

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty acid-binding protein, adipocyte (Homo sapiens (Human)) | BDBM50177675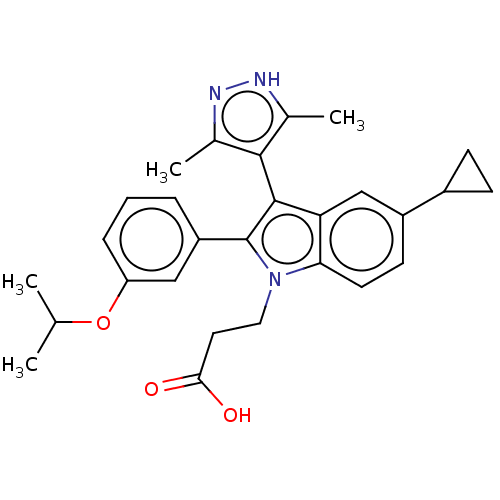 (CHEMBL3814634) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Binding affinity to His-tagged human recombinant FABP4 expressed in Escherichia coli BL21 (DE3) by fluorescence assay | ACS Med Chem Lett 7: 435-9 (2016) Article DOI: 10.1021/acsmedchemlett.6b00040 BindingDB Entry DOI: 10.7270/Q2RX9F0J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fatty acid-binding protein, adipocyte (Homo sapiens (Human)) | BDBM50177674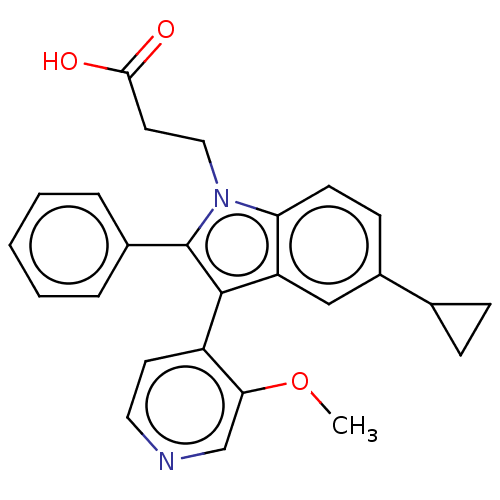 (CHEMBL3813892) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PDB Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Binding affinity to His-tagged human recombinant FABP4 expressed in Escherichia coli BL21 (DE3) by fluorescence assay | ACS Med Chem Lett 7: 435-9 (2016) Article DOI: 10.1021/acsmedchemlett.6b00040 BindingDB Entry DOI: 10.7270/Q2RX9F0J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fatty acid-binding protein, adipocyte (Homo sapiens (Human)) | BDBM50177673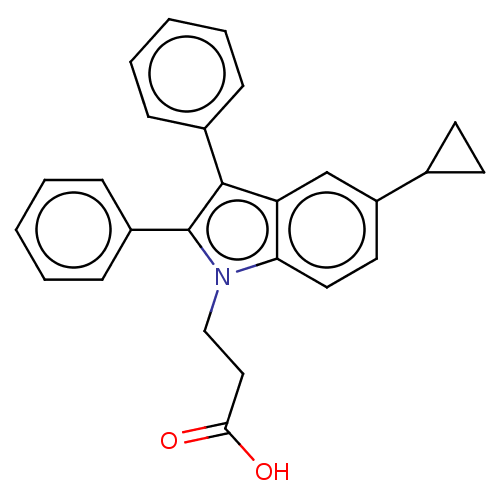 (CHEMBL3814575) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PDB Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Binding affinity to His-tagged human recombinant FABP4 expressed in Escherichia coli BL21 (DE3) by fluorescence assay | ACS Med Chem Lett 7: 435-9 (2016) Article DOI: 10.1021/acsmedchemlett.6b00040 BindingDB Entry DOI: 10.7270/Q2RX9F0J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fatty acid-binding protein, adipocyte (Homo sapiens (Human)) | BDBM50212873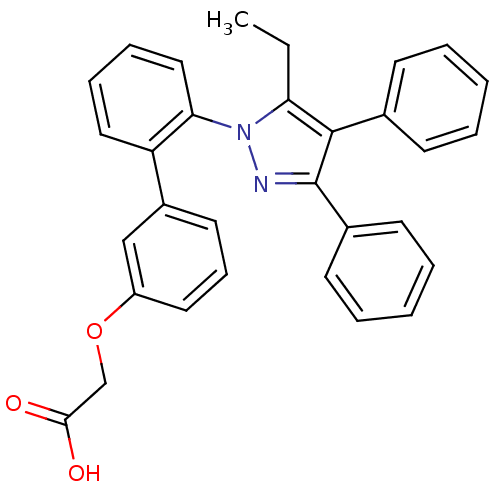 (((2'-(5-ETHYL-3,4-DIPHENYL-1H-PYRAZOL-1-YL)-3-BIPH...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Binding affinity to His-tagged human recombinant FABP4 expressed in Escherichia coli BL21 (DE3) by fluorescence assay | ACS Med Chem Lett 7: 435-9 (2016) Article DOI: 10.1021/acsmedchemlett.6b00040 BindingDB Entry DOI: 10.7270/Q2RX9F0J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fatty acid-binding protein, adipocyte (Homo sapiens (Human)) | BDBM50177672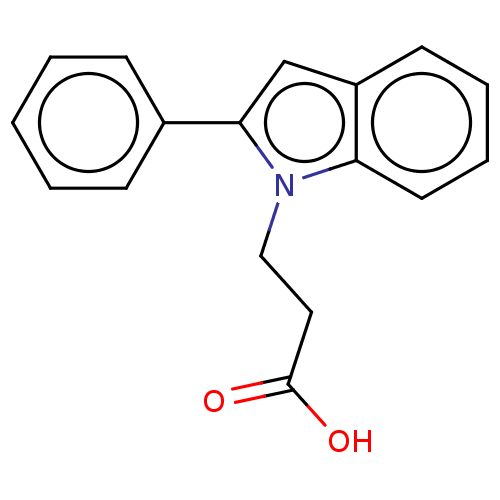 (CHEMBL1586017) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem | PDB Article PubMed | >1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Binding affinity to His-tagged human recombinant FABP4 expressed in Escherichia