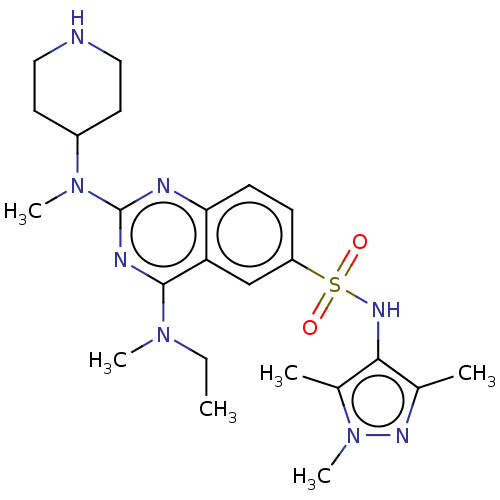
(CHEMBL4632704)Show SMILES CCN(C)c1nc(nc2ccc(cc12)S(=O)(=O)Nc1c(C)nn(C)c1C)N(C)C1CCNCC1 Show InChI InChI=1S/C23H34N8O2S/c1-7-29(4)22-19-14-18(34(32,33)28-21-15(2)27-31(6)16(21)3)8-9-20(19)25-23(26-22)30(5)17-10-12-24-13-11-17/h8-9,14,17,24,28H,7,10-13H2,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539990
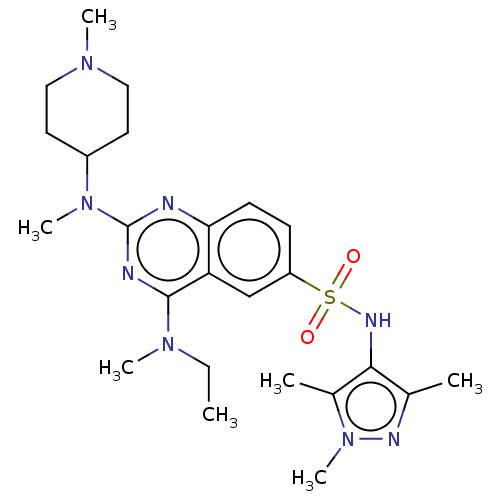
(CHEMBL4647034)Show SMILES CCN(C)c1nc(nc2ccc(cc12)S(=O)(=O)Nc1c(C)nn(C)c1C)N(C)C1CCN(C)CC1 Show InChI InChI=1S/C24H36N8O2S/c1-8-30(5)23-20-15-19(35(33,34)28-22-16(2)27-32(7)17(22)3)9-10-21(20)25-24(26-23)31(6)18-11-13-29(4)14-12-18/h9-10,15,18,28H,8,11-14H2,1-7H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539972

(CHEMBL4638503)Show SMILES COC[C@@H]1CCCN1c1nc(nc2ccsc12)N(C)C1CCNCC1 |r| Show InChI InChI=1S/C18H27N5OS/c1-22(13-5-8-19-9-6-13)18-20-15-7-11-25-16(15)17(21-18)23-10-3-4-14(23)12-24-2/h7,11,13-14,19H,3-6,8-10,12H2,1-2H3/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539956
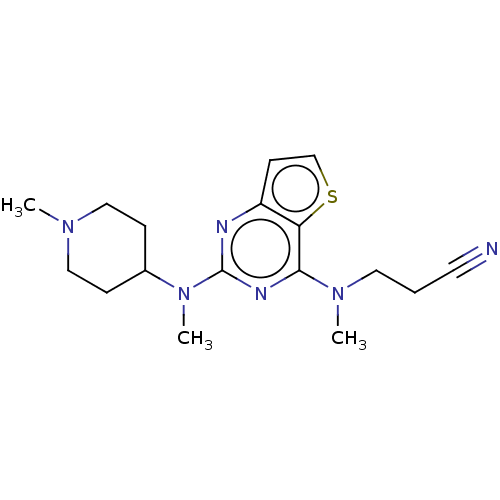
(CHEMBL4634852)Show InChI InChI=1S/C17H24N6S/c1-21-10-5-13(6-11-21)23(3)17-19-14-7-12-24-15(14)16(20-17)22(2)9-4-8-18/h7,12-13H,4-6,9-11H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Transcription activator BRG1
(Homo sapiens (Human)) | BDBM50184973
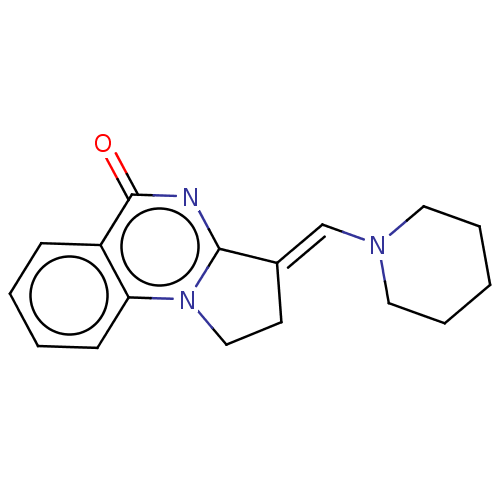
(CHEMBL3822828)Show InChI InChI=1S/C17H19N3O/c21-17-14-6-2-3-7-15(14)20-11-8-13(16(20)18-17)12-19-9-4-1-5-10-19/h2-3,6-7,12H,1,4-5,8-11H2/b13-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human recombinant SMARCA4 bromodomain expressed in Escherichia coli BL21(DE3)-R3-pRARE2 cells after 30 mins using biotinyla... |
J Med Chem 59: 5095-101 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01997
BindingDB Entry DOI: 10.7270/Q22R3TKH |
More data for this
Ligand-Target Pair | |
Transcription activator BRG1
(Homo sapiens (Human)) | BDBM50184975
