 Found 38 hits with Last Name = 'sweeney' and Initial = 'r'
Found 38 hits with Last Name = 'sweeney' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50129806
(CHEMBL3627987)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCNC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CCCCN)NC1=O)C(=O)N[C@@H](CC(C)C)C(N)=O)[C@H](C)O |r| Show InChI InChI=1S/C43H71N15O9S2/c1-22(2)17-31(35(47)60)55-41(66)33-21-69-68-20-27(46)36(61)50-16-13-26(45)37(62)53-30(12-8-15-51-43(48)49)38(63)56-32(19-25-18-24-9-4-5-10-28(24)52-25)40(65)58-34(23(3)59)42(67)54-29(39(64)57-33)11-6-7-14-44/h4-5,9-10,18,22-23,26-27,29-34,52,59H,6-8,11-17,19-21,44-46H2,1-3H3,(H2,47,60)(H,50,61)(H,53,62)(H,54,67)(H,55,66)(H,56,63)(H,57,64)(H,58,65)(H4,48,49,51)/t23-,26-,27-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain 448 residue preincubated for 30 mins by FRET assay |
Bioorg Med Chem 23: 7264-73 (2015)
Article DOI: 10.1016/j.bmc.2015.10.024
BindingDB Entry DOI: 10.7270/Q2MG7RB8 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50129806

(CHEMBL3627987)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCNC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CCCCN)NC1=O)C(=O)N[C@@H](CC(C)C)C(N)=O)[C@H](C)O |r| Show InChI InChI=1S/C43H71N15O9S2/c1-22(2)17-31(35(47)60)55-41(66)33-21-69-68-20-27(46)36(61)50-16-13-26(45)37(62)53-30(12-8-15-51-43(48)49)38(63)56-32(19-25-18-24-9-4-5-10-28(24)52-25)40(65)58-34(23(3)59)42(67)54-29(39(64)57-33)11-6-7-14-44/h4-5,9-10,18,22-23,26-27,29-34,52,59H,6-8,11-17,19-21,44-46H2,1-3H3,(H2,47,60)(H,50,61)(H,53,62)(H,54,67)(H,55,66)(H,56,63)(H,57,64)(H,58,65)(H4,48,49,51)/t23-,26-,27-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain 448 residue coincubated with TCEP for 30 mins and TCEP absent during reaction by FRET assay |
Bioorg Med Chem 23: 7264-73 (2015)
Article DOI: 10.1016/j.bmc.2015.10.024
BindingDB Entry DOI: 10.7270/Q2MG7RB8 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50129806

(CHEMBL3627987)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCNC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CCCCN)NC1=O)C(=O)N[C@@H](CC(C)C)C(N)=O)[C@H](C)O |r| Show InChI InChI=1S/C43H71N15O9S2/c1-22(2)17-31(35(47)60)55-41(66)33-21-69-68-20-27(46)36(61)50-16-13-26(45)37(62)53-30(12-8-15-51-43(48)49)38(63)56-32(19-25-18-24-9-4-5-10-28(24)52-25)40(65)58-34(23(3)59)42(67)54-29(39(64)57-33)11-6-7-14-44/h4-5,9-10,18,22-23,26-27,29-34,52,59H,6-8,11-17,19-21,44-46H2,1-3H3,(H2,47,60)(H,50,61)(H,53,62)(H,54,67)(H,55,66)(H,56,63)(H,57,64)(H,58,65)(H4,48,49,51)/t23-,26-,27-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum truncated-BoNT/A light chain 424 residue preincubated for 30 mins by FRET assay |
Bioorg Med Chem 23: 7264-73 (2015)
Article DOI: 10.1016/j.bmc.2015.10.024
BindingDB Entry DOI: 10.7270/Q2MG7RB8 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50129806