coli BL21 (DE3) by fluorescence assay | ACS Med Chem Lett 7: 435-9 (2016) Article DOI: 10.1021/acsmedchemlett.6b00040 BindingDB Entry DOI: 10.7270/Q2RX9F0J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Taste receptor type 1 member 2/3 (Homo sapiens (Human)) | BDBM50535463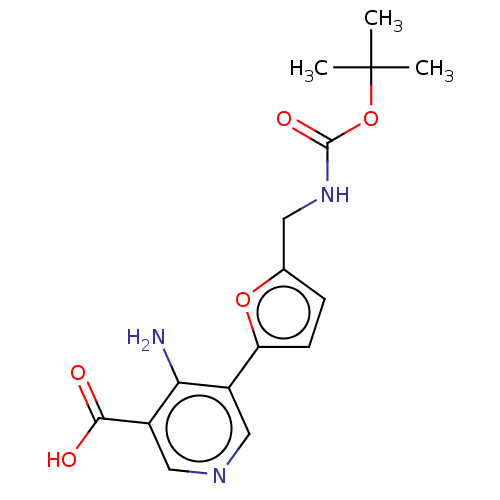 (CHEMBL4452237) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.45E+7 | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human T1R2/T1R3 expressed in PEAKrapid cells assessed as potentiation of sucrose-induced intracellular calc... | ACS Med Chem Lett 10: 800-805 (2019) Article DOI: 10.1021/acsmedchemlett.9b00051 BindingDB Entry DOI: 10.7270/Q2348PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 2/3 (Homo sapiens (Human)) | BDBM50535464 (CHEMBL4469334) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.16E+7 | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human T1R2/T1R3 expressed in PEAKrapid cells assessed as potentiation of sucrose-induced intracellular calc... | ACS Med Chem Lett 10: 800-805 (2019) Article DOI: 10.1021/acsmedchemlett.9b00051 BindingDB Entry DOI: 10.7270/Q2348PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 2/3 (Homo sapiens (Human)) | BDBM50535465 (CHEMBL4586146) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.11E+7 | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human T1R2/T1R3 expressed in PEAKrapid cells assessed as potentiation of sucrose-induced intracellular calc... | ACS Med Chem Lett 10: 800-805 (2019) Article DOI: 10.1021/acsmedchemlett.9b00051 BindingDB Entry DOI: 10.7270/Q2348PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 2/3 (Homo sapiens (Human)) | BDBM50535466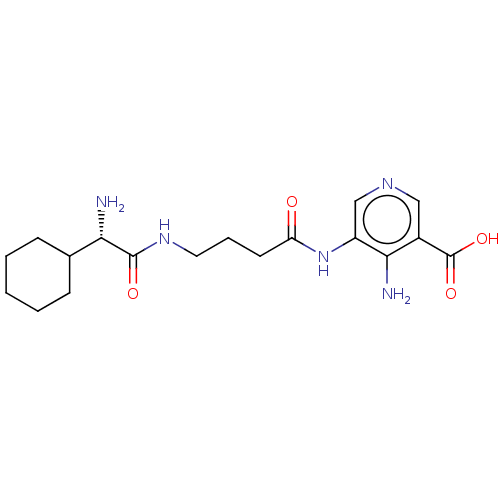 (CHEMBL4457270) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.62E+7 | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human T1R2/T1R3 expressed in PEAKrapid cells assessed as potentiation of sucrose-induced intracellular calc... | ACS Med Chem Lett 10: 800-805 (2019) Article DOI: 10.1021/acsmedchemlett.9b00051 BindingDB Entry DOI: 10.7270/Q2348PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 2/3 (Homo sapiens (Human)) | BDBM50535482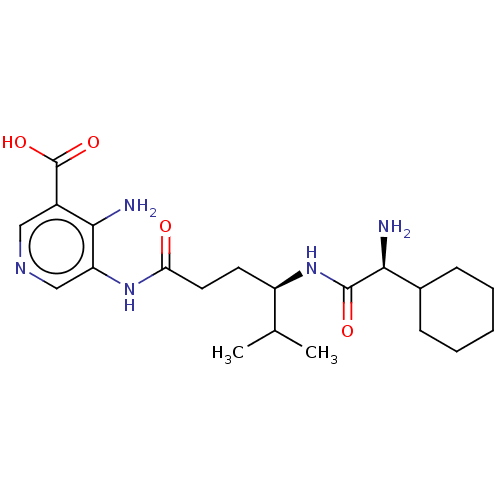 (CHEMBL4522824) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.05E+7 | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human T1R2/T1R3 expressed in PEAKrapid cells assessed as potentiation of sucrose-induced