(CHEMBL3823214)Show InChI InChI=1S/C18H22N4O/c1-2-20-9-11-21(12-10-20)13-14-7-8-22-16-6-4-3-5-15(16)18(23)19-17(14)22/h3-6,13H,2,7-12H2,1H3/b14-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human recombinant SMARCA4 bromodomain expressed in Escherichia coli BL21(DE3)-R3-pRARE2 cells after 30 mins using biotinyla... |
J Med Chem 59: 5095-101 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01997
BindingDB Entry DOI: 10.7270/Q22R3TKH |
More data for this
Ligand-Target Pair | |
Transcription activator BRG1
(Homo sapiens (Human)) | BDBM50184974
(CHEMBL3823057)Show InChI InChI=1S/C17H20N4O/c1-19-8-10-20(11-9-19)12-13-6-7-21-15-5-3-2-4-14(15)17(22)18-16(13)21/h2-5,12H,6-11H2,1H3/b13-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human recombinant SMARCA4 bromodomain expressed in Escherichia coli BL21(DE3)-R3-pRARE2 cells after 30 mins using biotinyla... |
J Med Chem 59: 5095-101 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01997
BindingDB Entry DOI: 10.7270/Q22R3TKH |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539977
(CHEMBL4634504)Show InChI InChI=1S/C16H25N5S/c1-5-20(3)15-14-13(8-11-22-14)17-16(18-15)21(4)12-6-9-19(2)10-7-12/h8,11-12H,5-7,9-10H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Transcription activator BRG1
(Homo sapiens (Human)) | BDBM50184972
(CHEMBL3823525)Show InChI InChI=1S/C16H17N3O/c20-16-13-5-1-2-6-14(13)19-10-7-12(15(19)17-16)11-18-8-3-4-9-18/h1-2,5-6,11H,3-4,7-10H2/b12-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human recombinant SMARCA4 bromodomain expressed in Escherichia coli BL21(DE3)-R3-pRARE2 cells after 30 mins using biotinyla... |
J Med Chem 59: 5095-101 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01997
BindingDB Entry DOI: 10.7270/Q22R3TKH |
More data for this
Ligand-Target Pair | |
Transcription activator BRG1
(Homo sapiens (Human)) | BDBM50184971
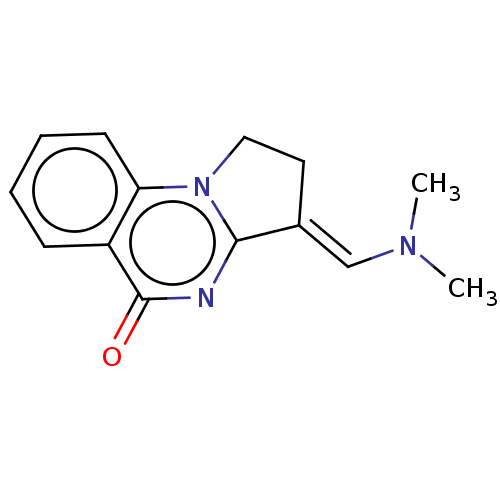
(CHEMBL3823210)Show InChI InChI=1S/C14H15N3O/c1-16(2)9-10-7-8-17-12-6-4-3-5-11(12)14(18)15-13(10)17/h3-6,9H,7-8H2,1-2H3/b10-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human recombinant SMARCA4 bromodomain expressed in Escherichia coli BL21(DE3)-R3-pRARE2 cells after 30 mins using biotinyla... |