(CHEMBL3627987)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCNC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CCCCN)NC1=O)C(=O)N[C@@H](CC(C)C)C(N)=O)[C@H](C)O |r| Show InChI InChI=1S/C43H71N15O9S2/c1-22(2)17-31(35(47)60)55-41(66)33-21-69-68-20-27(46)36(61)50-16-13-26(45)37(62)53-30(12-8-15-51-43(48)49)38(63)56-32(19-25-18-24-9-4-5-10-28(24)52-25)40(65)58-34(23(3)59)42(67)54-29(39(64)57-33)11-6-7-14-44/h4-5,9-10,18,22-23,26-27,29-34,52,59H,6-8,11-17,19-21,44-46H2,1-3H3,(H2,47,60)(H,50,61)(H,53,62)(H,54,67)(H,55,66)(H,56,63)(H,57,64)(H,58,65)(H4,48,49,51)/t23-,26-,27-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain 448 residue coincubated with TCEP for 60 mins and TCEP absent during reaction by FRET assay |
Bioorg Med Chem 23: 7264-73 (2015)
Article DOI: 10.1016/j.bmc.2015.10.024
BindingDB Entry DOI: 10.7270/Q2MG7RB8 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50129806

(CHEMBL3627987)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCNC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CCCCN)NC1=O)C(=O)N[C@@H](CC(C)C)C(N)=O)[C@H](C)O |r| Show InChI InChI=1S/C43H71N15O9S2/c1-22(2)17-31(35(47)60)55-41(66)33-21-69-68-20-27(46)36(61)50-16-13-26(45)37(62)53-30(12-8-15-51-43(48)49)38(63)56-32(19-25-18-24-9-4-5-10-28(24)52-25)40(65)58-34(23(3)59)42(67)54-29(39(64)57-33)11-6-7-14-44/h4-5,9-10,18,22-23,26-27,29-34,52,59H,6-8,11-17,19-21,44-46H2,1-3H3,(H2,47,60)(H,50,61)(H,53,62)(H,54,67)(H,55,66)(H,56,63)(H,57,64)(H,58,65)(H4,48,49,51)/t23-,26-,27-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain 448 residue coincubated with TCEP for 30 mins and TCEP present during reaction by FRET assay |
Bioorg Med Chem 23: 7264-73 (2015)
Article DOI: 10.1016/j.bmc.2015.10.024
BindingDB Entry DOI: 10.7270/Q2MG7RB8 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50129806

(CHEMBL3627987)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCNC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CCCCN)NC1=O)C(=O)N[C@@H](CC(C)C)C(N)=O)[C@H](C)O |r| Show InChI InChI=1S/C43H71N15O9S2/c1-22(2)17-31(35(47)60)55-41(66)33-21-69-68-20-27(46)36(61)50-16-13-26(45)37(62)53-30(12-8-15-51-43(48)49)38(63)56-32(19-25-18-24-9-4-5-10-28(24)52-25)40(65)58-34(23(3)59)42(67)54-29(39(64)57-33)11-6-7-14-44/h4-5,9-10,18,22-23,26-27,29-34,52,59H,6-8,11-17,19-21,44-46H2,1-3H3,(H2,47,60)(H,50,61)(H,53,62)(H,54,67)(H,55,66)(H,56,63)(H,57,64)(H,58,65)(H4,48,49,51)/t23-,26-,27-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain 448 residue coincubated with TCEP for 60 mins and TCEP present during reaction by FRET assay |
Bioorg Med Chem 23: 7264-73 (2015)
Article DOI: 10.1016/j.bmc.2015.10.024
BindingDB Entry DOI: 10.7270/Q2MG7RB8 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50129807

(CHEMBL3627988)Show SMILES [H][C@]1(NC(=O)[C@H](Cc2cc3ccccc3[nH]2)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCNC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CCCCN)NC1=O)C(N)=O)[C@H](C)O |r| Show InChI InChI=1S/C37H60N14O8S2/c1-19(52)29-36(59)48-25(9-4-5-12-38)34(57)50-28(30(41)53)18-61-60-17-23(40)31(54)44-14-11-22(39)32(55)47-26(10-6-13-45-37(42)43)33(56)49-27(35(58)51-29)16-21-15-20-7-2-3-8-24(20)46-21/h2-3,7-8,15,19,22-23,25-29,46,52H,4-6,9-14,16-18,38-40H2,1H3,(H2,41,53)(H,44,54)(H,47,55)(H,48,59)(H,49,56)(H,50,57)(H,51,58)(H4,42,43,45)/t19-,22-,23-,25-,26-,27-,28-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 468 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain 448 residue preincubated for 30 mins by FRET assay |
Bioorg Med Chem 23: 7264-73 (2015)
Article DOI: 10.1016/j.bmc.2015.10.024
BindingDB Entry DOI: 10.7270/Q2MG7RB8 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009661
(CHEMBL3235218)Show InChI InChI=1S/C21H28N4O2/c26-20(15-24-27)22-13-7-1-2-8-14-23-21-16-9-3-5-11-18(16)25-19-12-6-4-10-17(19)21/h3,5,9,11,15,27H,1-2,4,6-8,10,12-14H2,(H,22,26)(H,23,25)/b24-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 588 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009659

