 Found 209 hits with Last Name = 'tan' and Initial = 'gt'
Found 209 hits with Last Name = 'tan' and Initial = 'gt' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50368504
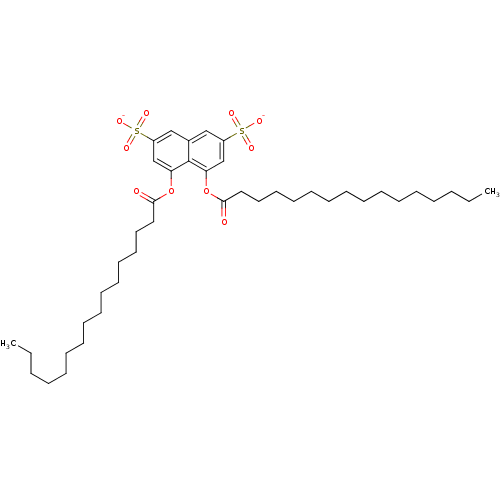
(CHEMBL1788126)Show SMILES CCCCCCCCCCCCCCCC(=O)Oc1cc(cc2cc(cc(OC(=O)CCCCCCCCCCCCCCC)c12)S([O-])(=O)=O)S([O-])(=O)=O Show InChI InChI=1S/C42H68O10S2/c1-3-5-7-9-11-13-15-17-19-21-23-25-27-29-40(43)51-38-33-36(53(45,46)47)31-35-32-37(54(48,49)50)34-39(42(35)38)52-41(44)30-28-26-24-22-20-18-16-14-12-10-8-6-4-2/h31-34H,3-30H2,1-2H3,(H,45,46,47)(H,48,49,50)/p-2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Universityof Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-2 reverse transcriptase |
J Med Chem 35: 4846-53 (1992)
BindingDB Entry DOI: 10.7270/Q2M0461N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50368504
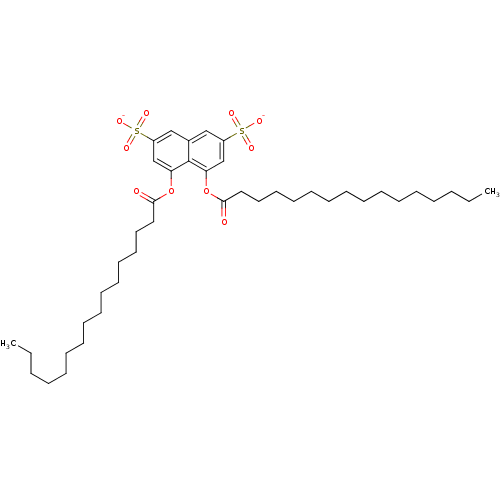
(CHEMBL1788126)Show SMILES CCCCCCCCCCCCCCCC(=O)Oc1cc(cc2cc(cc(OC(=O)CCCCCCCCCCCCCCC)c12)S([O-])(=O)=O)S([O-])(=O)=O Show InChI InChI=1S/C42H68O10S2/c1-3-5-7-9-11-13-15-17-19-21-23-25-27-29-40(43)51-38-33-36(53(45,46)47)31-35-32-37(54(48,49)50)34-39(42(35)38)52-41(44)30-28-26-24-22-20-18-16-14-12-10-8-6-4-2/h31-34H,3-30H2,1-2H3,(H,45,46,47)(H,48,49,50)/p-2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Universityof Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-2 reverse transcriptase |
J Med Chem 35: 4846-53 (1992)
BindingDB Entry DOI: 10.7270/Q2M0461N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50368515
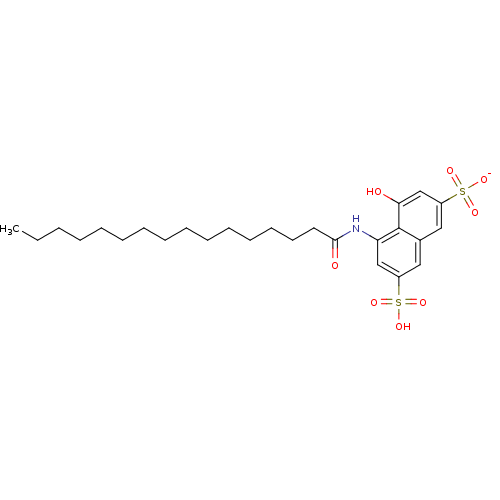
(CHEMBL1788125)Show SMILES CCCCCCCCCCCCCCCC(=O)Nc1cc(cc2cc(cc(O)c12)S([O-])(=O)=O)S(O)(=O)=O Show InChI InChI=1S/C26H39NO8S2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-25(29)27-23-18-21(36(30,31)32)16-20-17-22(37(33,34)35)19-24(28)26(20)23/h16-19,28H,2-15H2,1H3,(H,27,29)(H,30,31,32)(H,33,34,35)/p-1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universityof Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-2 reverse transcriptase |
J Med Chem 35: 4846-53 (1992)
BindingDB Entry DOI: 10.7270/Q2M0461N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50368515