J Med Chem 59: 5095-101 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01997
BindingDB Entry DOI: 10.7270/Q22R3TKH |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539962
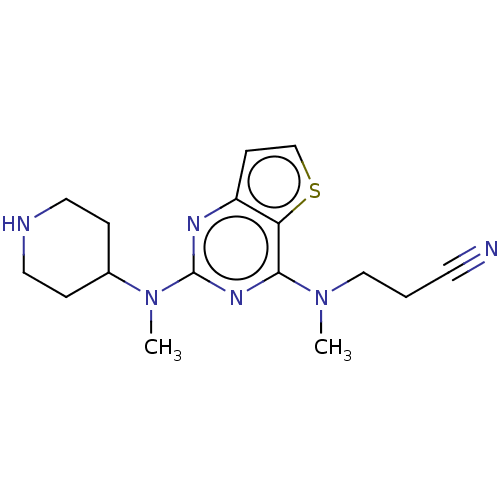
(CHEMBL4638689)Show InChI InChI=1S/C16H22N6S/c1-21(10-3-7-17)15-14-13(6-11-23-14)19-16(20-15)22(2)12-4-8-18-9-5-12/h6,11-12,18H,3-5,8-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539958
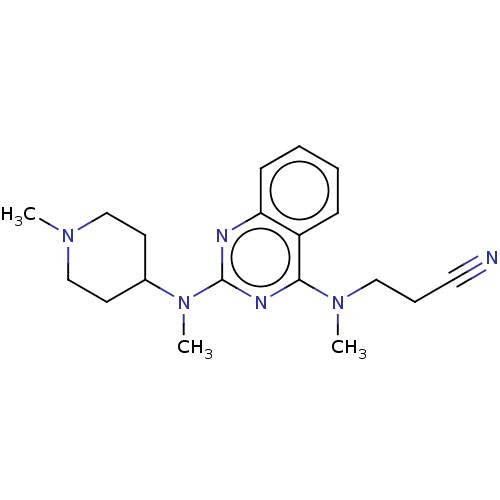
(CHEMBL4642741)Show InChI InChI=1S/C19H26N6/c1-23-13-9-15(10-14-23)25(3)19-21-17-8-5-4-7-16(17)18(22-19)24(2)12-6-11-20/h4-5,7-8,15H,6,9-10,12-14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539976
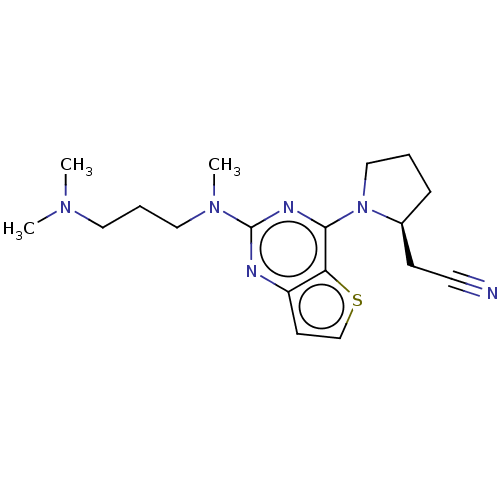
(CHEMBL4641086)Show SMILES CN(C)CCCN(C)c1nc(N2CCC[C@H]2CC#N)c2sccc2n1 |r| Show InChI InChI=1S/C18H26N6S/c1-22(2)10-5-11-23(3)18-20-15-8-13-25-16(15)17(21-18)24-12-4-6-14(24)7-9-19/h8,13-14H,4-7,10-12H2,1-3H3/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539963
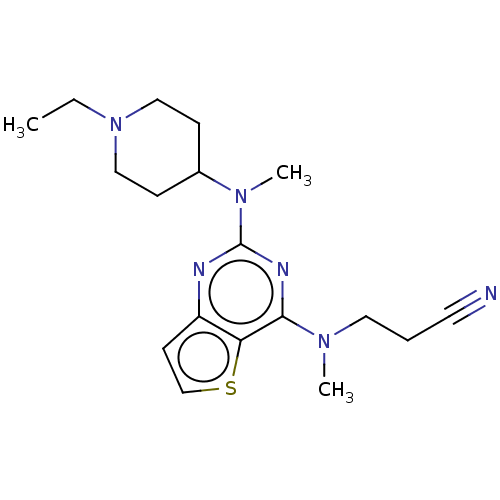
(CHEMBL4637123)Show InChI InChI=1S/C18H26N6S/c1-4-24-11-6-14(7-12-24)23(3)18-20-15-8-13-25-16(15)17(21-18)22(2)10-5-9-19/h8,13-14H,4-7,10-12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Transcription activator BRG1
(Homo sapiens (Human)) | BDBM50184976
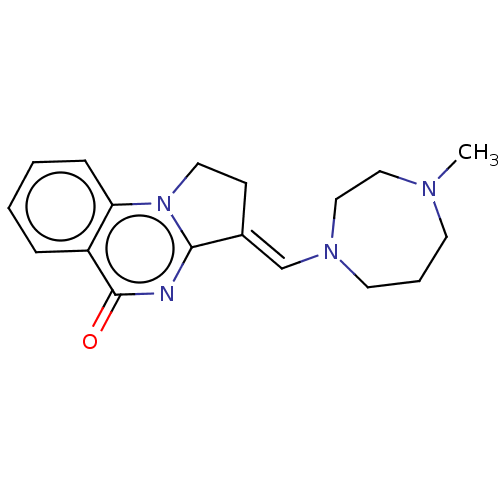
(CHEMBL3823365)Show InChI InChI=1S/C18H22N4O/c1-20-8-4-9-21(12-11-20)13-14-7-10-22-16-6-3-2-5-15(16)18(23)19-17(14)22/h2-3,5-6,13H,4,7-12H2,1H3/b14-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human recombinant SMARCA4 bromodomain expressed in Escherichia coli BL21(DE3)-R3-pRARE2 cells after 30 mins using biotinyla... |
J Med Chem 59: 5095-101 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01997
BindingDB Entry DOI: 10.7270/Q22R3TKH |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539973
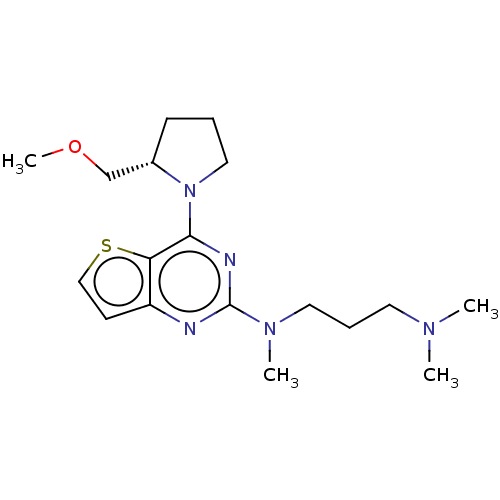
(CHEMBL4641649)Show SMILES COC[C@@H]1CCCN1c1nc(nc2ccsc12)N(C)CCCN(C)C |r| Show InChI InChI=1S/C18H29N5OS/c1-21(2)9-6-10-22(3)18-19-15-8-12-25-16(15)17(20-18)23-11-5-7-14(23)13-24-4/h8,12,14H,5-7,9-11,13H2,1-4H3/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539981