intracellular calc... | ACS Med Chem Lett 10: 800-805 (2019) Article DOI: 10.1021/acsmedchemlett.9b00051 BindingDB Entry DOI: 10.7270/Q2348PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 2/3 (Homo sapiens (Human)) | BDBM50535468 (CHEMBL4525425) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.02E+7 | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human T1R2/T1R3 expressed in PEAKrapid cells assessed as potentiation of sucrose-induced intracellular calc... | ACS Med Chem Lett 10: 800-805 (2019) Article DOI: 10.1021/acsmedchemlett.9b00051 BindingDB Entry DOI: 10.7270/Q2348PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 2/3 (Homo sapiens (Human)) | BDBM50535469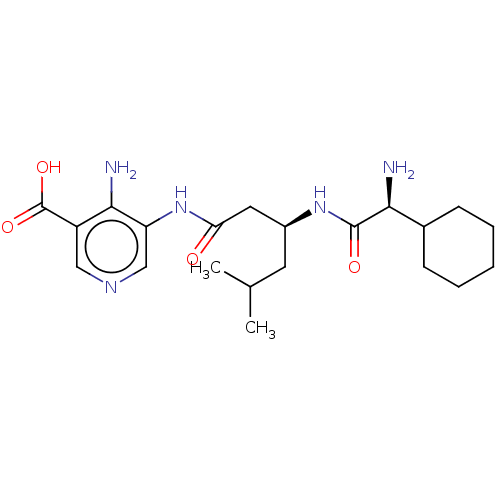 (CHEMBL4554779) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.27E+7 | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human T1R2/T1R3 expressed in PEAKrapid cells assessed as potentiation of sucrose-induced intracellular calc... | ACS Med Chem Lett 10: 800-805 (2019) Article DOI: 10.1021/acsmedchemlett.9b00051 BindingDB Entry DOI: 10.7270/Q2348PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 2/3 (Homo sapiens (Human)) | BDBM50535470 (CHEMBL4483698) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 8.90E+6 | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human T1R2/T1R3 expressed in PEAKrapid cells assessed as potentiation of sucrose-induced intracellular calc... | ACS Med Chem Lett 10: 800-805 (2019) Article DOI: 10.1021/acsmedchemlett.9b00051 BindingDB Entry DOI: 10.7270/Q2348PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 2/3 (Homo sapiens (Human)) | BDBM50535471 (CHEMBL4549509) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.15E+7 | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human T1R2/T1R3 expressed in PEAKrapid cells assessed as potentiation of sucrose-induced intracellular calc... | ACS Med Chem Lett 10: 800-805 (2019) Article DOI: 10.1021/acsmedchemlett.9b00051 BindingDB Entry DOI: 10.7270/Q2348PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 2/3 (Homo sapiens (Human)) | BDBM50535472 (CHEMBL4464976) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.10E+7 | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human T1R2/T1R3 expressed in PEAKrapid cells assessed as potentiation of sucrose-induced intracellular calc... | ACS Med Chem Lett 10: 800-805 (2019) Article DOI: 10.1021/acsmedchemlett.9b00051 BindingDB