(CHEMBL1788125)Show SMILES CCCCCCCCCCCCCCCC(=O)Nc1cc(cc2cc(cc(O)c12)S([O-])(=O)=O)S(O)(=O)=O Show InChI InChI=1S/C26H39NO8S2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-25(29)27-23-18-21(36(30,31)32)16-20-17-22(37(33,34)35)19-24(28)26(20)23/h16-19,28H,2-15H2,1H3,(H,27,29)(H,30,31,32)(H,33,34,35)/p-1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universityof Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-1 reverse transcriptase |
J Med Chem 35: 4846-53 (1992)
BindingDB Entry DOI: 10.7270/Q2M0461N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50336799
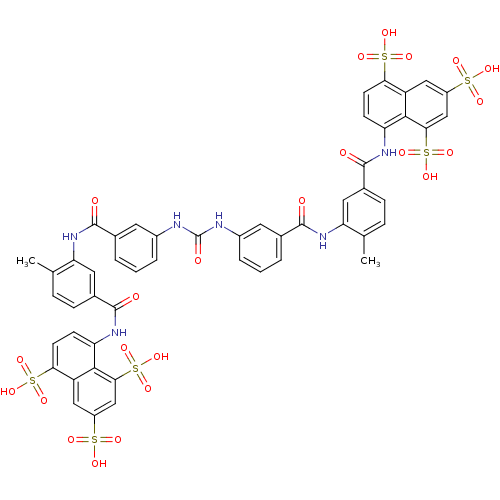
(5,5',5''-[1,3,6-naphthalenetriyltris(sulfonylimino...)Show SMILES Cc1ccc(cc1NC(=O)c1cccc(NC(=O)Nc2cccc(c2)C(=O)Nc2cc(ccc2C)C(=O)Nc2ccc(c3cc(cc(c23)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O)c1)C(=O)Nc1ccc(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O Show InChI InChI=1S/C51H40N6O23S6/c1-25-9-11-29(49(60)54-37-13-15-41(83(69,70)71)35-21-33(81(63,64)65)23-43(45(35)37)85(75,76)77)19-39(25)56-47(58)27-5-3-7-31(17-27)52-51(62)53-32-8-4-6-28(18-32)48(59)57-40-20-30(12-10-26(40)2)50(61)55-38-14-16-42(84(72,73)74)36-22-34(82(66,67)68)24-44(46(36)38)86(78,79)80/h3-24H,1-2H3,(H,54,60)(H,55,61)(H,56,58)(H,57,59)(H2,52,53,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT |
J Nat Prod 54: 143-54
Article DOI: 10.1021/np50073a012
BindingDB Entry DOI: 10.7270/Q2NK3HTG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50336799

(5,5',5''-[1,3,6-naphthalenetriyltris(sulfonylimino...)Show SMILES Cc1ccc(cc1NC(=O)c1cccc(NC(=O)Nc2cccc(c2)C(=O)Nc2cc(ccc2C)C(=O)Nc2ccc(c3cc(cc(c23)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O)c1)C(=O)Nc1ccc(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O Show InChI InChI=1S/C51H40N6O23S6/c1-25-9-11-29(49(60)54-37-13-15-41(83(69,70)71)35-21-33(81(63,64)65)23-43(45(35)37)85(75,76)77)19-39(25)56-47(58)27-5-3-7-31(17-27)52-51(62)53-32-8-4-6-28(18-32)48(59)57-40-20-30(12-10-26(40)2)50(61)55-38-14-16-42(84(72,73)74)36-22-34(82(66,67)68)24-44(46(36)38)86(78,79)80/h3-24H,1-2H3,(H,54,60)(H,55,61)(H,56,58)(H,57,59)(H2,52,53,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universityof Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-2 reverse transcriptase |
J Med Chem 35: 4846-53 (1992)
BindingDB Entry DOI: 10.7270/Q2M0461N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50336799