(CHEMBL3235216)Show InChI InChI=1S/C19H24N4O2/c24-18(13-22-25)20-11-5-6-12-21-19-14-7-1-3-9-16(14)23-17-10-4-2-8-15(17)19/h1,3,7,9,13,25H,2,4-6,8,10-12H2,(H,20,24)(H,21,23)/b22-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009660
(CHEMBL3235217)Show InChI InChI=1S/C19H21N5O2/c20-12-17(24-26)19(25)22-11-5-10-21-18-13-6-1-3-8-15(13)23-16-9-4-2-7-14(16)18/h1,3,6,8,26H,2,4-5,7,9-11H2,(H,21,23)(H,22,25)/b24-17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009658
(CHEMBL3235215)Show InChI InChI=1S/C18H22N4O2.ClH/c23-17(12-21-24)19-10-5-11-20-18-13-6-1-3-8-15(13)22-16-9-4-2-7-14(16)18;/h1,3,6,8,12,24H,2,4-5,7,9-11H2,(H,19,23)(H,20,22);1H/b21-12+; | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009664
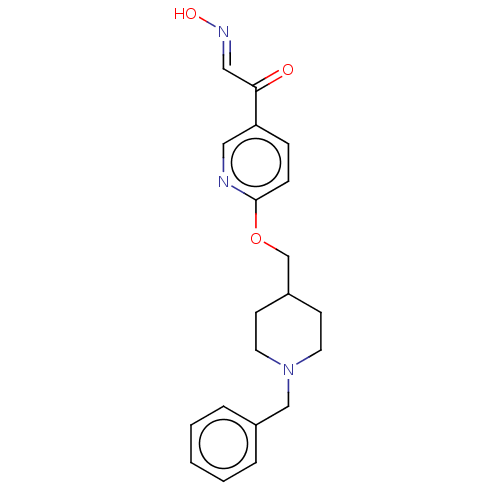
(CHEMBL3235221)Show InChI InChI=1S/C20H23N3O3/c24-19(13-22-25)18-6-7-20(21-12-18)26-15-17-8-10-23(11-9-17)14-16-4-2-1-3-5-16/h1-7,12-13,17,25H,8-11,14-15H2/b22-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009670

(CHEMBL3235227)Show InChI InChI=1S/C19H22N4O3/c24-18(12-22-25)17-10-20-19(21-11-17)26-14-16-6-8-23(9-7-16)13-15-4-2-1-3-5-15/h1-5,10-12,16,25H,6-9,13-14H2/b22-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009694
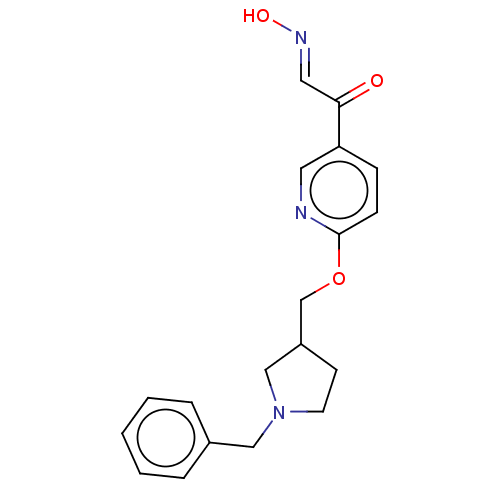
(CHEMBL3235232)Show InChI InChI=1S/C19H21N3O3/c23-18(11-21-24)17-6-7-19(20-10-17)25-14-16-8-9-22(13-16)12-15-4-2-1-3-5-15/h1-7,10-11,16,24H,8-9,12-14H2/b21-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.37E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009663