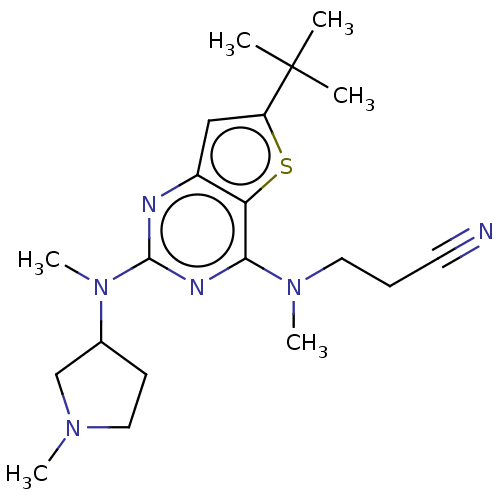
(CHEMBL4640420)Show SMILES CN(CCC#N)c1nc(nc2cc(sc12)C(C)(C)C)N(C)C1CCN(C)C1 Show InChI InChI=1S/C20H30N6S/c1-20(2,3)16-12-15-17(27-16)18(25(5)10-7-9-21)23-19(22-15)26(6)14-8-11-24(4)13-14/h12,14H,7-8,10-11,13H2,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539978
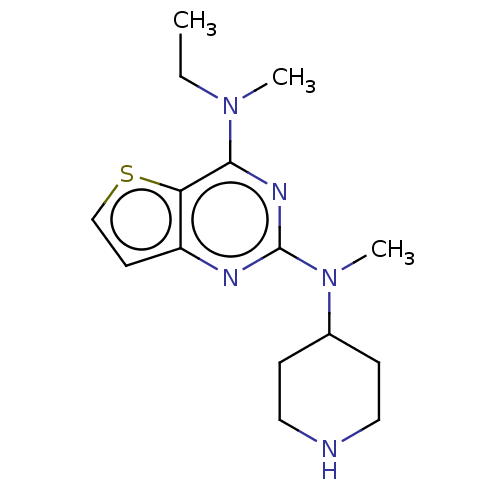
(CHEMBL4639250)Show InChI InChI=1S/C15H23N5S/c1-4-19(2)14-13-12(7-10-21-13)17-15(18-14)20(3)11-5-8-16-9-6-11/h7,10-11,16H,4-6,8-9H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539966
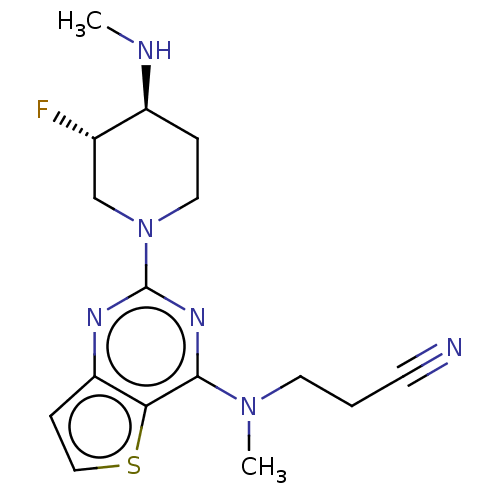
(CHEMBL4634547)Show SMILES CN[C@H]1CCN(C[C@@H]1F)c1nc(N(C)CCC#N)c2sccc2n1 |r| Show InChI InChI=1S/C16H21FN6S/c1-19-12-4-8-23(10-11(12)17)16-20-13-5-9-24-14(13)15(21-16)22(2)7-3-6-18/h5,9,11-12,19H,3-4,7-8,10H2,1-2H3/t11-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539965
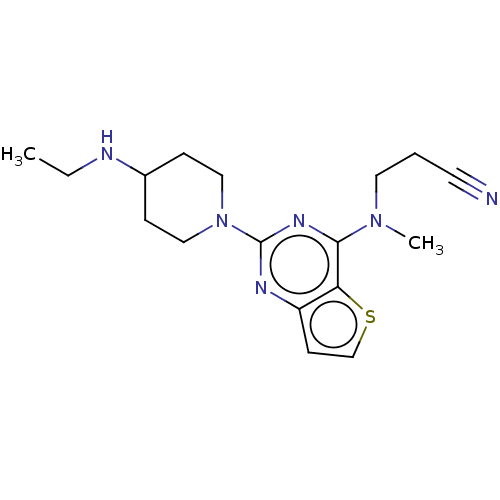
(CHEMBL4642301)Show InChI InChI=1S/C17H24N6S/c1-3-19-13-5-10-23(11-6-13)17-20-14-7-12-24-15(14)16(21-17)22(2)9-4-8-18/h7,12-13,19H,3-6,9-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539983
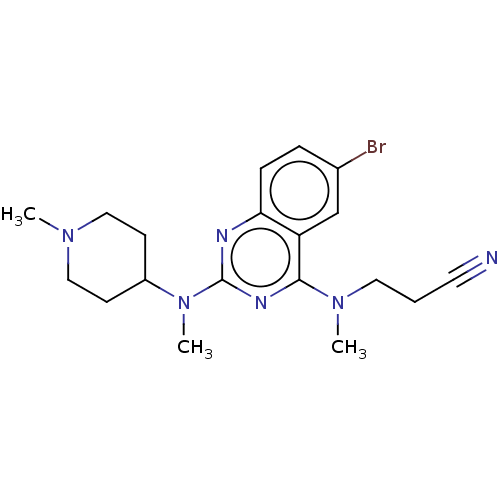
(CHEMBL4643474)Show SMILES CN(CCC#N)c1nc(nc2ccc(Br)cc12)N(C)C1CCN(C)CC1 Show InChI InChI=1S/C19H25BrN6/c1-24-11-7-15(8-12-24)26(3)19-22-17-6-5-14(20)13-16(17)18(23-19)25(2)10-4-9-21/h5-6,13,15H,4,7-8,10-12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539985
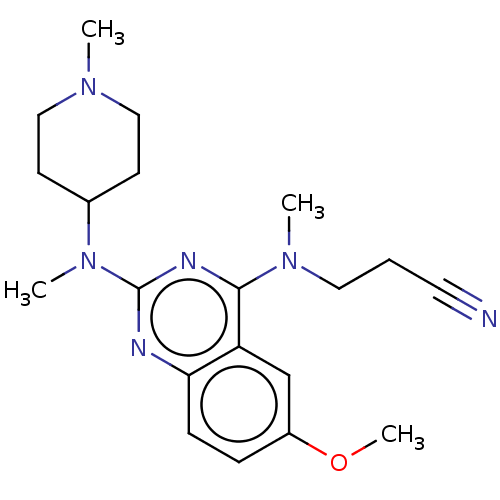
(CHEMBL4646966)Show SMILES COc1ccc2nc(nc(N(C)CCC#N)c2c1)N(C)C1CCN(C)CC1 Show InChI InChI=1S/C20H28N6O/c1-24-12-8-15(9-13-24)26(3)20-22-18-7-6-16(27-4)14-17(18)19(23-20)25(2)11-5-10-21/h6-7,14-15H,5,8-9,11-13H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539964
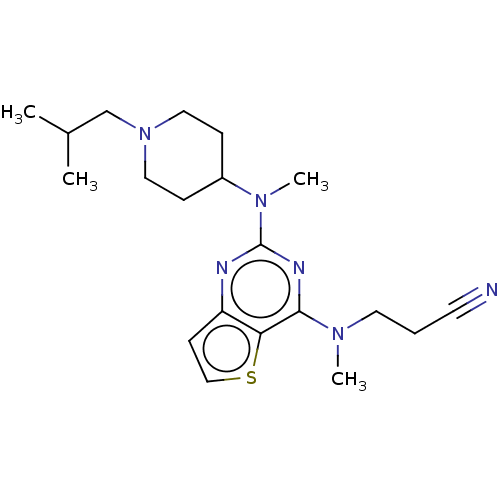
(CHEMBL4636010)Show SMILES CC(C)CN1CCC(CC1)N(C)c1nc(N(C)CCC#N)c2sccc2n1 Show InChI InChI=1S/C20H30N6S/c1-15(2)14-26-11-6-16(7-12-26)25(4)20-22-17-8-13-27-18(17)19(23-20)24(3)10-5-9-21/h8,13,15-16H,5-7,10-12,14H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539986