(5,5',5''-[1,3,6-naphthalenetriyltris(sulfonylimino...)Show SMILES Cc1ccc(cc1NC(=O)c1cccc(NC(=O)Nc2cccc(c2)C(=O)Nc2cc(ccc2C)C(=O)Nc2ccc(c3cc(cc(c23)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O)c1)C(=O)Nc1ccc(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O Show InChI InChI=1S/C51H40N6O23S6/c1-25-9-11-29(49(60)54-37-13-15-41(83(69,70)71)35-21-33(81(63,64)65)23-43(45(35)37)85(75,76)77)19-39(25)56-47(58)27-5-3-7-31(17-27)52-51(62)53-32-8-4-6-28(18-32)48(59)57-40-20-30(12-10-26(40)2)50(61)55-38-14-16-42(84(72,73)74)36-22-34(82(66,67)68)24-44(46(36)38)86(78,79)80/h3-24H,1-2H3,(H,54,60)(H,55,61)(H,56,58)(H,57,59)(H2,52,53,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universityof Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-2 reverse transcriptase |
J Med Chem 35: 4846-53 (1992)
BindingDB Entry DOI: 10.7270/Q2M0461N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50478516
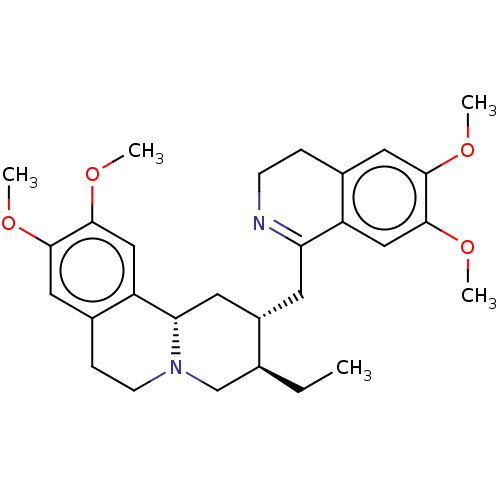
(CHEBI:81067 | CHEMBL471812 | O-Methylpsychotrine)Show SMILES [H][C@]1(CC2=NCCc3cc(OC)c(OC)cc23)C[C@]2([H])N(CCc3cc(OC)c(OC)cc23)C[C@@H]1CC |r,t:3| Show InChI InChI=1S/C29H38N2O4/c1-6-18-17-31-10-8-20-14-27(33-3)29(35-5)16-23(20)25(31)12-21(18)11-24-22-15-28(34-4)26(32-2)13-19(22)7-9-30-24/h13-16,18,21,25H,6-12,17H2,1-5H3/t18-,21-,25-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT |
J Nat Prod 54: 143-54
Article DOI: 10.1021/np50073a012
BindingDB Entry DOI: 10.7270/Q2NK3HTG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50478514
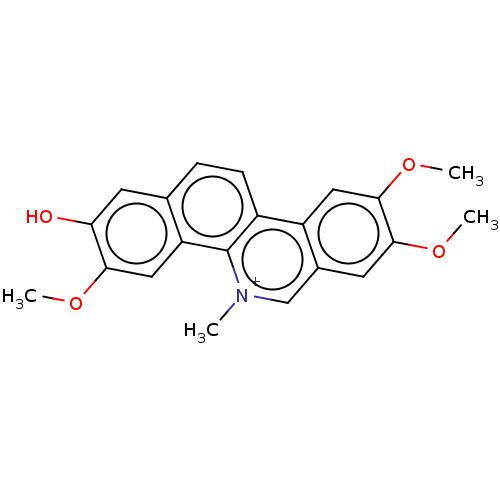
(Fagaronine | Fagaronine Chloride)Show SMILES [Cl-].COc1cc2c(ccc3c4cc(OC)c(OC)cc4c[n+](C)c23)cc1O Show InChI InChI=1S/C21H19NO4/c1-22-11-13-8-19(25-3)20(26-4)9-15(13)14-6-5-12-7-17(23)18(24-2)10-16(12)21(14)22/h5-11H,1-4H3/p+1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT using poly A oligo(dT) template primer |