(CHEMBL3235220)Show InChI InChI=1S/C20H23N3O3/c24-19(13-22-25)18-6-9-21-20(12-18)26-15-17-7-10-23(11-8-17)14-16-4-2-1-3-5-16/h1-6,9,12-13,17,25H,7-8,10-11,14-15H2/b22-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.44E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009667
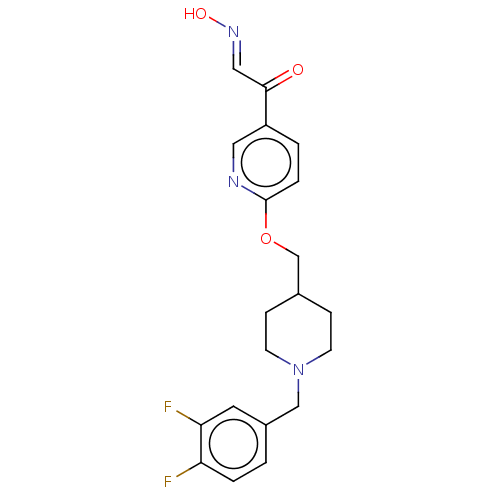
(CHEMBL3235224)Show SMILES O\N=C\C(=O)c1ccc(OCC2CCN(Cc3ccc(F)c(F)c3)CC2)nc1 Show InChI InChI=1S/C20H21F2N3O3/c21-17-3-1-15(9-18(17)22)12-25-7-5-14(6-8-25)13-28-20-4-2-16(10-23-20)19(26)11-24-27/h1-4,9-11,14,27H,5-8,12-13H2/b24-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.59E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009662

(CHEMBL3235219 | US9162983, C)Show InChI InChI=1S/C20H23N3O3/c24-19(13-22-25)18-7-4-10-21-20(18)26-15-17-8-11-23(12-9-17)14-16-5-2-1-3-6-16/h1-7,10,13,17,25H,8-9,11-12,14-15H2/b22-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009715
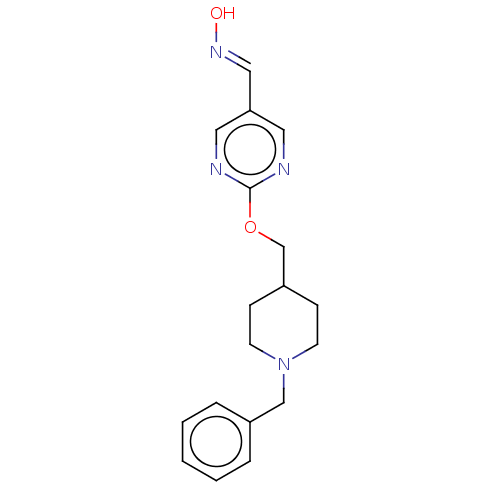
(CHEMBL3235235)Show InChI InChI=1S/C18H22N4O2/c23-21-12-17-10-19-18(20-11-17)24-14-16-6-8-22(9-7-16)13-15-4-2-1-3-5-15/h1-5,10-12,16,23H,6-9,13-14H2/b21-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.99E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009665
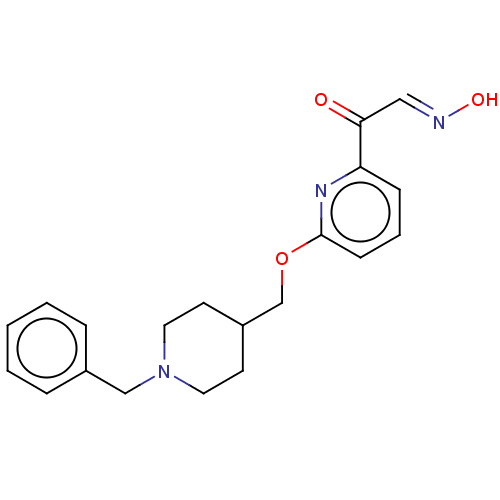
(CHEMBL3235222)Show InChI InChI=1S/C20H23N3O3/c24-19(13-21-25)18-7-4-8-20(22-18)26-15-17-9-11-23(12-10-17)14-16-5-2-1-3-6-16/h1-8,13,17,25H,9-12,14-15H2/b21-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.99E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009675
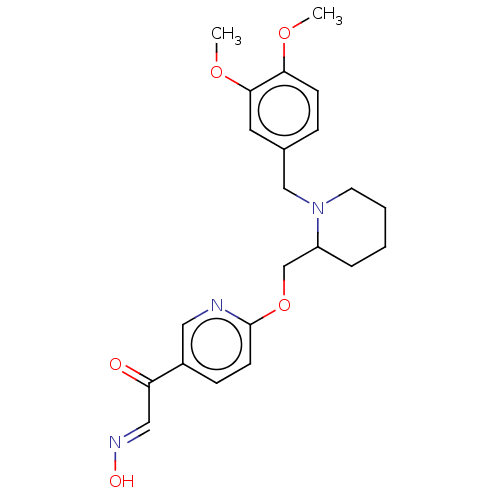
(CHEMBL3235230)Show SMILES COc1ccc(CN2CCCCC2COc2ccc(cn2)C(=O)\C=N\O)cc1OC Show InChI InChI=1S/C22H27N3O5/c1-28-20-8-6-16(11-21(20)29-2)14-25-10-4-3-5-18(25)15-30-22-9-7-17(12-23-22)19(26)13-24-27/h6-9,11-13,18,27H,3-5,10,14-15H2,1-2H3/b24-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009650