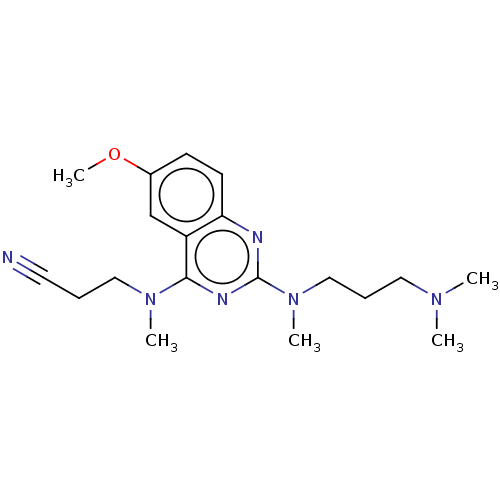
(CHEMBL4638924)Show InChI InChI=1S/C19H28N6O/c1-23(2)11-7-13-25(4)19-21-17-9-8-15(26-5)14-16(17)18(22-19)24(3)12-6-10-20/h8-9,14H,6-7,11-13H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539967
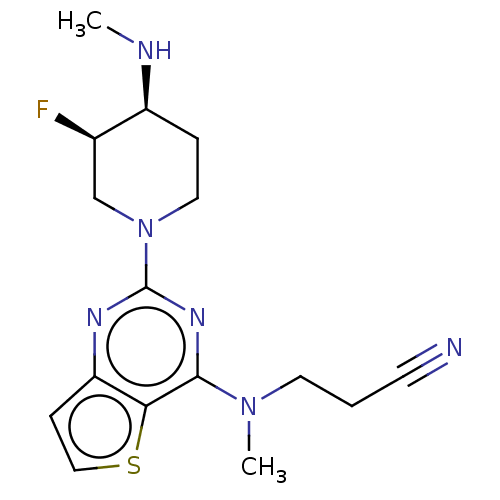
(CHEMBL4649184)Show SMILES CN[C@H]1CCN(C[C@H]1F)c1nc(N(C)CCC#N)c2sccc2n1 |r| Show InChI InChI=1S/C16H21FN6S/c1-19-12-4-8-23(10-11(12)17)16-20-13-5-9-24-14(13)15(21-16)22(2)7-3-6-18/h5,9,11-12,19H,3-4,7-8,10H2,1-2H3/t11-,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539968
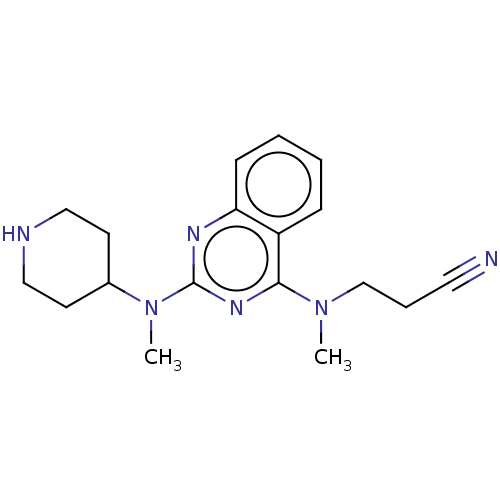
(CHEMBL4645634)Show InChI InChI=1S/C18H24N6/c1-23(13-5-10-19)17-15-6-3-4-7-16(15)21-18(22-17)24(2)14-8-11-20-12-9-14/h3-4,6-7,14,20H,5,8-9,11-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539969
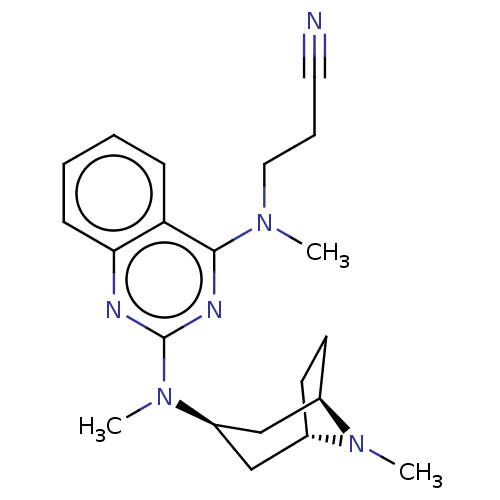
(CHEMBL4644983)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)N(C)c1nc(N(C)CCC#N)c3ccccc3n1)N2C |r,TLB:28:27:2.3:7.8.6| Show InChI InChI=1S/C21H28N6/c1-25(12-6-11-22)20-18-7-4-5-8-19(18)23-21(24-20)27(3)17-13-15-9-10-16(14-17)26(15)2/h4-5,7-8,15-17H,6,9-10,12-14H2,1-3H3/t15-,16+,17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539970
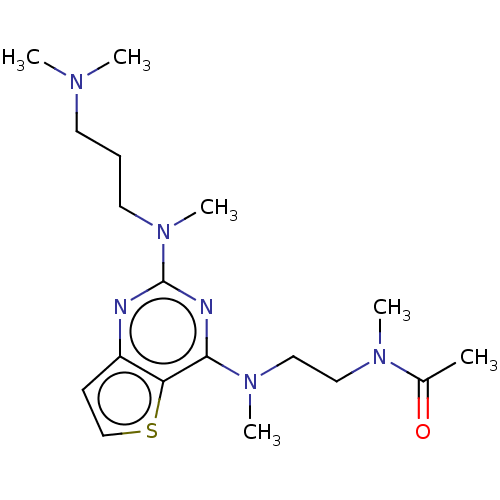
(CHEMBL4648485)Show SMILES CN(C)CCCN(C)c1nc(N(C)CCN(C)C(C)=O)c2sccc2n1 Show InChI InChI=1S/C18H30N6OS/c1-14(25)22(4)11-12-23(5)17-16-15(8-13-26-16)19-18(20-17)24(6)10-7-9-21(2)3/h8,13H,7,9-12H2,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539971
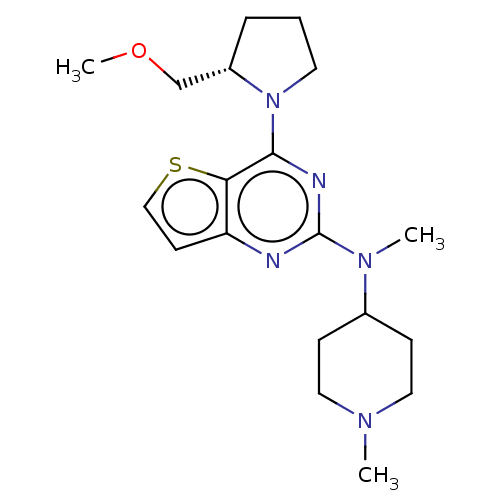
(CHEMBL4639906)Show SMILES COC[C@@H]1CCCN1c1nc(nc2ccsc12)N(C)C1CCN(C)CC1 |r| Show InChI InChI=1S/C19H29N5OS/c1-22-10-6-14(7-11-22)23(2)19-20-16-8-12-26-17(16)18(21-19)24-9-4-5-15(24)13-25-3/h8,12,14-15H,4-7,9-11,13H2,1-3H3/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539957
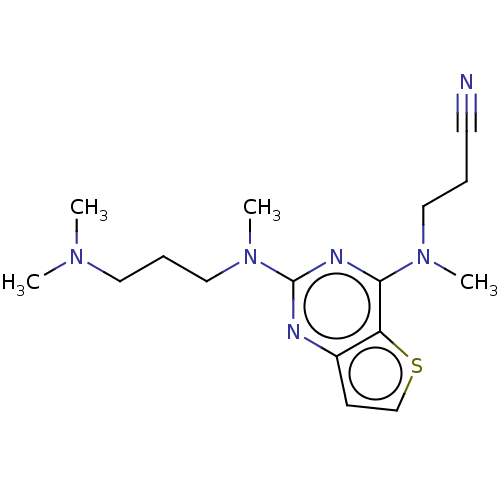