J Nat Prod 54: 143-54
Article DOI: 10.1021/np50073a012
BindingDB Entry DOI: 10.7270/Q2NK3HTG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM60986
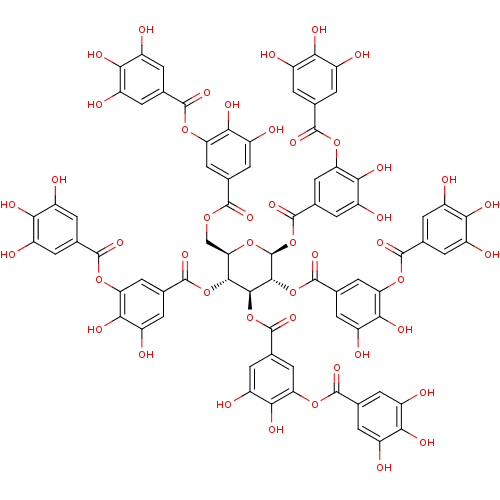
(MLS001335996 | SMR000857330 | TANNIC ACID | cid_16...)Show SMILES Oc1cc(cc(O)c1O)C(=O)Oc1cc(cc(O)c1O)C(=O)OC[C@H]1O[C@@H](OC(=O)c2cc(O)c(O)c(OC(=O)c3cc(O)c(O)c(O)c3)c2)[C@H](OC(=O)c2cc(O)c(O)c(OC(=O)c3cc(O)c(O)c(O)c3)c2)[C@@H](OC(=O)c2cc(O)c(O)c(OC(=O)c3cc(O)c(O)c(O)c3)c2)[C@@H]1OC(=O)c1cc(O)c(O)c(OC(=O)c2cc(O)c(O)c(O)c2)c1 Show InChI InChI=1S/C76H52O46/c77-32-1-22(2-33(78)53(32)92)67(103)113-47-16-27(11-42(87)58(47)97)66(102)112-21-52-63(119-72(108)28-12-43(88)59(98)48(17-28)114-68(104)23-3-34(79)54(93)35(80)4-23)64(120-73(109)29-13-44(89)60(99)49(18-29)115-69(105)24-5-36(81)55(94)37(82)6-24)65(121-74(110)30-14-45(90)61(100)50(19-30)116-70(106)25-7-38(83)56(95)39(84)8-25)76(118-52)122-75(111)31-15-46(91)62(101)51(20-31)117-71(107)26-9-40(85)57(96)41(86)10-26/h1-20,52,63-65,76-101H,21H2/t52-,63-,64+,65-,76+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <2.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT |
J Nat Prod 54: 143-54
Article DOI: 10.1021/np50073a012
BindingDB Entry DOI: 10.7270/Q2NK3HTG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50000024
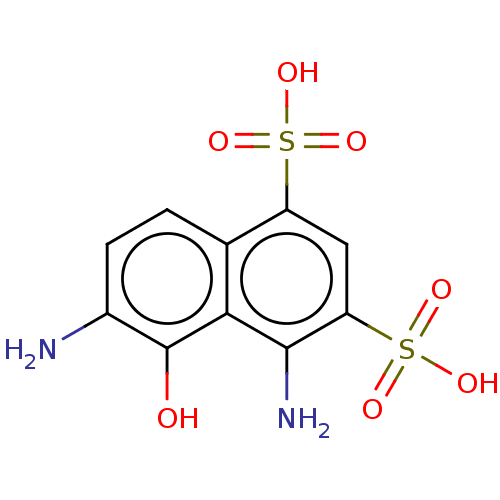
(4,6-Diamino-5-hydroxy-naphthalene-1,3-disulfonic a...)Show SMILES Nc1ccc2c(cc(c(N)c2c1O)S(O)(=O)=O)S(O)(=O)=O Show InChI InChI=1S/C10H10N2O7S2/c11-5-2-1-4-6(20(14,15)16)3-7(21(17,18)19)9(12)8(4)10(5)13/h1-3,13H,11-12H2,(H,14,15,16)(H,17,18,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universityof Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-1 reverse transcriptase |
J Med Chem 35: 4846-53 (1992)
BindingDB Entry DOI: 10.7270/Q2M0461N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50478514