(CHEMBL3235207)Show InChI InChI=1S/C16H21N3O3/c1-12(20)15(18-22)16(21)17-14-7-9-19(10-8-14)11-13-5-3-2-4-6-13/h2-6,14,22H,7-11H2,1H3,(H,17,21)/b18-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.34E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009713
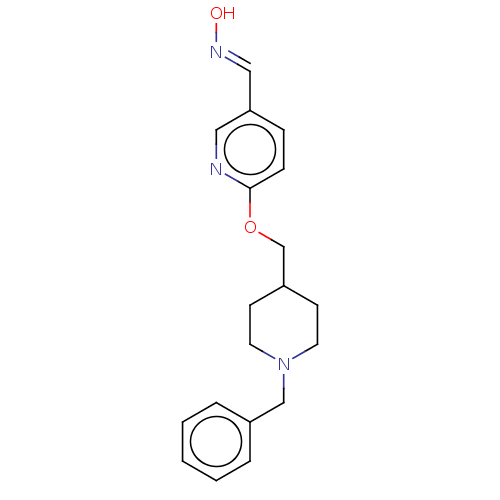
(CHEMBL3235234)Show InChI InChI=1S/C19H23N3O2/c23-21-13-18-6-7-19(20-12-18)24-15-17-8-10-22(11-9-17)14-16-4-2-1-3-5-16/h1-7,12-13,17,23H,8-11,14-15H2/b21-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.66E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009671
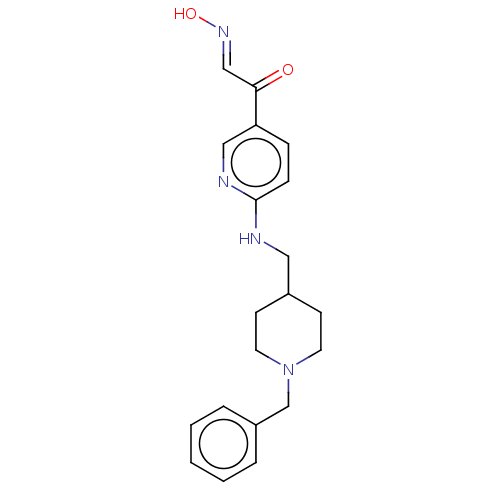
(CHEMBL3235228)Show InChI InChI=1S/C20H24N4O2/c25-19(14-23-26)18-6-7-20(22-13-18)21-12-16-8-10-24(11-9-16)15-17-4-2-1-3-5-17/h1-7,13-14,16,26H,8-12,15H2,(H,21,22)/b23-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.86E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009669