(CHEMBL4649163)Show InChI InChI=1S/C16H24N6S/c1-20(2)9-6-11-22(4)16-18-13-7-12-23-14(13)15(19-16)21(3)10-5-8-17/h7,12H,5-6,9-11H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539987
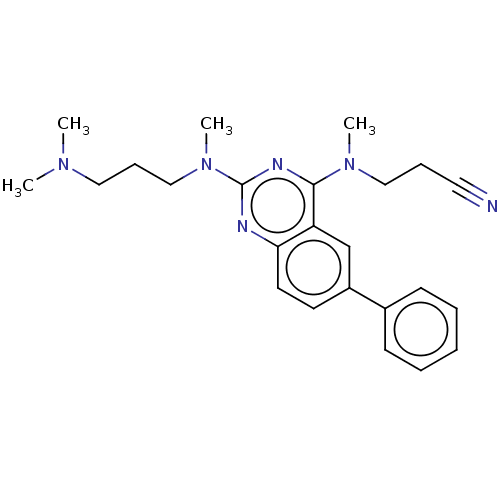
(CHEMBL4643976)Show SMILES CN(C)CCCN(C)c1nc(N(C)CCC#N)c2cc(ccc2n1)-c1ccccc1 Show InChI InChI=1S/C24H30N6/c1-28(2)15-9-17-30(4)24-26-22-13-12-20(19-10-6-5-7-11-19)18-21(22)23(27-24)29(3)16-8-14-25/h5-7,10-13,18H,8-9,15-17H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539974
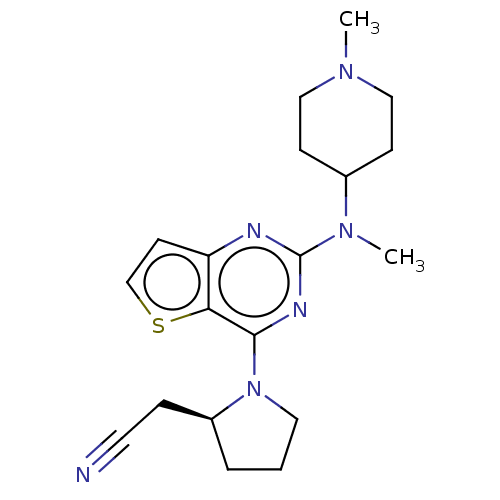
(CHEMBL4635262)Show SMILES CN(C1CCN(C)CC1)c1nc(N2CCC[C@H]2CC#N)c2sccc2n1 |r| Show InChI InChI=1S/C19H26N6S/c1-23-11-6-14(7-12-23)24(2)19-21-16-8-13-26-17(16)18(22-19)25-10-3-4-15(25)5-9-20/h8,13-15H,3-7,10-12H2,1-2H3/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539988
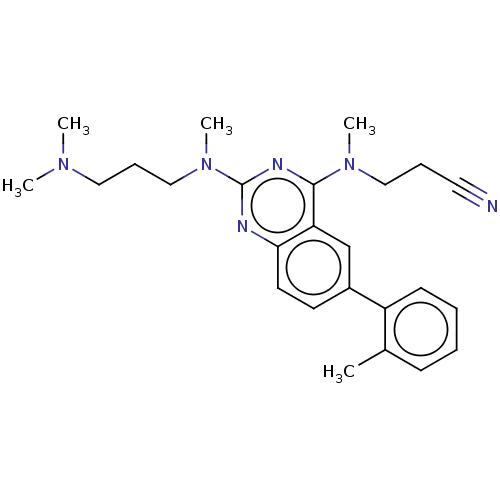
(CHEMBL4641823)Show SMILES CN(C)CCCN(C)c1nc(N(C)CCC#N)c2cc(ccc2n1)-c1ccccc1C Show InChI InChI=1S/C25H32N6/c1-19-10-6-7-11-21(19)20-12-13-23-22(18-20)24(30(4)16-8-14-26)28-25(27-23)31(5)17-9-15-29(2)3/h6-7,10-13,18H,8-9,15-17H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539989
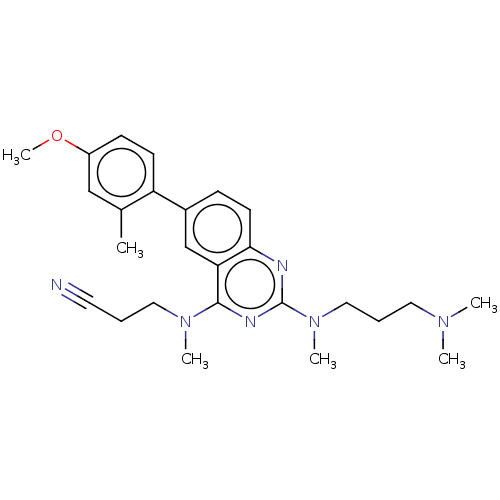
(CHEMBL4644841)Show SMILES COc1ccc(c(C)c1)-c1ccc2nc(nc(N(C)CCC#N)c2c1)N(C)CCCN(C)C Show InChI InChI=1S/C26H34N6O/c1-19-17-21(33-6)10-11-22(19)20-9-12-24-23(18-20)25(31(4)15-7-13-27)29-26(28-24)32(5)16-8-14-30(2)3/h9-12,17-18H,7-8,14-16H2,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539959
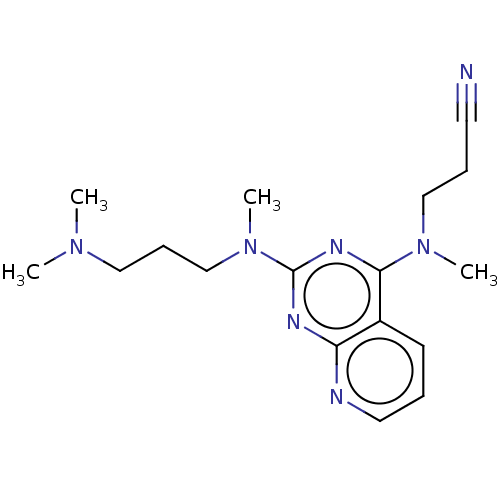
(CHEMBL4643929)Show InChI InChI=1S/C17H25N7/c1-22(2)11-7-13-24(4)17-20-15-14(8-5-10-19-15)16(21-17)23(3)12-6-9-18/h5,8,10H,6-7,11-13H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539961
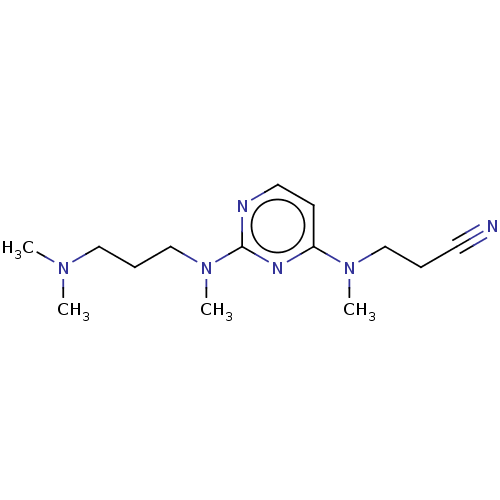
(CHEMBL4642415)Show InChI InChI=1S/C14H24N6/c1-18(2)10-6-12-20(4)14-16-9-7-13(17-14)19(3)11-5-8-15/h7,9H,5-6,10-12H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539979
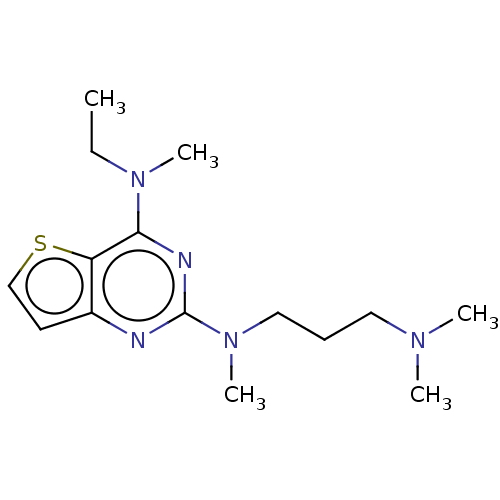
(CHEMBL4649765)Show InChI InChI=1S/C15H25N5S/c1-6-19(4)14-13-12(8-11-21-13)16-15(17-14)20(5)10-7-9-18(2)3/h8,11H,6-7,9-10H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539960