(Fagaronine | Fagaronine Chloride)Show SMILES [Cl-].COc1cc2c(ccc3c4cc(OC)c(OC)cc4c[n+](C)c23)cc1O Show InChI InChI=1S/C21H19NO4/c1-22-11-13-8-19(25-3)20(26-4)9-15(13)14-6-5-12-7-17(23)18(24-2)10-16(12)21(14)22/h5-11H,1-4H3/p+1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT |
J Nat Prod 54: 143-54
Article DOI: 10.1021/np50073a012
BindingDB Entry DOI: 10.7270/Q2NK3HTG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50000024

(4,6-Diamino-5-hydroxy-naphthalene-1,3-disulfonic a...)Show SMILES Nc1ccc2c(cc(c(N)c2c1O)S(O)(=O)=O)S(O)(=O)=O Show InChI InChI=1S/C10H10N2O7S2/c11-5-2-1-4-6(20(14,15)16)3-7(21(17,18)19)9(12)8(4)10(5)13/h1-3,13H,11-12H2,(H,14,15,16)(H,17,18,19) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universityof Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-2 reverse transcriptase |
J Med Chem 35: 4846-53 (1992)
BindingDB Entry DOI: 10.7270/Q2M0461N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50000039

(3,4-Diamino-naphthalene-1-sulfonic acid | CHEMBL44...)Show InChI InChI=1S/C10H10N2O3S/c11-8-5-9(16(13,14)15)6-3-1-2-4-7(6)10(8)12/h1-5H,11-12H2,(H,13,14,15) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universityof Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-1 reverse transcriptase |
J Med Chem 35: 4846-53 (1992)
BindingDB Entry DOI: 10.7270/Q2M0461N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50000039

(3,4-Diamino-naphthalene-1-sulfonic acid | CHEMBL44...)Show InChI InChI=1S/C10H10N2O3S/c11-8-5-9(16(13,14)15)6-3-1-2-4-7(6)10(8)12/h1-5H,11-12H2,(H,13,14,15) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universityof Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-1 reverse transcriptase |
J Med Chem 35: 4846-53 (1992)
BindingDB Entry DOI: 10.7270/Q2M0461N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50368508
(CHEMBL1788186)Show SMILES Oc1cc(cc2cc(cc(NC(=O)CCCCCCCCC(=O)Nc3cc(cc4cc(cc(O)c34)S([O-])(=O)=O)S([O-])(=O)=O)c12)S([O-])(=O)=O)S([O-])(=O)=O Show InChI InChI=1S/C30H32N2O16S4/c33-25-15-21(51(43,44)45)11-17-9-19(49(37,38)39)13-23(29(17)25)31-27(35)7-5-3-1-2-4-6-8-28(36)32-24-14-20(50(40,41)42)10-18-12-22(52(46,47)48)16-26(34)30(18)24/h9-16,33-34H,1-8H2,(H,31,35)(H,32,36)(H,37,38,39)(H,40,41,42)(H,43,44,45)(H,46,47,48)/p-4 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universityof Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-1 reverse transcriptase |
J Med Chem 35: 4846-53 (1992)
BindingDB Entry DOI: 10.7270/Q2M0461N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50368509
(CHEMBL1744147)Show SMILES CCCCCCCCCCCCCCCC(=O)Nc1cc(cc2cc(cc(c12)S([O-])(=O)=O)S([O-])(=O)=O)S([O-])(=O)=O Show InChI InChI=1S/C26H39NO10S3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-25(28)27-23-18-21(38(29,30)31)16-20-17-22(39(32,33)34)19-24(26(20)23)40(35,36)37/h16-19H,2-15H2,1H3,(H,27,28)(H,29,30,31)(H,32,33,34)(H,35,36,37)/p-3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universityof Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-2 reverse transcriptase |
J Med Chem 35: 4846-53 (1992)
BindingDB Entry DOI: 10.7270/Q2M0461N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50368509

(CHEMBL1744147)Show SMILES CCCCCCCCCCCCCCCC(=O)Nc1cc(cc2cc(cc(c12)S([O-])(=O)=O)S([O-])(=O)=O)S([O-])(=O)=O Show InChI InChI=1S/C26H39NO10S3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-25(28)27-23-18-21(38(29,30)31)16-20-17-22(39(32,33)34)19-24(26(20)23)40(35,36)37/h16-19H,2-15H2,1H3,(H,27,28)(H,29,30,31)(H,32,33,34)(H,35,36,37)/p-3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universityof Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-2 reverse transcriptase |
J Med Chem 35: 4846-53 (1992)
BindingDB Entry DOI: 10.7270/Q2M0461N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50368508