Entry DOI: 10.7270/Q2348PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 2/3 (Homo sapiens (Human)) | BDBM50535473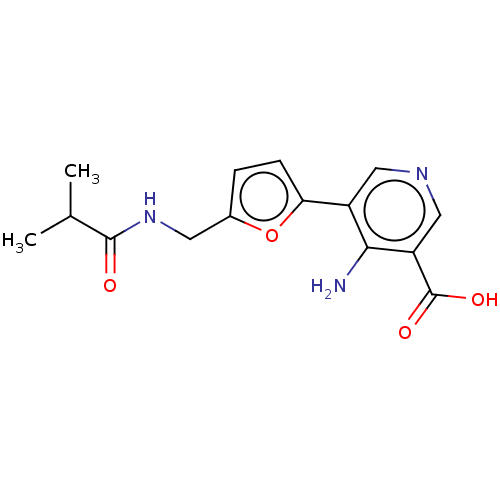 (CHEMBL4473744) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.21E+7 | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human T1R2/T1R3 expressed in PEAKrapid cells assessed as potentiation of sucrose-induced intracellular calc... | ACS Med Chem Lett 10: 800-805 (2019) Article DOI: 10.1021/acsmedchemlett.9b00051 BindingDB Entry DOI: 10.7270/Q2348PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 2/3 (Homo sapiens (Human)) | BDBM50535474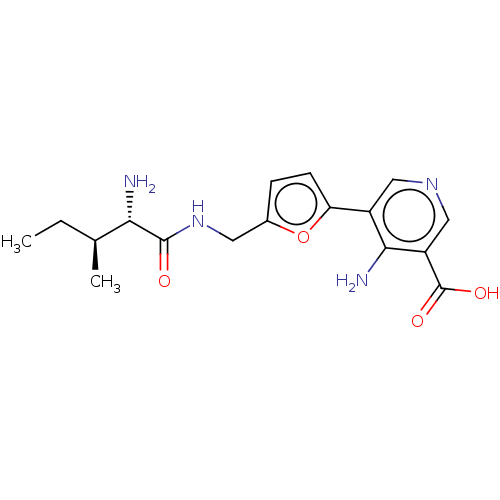 (CHEMBL4471408) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+7 | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human T1R2/T1R3 expressed in PEAKrapid cells assessed as potentiation of sucrose-induced intracellular calc... | ACS Med Chem Lett 10: 800-805 (2019) Article DOI: 10.1021/acsmedchemlett.9b00051 BindingDB Entry DOI: 10.7270/Q2348PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 2/3 (Homo sapiens (Human)) | BDBM50535475 (CHEMBL4450078) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 8.50E+6 | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human T1R2/T1R3 expressed in PEAKrapid cells assessed as potentiation of sucrose-induced intracellular calc... | ACS Med Chem Lett 10: 800-805 (2019) Article DOI: 10.1021/acsmedchemlett.9b00051 BindingDB Entry DOI: 10.7270/Q2348PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 2/3 (Homo sapiens (Human)) | BDBM50535476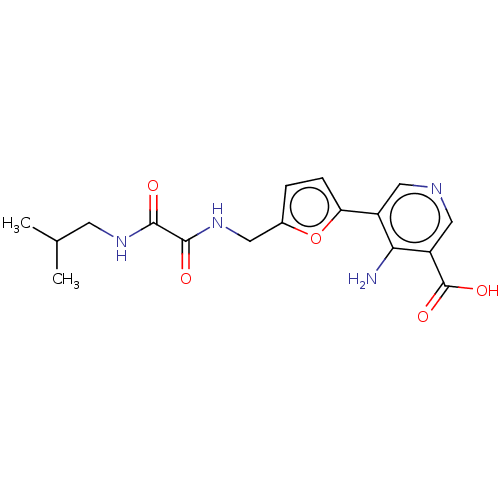 (CHEMBL4585719) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.65E+7 | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human T1R2/T1R3 expressed in PEAKrapid cells assessed as potentiation of sucrose-induced intracellular calc... | ACS Med Chem Lett 10: 800-805 (2019) Article DOI: 10.1021/acsmedchemlett.9b00051 BindingDB Entry DOI: 10.7270/Q2348PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 2/3 (Homo sapiens (Human)) | BDBM50535477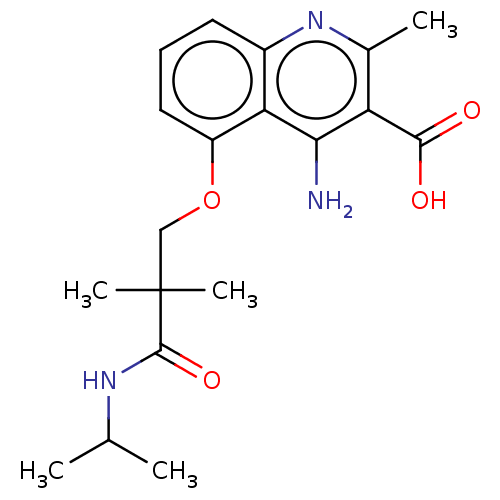 (CHEMBL4561660) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 6.00E+5 | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human T1R2/T1R3 expressed in PEAKrapid cells assessed as potentiation of sucrose-induced intracellular calc... | ACS Med Chem Lett 10: 800-805 (2019) Article DOI: 10.1021/acsmedchemlett.9b00051 BindingDB