(CHEMBL3235226)Show SMILES O\N=C\C(=O)c1ccc(OCC2CCN(Cc3cccc(Cl)c3)CC2)nc1 Show InChI InChI=1S/C20H22ClN3O3/c21-18-3-1-2-16(10-18)13-24-8-6-15(7-9-24)14-27-20-5-4-17(11-22-20)19(25)12-23-26/h1-5,10-12,15,26H,6-9,13-14H2/b23-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.98E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009673
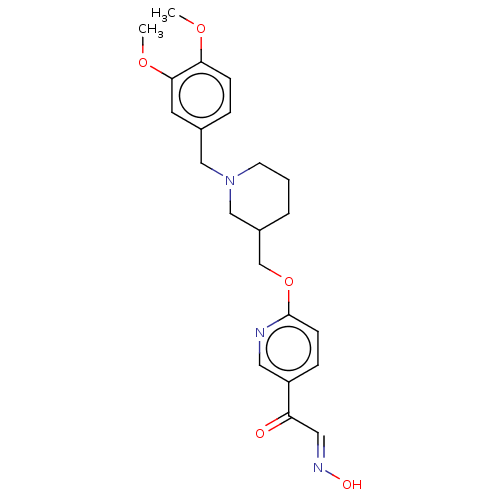
(CHEMBL3235229)Show SMILES COc1ccc(CN2CCCC(COc3ccc(cn3)C(=O)\C=N\O)C2)cc1OC Show InChI InChI=1S/C22H27N3O5/c1-28-20-7-5-16(10-21(20)29-2)13-25-9-3-4-17(14-25)15-30-22-8-6-18(11-23-22)19(26)12-24-27/h5-8,10-12,17,27H,3-4,9,13-15H2,1-2H3/b24-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009692

(CHEMBL3235231)Show InChI InChI=1S/C20H23N3O3/c24-19(13-22-25)17-9-10-20(21-12-17)26-15-18-8-4-5-11-23(18)14-16-6-2-1-3-7-16/h1-3,6-7,9-10,12-13,18,25H,4-5,8,11,14-15H2/b22-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.51E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009655
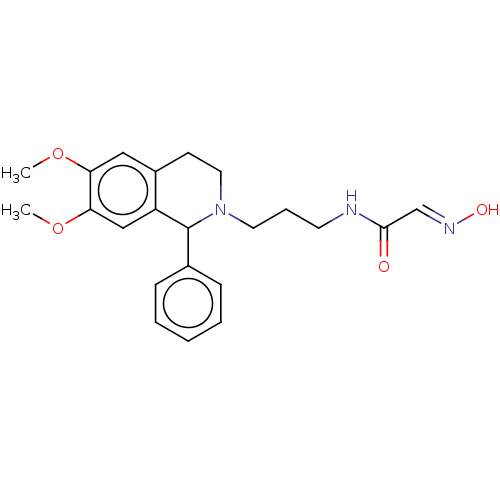
(CHEMBL3235212)Show SMILES COc1cc2CCN(CCCNC(=O)\C=N\O)C(c3ccccc3)c2cc1OC Show InChI InChI=1S/C22H27N3O4/c1-28-19-13-17-9-12-25(11-6-10-23-21(26)15-24-27)22(16-7-4-3-5-8-16)18(17)14-20(19)29-2/h3-5,7-8,13-15,22,27H,6,9-12H2,1-2H3,(H,23,26)/b24-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.95E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009652

(CHEMBL3235209)Show InChI InChI=1S/C15H18N4O2/c16-10-14(18-21)15(20)17-13-6-8-19(9-7-13)11-12-4-2-1-3-5-12/h1-5,13,21H,6-9,11H2,(H,17,20)/b18-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009668
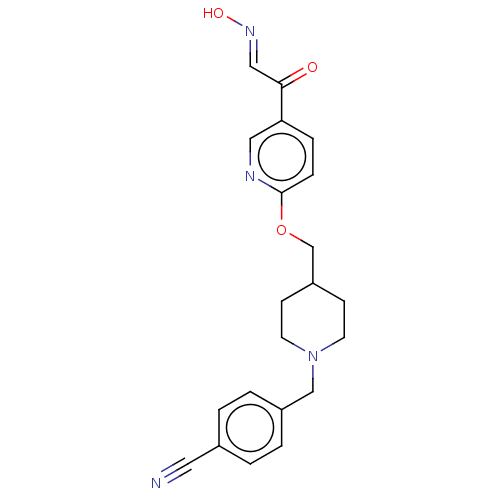
(CHEMBL3235225)Show SMILES O\N=C\C(=O)c1ccc(OCC2CCN(Cc3ccc(cc3)C#N)CC2)nc1 Show InChI InChI=1S/C21H22N4O3/c22-11-16-1-3-17(4-2-16)14-25-9-7-18(8-10-25)15-28-21-6-5-19(12-23-21)20(26)13-24-27/h1-6,12-13,18,27H,7-10,14-15H2/b24-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.61E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009654