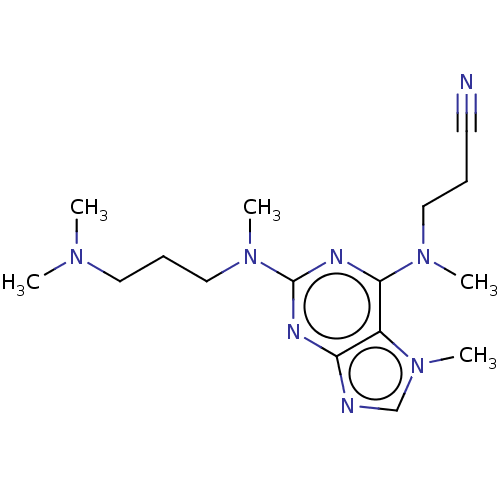
(CHEMBL4644148)Show InChI InChI=1S/C16H26N8/c1-21(2)9-7-11-23(4)16-19-14-13(24(5)12-18-14)15(20-16)22(3)10-6-8-17/h12H,6-7,9-11H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539982
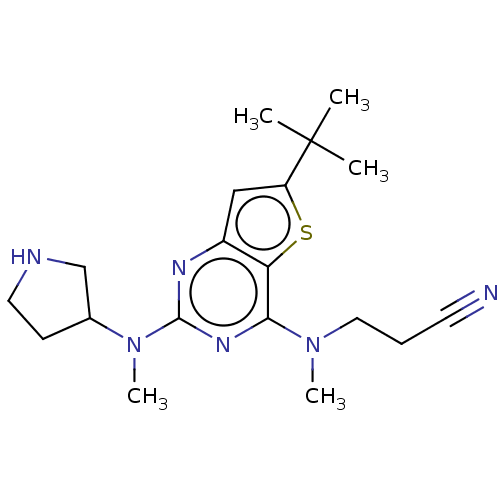
(CHEMBL4642112)Show SMILES CN(CCC#N)c1nc(nc2cc(sc12)C(C)(C)C)N(C)C1CCNC1 Show InChI InChI=1S/C19H28N6S/c1-19(2,3)15-11-14-16(26-15)17(24(4)10-6-8-20)23-18(22-14)25(5)13-7-9-21-12-13/h11,13,21H,6-7,9-10,12H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50539984
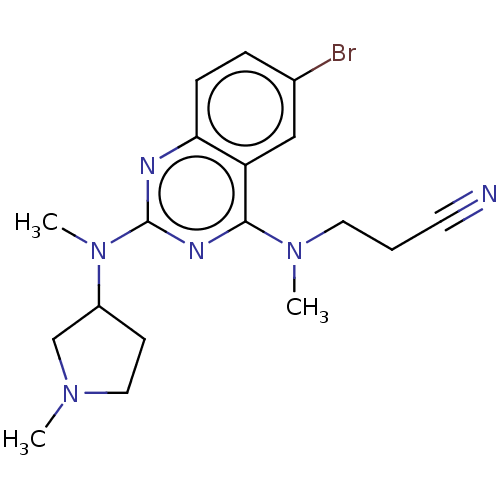
(CHEMBL4641691)Show SMILES CN(CCC#N)c1nc(nc2ccc(Br)cc12)N(C)C1CCN(C)C1 Show InChI InChI=1S/C18H23BrN6/c1-23-10-7-14(12-23)25(3)18-21-16-6-5-13(19)11-15(16)17(22-18)24(2)9-4-8-20/h5-6,11,14H,4,7,9-10,12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human NMT1 using p60 SRC(2 to 16) as substrate by CPM dye based fluorescence assay |
J Med Chem 63: 7740-7765 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00570
BindingDB Entry DOI: 10.7270/Q2HX1H6V |
More data for this
Ligand-Target Pair | |
Protein polybromo-1
(Homo sapiens (Human)) | BDBM50184972

(CHEMBL3823525)Show InChI InChI=1S/C16H17N3O/c20-16-13-5-1-2-6-14(13)19-10-7-12(15(19)17-16)11-18-8-3-4-9-18/h1-2,5-6,11H,3-4,7-10H2/b12-11+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Binding affinity to His6-tagged human recombinant PB1 bromodomain isoform 5 expressed in Escherichia coli BL21(DE3)-R3-pRARE2 cells by VP-ITC microca... |
J Med Chem 59: 5095-101 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01997
BindingDB Entry DOI: 10.7270/Q22R3TKH |
More data for this
Ligand-Target Pair | |
Protein polybromo-1
(Homo sapiens (Human)) | BDBM50184973

(CHEMBL3822828)Show InChI InChI=1S/C17H19N3O/c21-17-14-6-2-3-7-15(14)20-11-8-13(16(20)18-17)12-19-9-4-1-5-10-19/h2-3,6-7,12H,1,4-5,8-11H2/b13-12+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Binding affinity to His6-tagged human recombinant PB1 bromodomain isoform 5 expressed in Escherichia coli BL21(DE3)-R3-pRARE2 cells by VP-ITC microca... |