Entry DOI: 10.7270/Q2348PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 2/3 (Homo sapiens (Human)) | BDBM50535478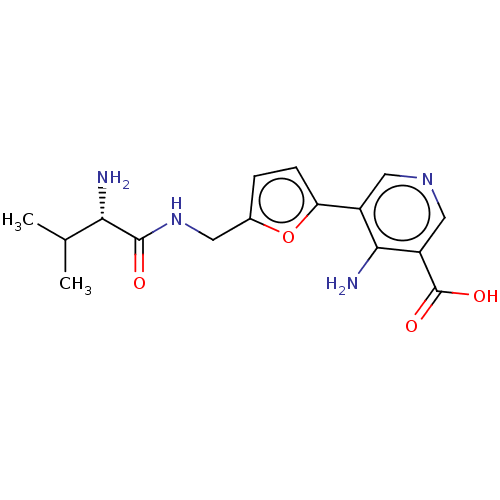 (CHEMBL4459681) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.46E+7 | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human T1R2/T1R3 expressed in PEAKrapid cells assessed as potentiation of sucrose-induced intracellular calc... | ACS Med Chem Lett 10: 800-805 (2019) Article DOI: 10.1021/acsmedchemlett.9b00051 BindingDB Entry DOI: 10.7270/Q2348PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 2/3 (Homo sapiens (Human)) | BDBM50535479 (CHEMBL4440803) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.02E+7 | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human T1R2/T1R3 expressed in PEAKrapid cells assessed as potentiation of sucrose-induced intracellular calc... | ACS Med Chem Lett 10: 800-805 (2019) Article DOI: 10.1021/acsmedchemlett.9b00051 BindingDB Entry DOI: 10.7270/Q2348PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 2/3 (Homo sapiens (Human)) | BDBM50535480 (CHEMBL4458064) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.68E+7 | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human T1R2/T1R3 expressed in PEAKrapid cells assessed as potentiation of sucrose-induced intracellular calc... | ACS Med Chem Lett 10: 800-805 (2019) Article DOI: 10.1021/acsmedchemlett.9b00051 BindingDB Entry DOI: 10.7270/Q2348PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 2/3 (Homo sapiens (Human)) | BDBM50535481 (CHEMBL4454097) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.20E+6 | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human T1R2/T1R3 expressed in PEAKrapid cells assessed as potentiation of sucrose-induced intracellular calc... | ACS Med Chem Lett 10: 800-805 (2019) Article DOI: 10.1021/acsmedchemlett.9b00051 BindingDB Entry DOI: 10.7270/Q2348PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 2/3 (Homo sapiens (Human)) | BDBM50535462 (CHEMBL4561537) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.59E+7 | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human T1R2/T1R3 expressed in PEAKrapid cells assessed as potentiation of sucrose-induced intracellular calc... | ACS Med Chem Lett 10: 800-805 (2019) Article DOI: 10.1021/acsmedchemlett.9b00051 BindingDB Entry DOI: 10.7270/Q2348PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 2/3 (Homo sapiens (Human)) | BDBM50535461 (CHEMBL4531239) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 5.80E+6 | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human T1R2/T1R3 expressed in PEAKrapid cells assessed as potentiation of sucrose-induced intracellular calc... | ACS Med Chem Lett 10: 800-805 (2019) Article DOI: 10.1021/acsmedchemlett.9b00051 BindingDB Entry DOI: 10.7270/Q2348PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 2/3 (Homo sapiens (Human)) | BDBM50535460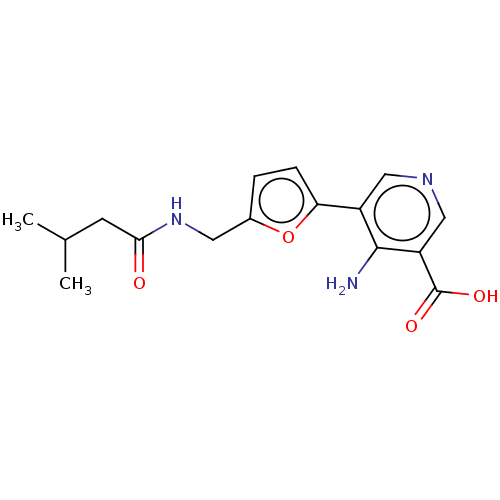 (CHEMBL4517824) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.83E+7 | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human T1R2/T1R3 expressed in PEAKrapid cells assessed as potentiation of sucrose-induced intracellular calc... | ACS Med Chem Lett 10: 800-805 (2019) Article DOI: 10.1021/acsmedchemlett.9b00051 BindingDB Entry DOI: 10.7270/Q2348PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 2/3 (Homo sapiens (Human)) | BDBM50535459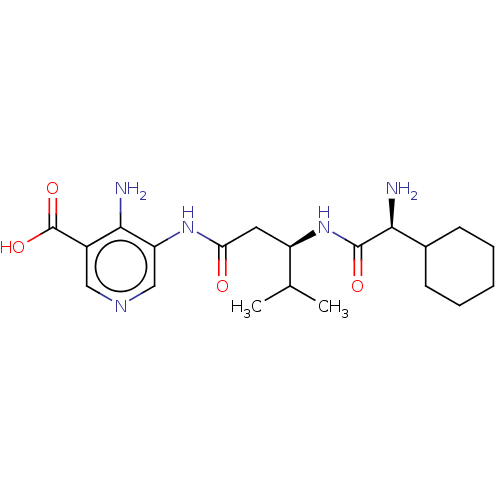 (CHEMBL4531637) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.84E+7 | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human T1R2/T1R3 expressed in PEAKrapid cells assessed as potentiation of sucrose-induced intracellular calc... | ACS Med Chem Lett 10: 800-805 (2019) Article DOI: 10.1021/acsmedchemlett.9b00051 BindingDB Entry DOI: 10.7270/Q2348PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 2/3 (Homo sapiens (Human)) | BDBM50535458 (CHEMBL4525445) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.71E+7 | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human T1R2/T1R3 expressed in PEAKrapid cells assessed as potentiation of sucrose-induced intracellular calc... | ACS Med Chem Lett 10: 800-805 (2019) Article DOI: 10.1021/acsmedchemlett.9b00051 BindingDB Entry DOI: 10.7270/Q2348PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Taste receptor type 1 member 2/3 (Homo sapiens (Human)) | BDBM50535467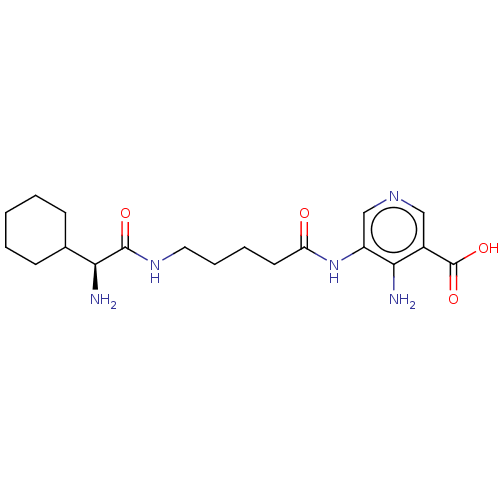 (CHEMBL4542114) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 7.80E+6 | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description Positive allosteric modulator activity at human T1R2/T1R3 expressed in PEAKrapid cells assessed as potentiation of sucrose-induced intracellular calc... | ACS Med Chem Lett 10: 800-805 (2019) Article DOI: 10.1021/acsmedchemlett.9b00051 BindingDB Entry DOI: 10.7270/Q2348PXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||