(CHEMBL3235211)Show SMILES COc1ccc(CN2CCC(CNC(=O)C(=N\O)\C(C)=O)CC2)cc1OC Show InChI InChI=1S/C19H27N3O5/c1-13(23)18(21-25)19(24)20-11-14-6-8-22(9-7-14)12-15-4-5-16(26-2)17(10-15)27-3/h4-5,10,14,25H,6-9,11-12H2,1-3H3,(H,20,24)/b21-18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.63E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009656

(CHEMBL3235213)Show SMILES COc1cc2CCN(CCCNC(=O)C(=N\O)\C(C)=O)C(c3ccccc3)c2cc1OC Show InChI InChI=1S/C24H29N3O5/c1-16(28)22(26-30)24(29)25-11-7-12-27-13-10-18-14-20(31-2)21(32-3)15-19(18)23(27)17-8-5-4-6-9-17/h4-6,8-9,14-15,23,30H,7,10-13H2,1-3H3,(H,25,29)/b26-22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.93E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009666

(CHEMBL3235223)Show SMILES COc1ccc(CN2CCC(COc3ccc(cn3)C(=O)\C=N\O)CC2)cc1OC Show InChI InChI=1S/C22H27N3O5/c1-28-20-5-3-17(11-21(20)29-2)14-25-9-7-16(8-10-25)15-30-22-6-4-18(12-23-22)19(26)13-24-27/h3-6,11-13,16,27H,7-10,14-15H2,1-2H3/b24-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.93E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009651
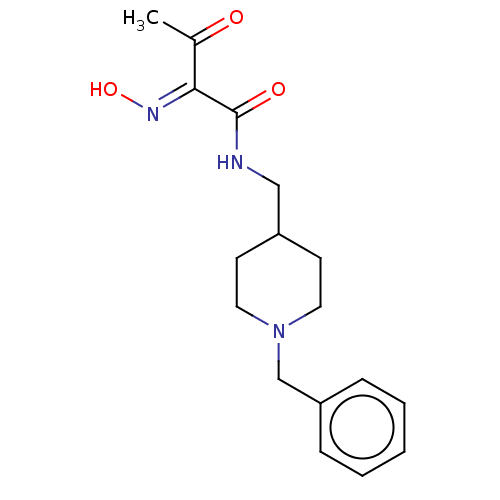
(CHEMBL3235208 | US9162983, E)Show InChI InChI=1S/C17H23N3O3/c1-13(21)16(19-23)17(22)18-11-14-7-9-20(10-8-14)12-15-5-3-2-4-6-15/h2-6,14,23H,7-12H2,1H3,(H,18,22)/b19-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009648

(CHEMBL3235205)Show InChI InChI=1S/C14H19N3O2/c18-14(10-15-19)16-13-6-8-17(9-7-13)11-12-4-2-1-3-5-12/h1-5,10,13,19H,6-9,11H2,(H,16,18)/b15-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.43E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009711
(CHEMBL3235233)Show SMILES COc1ccc(CN2CCC(COc3ccc(cn3)C(=O)\C=N\O)C2)cc1OC Show InChI InChI=1S/C21H25N3O5/c1-27-19-5-3-15(9-20(19)28-2)12-24-8-7-16(13-24)14-29-21-6-4-17(10-22-21)18(25)11-23-26/h3-6,9-11,16,26H,7-8,12-14H2,1-2H3/b23-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.52E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009657

(CHEMBL3235214)Show SMILES COc1cc2CCN(CCCNC(=O)C(=N\O)\C(C)=O)Cc2cc1OC Show InChI InChI=1S/C18H25N3O5/c1-12(22)17(20-24)18(23)19-6-4-7-21-8-5-13-9-15(25-2)16(26-3)10-14(13)11-21/h9-10,24H,4-8,11H2,1-3H3,(H,19,23)/b20-17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.67E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009649

(CHEMBL3235206)Show InChI InChI=1S/C15H21N3O2/c19-15(11-17-20)16-10-13-6-8-18(9-7-13)12-14-4-2-1-3-5-14/h1-5,11,13,20H,6-10,12H2,(H,16,19)/b17-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.18E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009653

(CHEMBL3235210)Show InChI InChI=1S/C16H20N4O2/c17-10-15(19-22)16(21)18-11-13-6-8-20(9-7-13)12-14-4-2-1-3-5-14/h1-5,13,22H,6-9,11-12H2,(H,18,21)/b19-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.50E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data