J Med Chem 59: 5095-101 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01997
BindingDB Entry DOI: 10.7270/Q22R3TKH |
More data for this
Ligand-Target Pair | |
Protein polybromo-1
(Homo sapiens (Human)) | BDBM50184977

(CHEMBL3823132)Show InChI InChI=1S/C14H14ClN3O/c1-17(2)8-9-6-7-18-11-5-3-4-10(15)12(11)14(19)16-13(9)18/h3-5,8H,6-7H2,1-2H3/b9-8+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Binding affinity to His6-tagged human recombinant PB1 bromodomain isoform 5 expressed in Escherichia coli BL21(DE3)-R3-pRARE2 cells by VP-ITC microca... |
J Med Chem 59: 5095-101 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01997
BindingDB Entry DOI: 10.7270/Q22R3TKH |
More data for this
Ligand-Target Pair | |
Protein polybromo-1
(Homo sapiens (Human)) | BDBM50184978
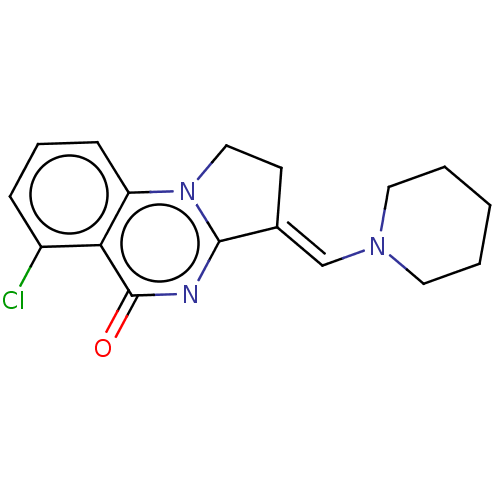
(CHEMBL3823524)Show InChI InChI=1S/C17H18ClN3O/c18-13-5-4-6-14-15(13)17(22)19-16-12(7-10-21(14)16)11-20-8-2-1-3-9-20/h4-6,11H,1-3,7-10H2/b12-11+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Binding affinity to His6-tagged human recombinant PB1 bromodomain isoform 5 expressed in Escherichia coli BL21(DE3)-R3-pRARE2 cells by VP-ITC microca... |
J Med Chem 59: 5095-101 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01997
BindingDB Entry DOI: 10.7270/Q22R3TKH |
More data for this
Ligand-Target Pair | |
Protein polybromo-1
(Homo sapiens (Human)) | BDBM50184979
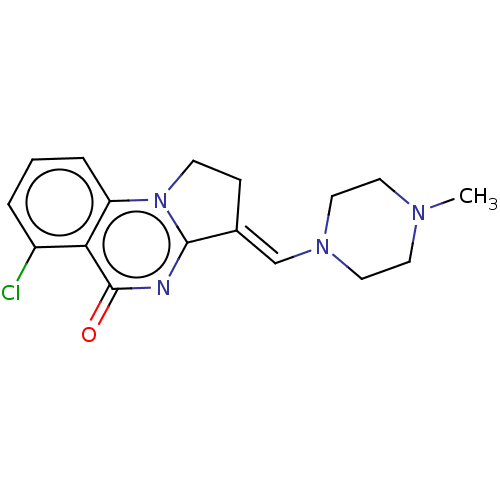
(CHEMBL3822689)Show InChI InChI=1S/C17H19ClN4O/c1-20-7-9-21(10-8-20)11-12-5-6-22-14-4-2-3-13(18)15(14)17(23)19-16(12)22/h2-4,11H,5-10H2,1H3/b12-11+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Binding affinity to His6-tagged human recombinant PB1 bromodomain isoform 5 expressed in Escherichia coli BL21(DE3)-R3-pRARE2 cells by VP-ITC microca... |
J Med Chem 59: 5095-101 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01997
BindingDB Entry DOI: 10.7270/Q22R3TKH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data