(CHEMBL1788186)Show SMILES Oc1cc(cc2cc(cc(NC(=O)CCCCCCCCC(=O)Nc3cc(cc4cc(cc(O)c34)S([O-])(=O)=O)S([O-])(=O)=O)c12)S([O-])(=O)=O)S([O-])(=O)=O Show InChI InChI=1S/C30H32N2O16S4/c33-25-15-21(51(43,44)45)11-17-9-19(49(37,38)39)13-23(29(17)25)31-27(35)7-5-3-1-2-4-6-8-28(36)32-24-14-20(50(40,41)42)10-18-12-22(52(46,47)48)16-26(34)30(18)24/h9-16,33-34H,1-8H2,(H,31,35)(H,32,36)(H,37,38,39)(H,40,41,42)(H,43,44,45)(H,46,47,48)/p-4 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.22E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universityof Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-2 reverse transcriptase |
J Med Chem 35: 4846-53 (1992)
BindingDB Entry DOI: 10.7270/Q2M0461N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50368502
(CHEMBL1788183)Show SMILES [O-]S(=O)(=O)c1cc(c2cc(NS(=O)(=O)c3ccc(cc3)-c3ccc(cc3)S(=O)(=O)Nc3ccc4cc(cc(c4c3)S([O-])(=O)=O)S([O-])(=O)=O)ccc2c1)S([O-])(=O)=O Show InChI InChI=1S/C32H24N2O16S6/c35-51(36,33-23-7-1-21-13-27(53(39,40)41)17-31(29(21)15-23)55(45,46)47)25-9-3-19(4-10-25)20-5-11-26(12-6-20)52(37,38)34-24-8-2-22-14-28(54(42,43)44)18-32(30(22)16-24)56(48,49)50/h1-18,33-34H,(H,39,40,41)(H,42,43,44)(H,45,46,47)(H,48,49,50)/p-4 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universityof Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-1 reverse transcriptase |
J Med Chem 35: 4846-53 (1992)
BindingDB Entry DOI: 10.7270/Q2M0461N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50368502

(CHEMBL1788183)Show SMILES [O-]S(=O)(=O)c1cc(c2cc(NS(=O)(=O)c3ccc(cc3)-c3ccc(cc3)S(=O)(=O)Nc3ccc4cc(cc(c4c3)S([O-])(=O)=O)S([O-])(=O)=O)ccc2c1)S([O-])(=O)=O Show InChI InChI=1S/C32H24N2O16S6/c35-51(36,33-23-7-1-21-13-27(53(39,40)41)17-31(29(21)15-23)55(45,46)47)25-9-3-19(4-10-25)20-5-11-26(12-6-20)52(37,38)34-24-8-2-22-14-28(54(42,43)44)18-32(30(22)16-24)56(48,49)50/h1-18,33-34H,(H,39,40,41)(H,42,43,44)(H,45,46,47)(H,48,49,50)/p-4 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universityof Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-2 reverse transcriptase |
J Med Chem 35: 4846-53 (1992)
BindingDB Entry DOI: 10.7270/Q2M0461N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50143421
(CHEMBL425511 | Di sodium; 4,5-Dihydroxy-7-sulfo-na...)Show SMILES Oc1cc(cc2cc(cc(O)c12)S([O-])(=O)=O)S([O-])(=O)=O Show InChI InChI=1S/C10H8O8S2/c11-8-3-6(19(13,14)15)1-5-2-7(20(16,17)18)4-9(12)10(5)8/h1-4,11-12H,(H,13,14,15)(H,16,17,18)/p-2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universityof Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-1 reverse transcriptase |
J Med Chem 35: 4846-53 (1992)
BindingDB Entry DOI: 10.7270/Q2M0461N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50017566
(CHEBI:7578 | NITIDINE)Show SMILES COc1cc2c[n+](C)c3c(ccc4cc5OCOc5cc34)c2cc1OC Show InChI InChI=1S/C21H18NO4/c1-22-10-13-7-17(23-2)18(24-3)8-15(13)14-5-4-12-6-19-20(26-11-25-19)9-16(12)21(14)22/h4-10H,11H2,1-3H3/q+1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT using poly A oligo(dT) template primer |
J Nat Prod 54: 143-54
Article DOI: 10.1021/np50073a012
BindingDB Entry DOI: 10.7270/Q2NK3HTG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50017566

(CHEBI:7578 | NITIDINE)Show SMILES COc1cc2c[n+](C)c3c(ccc4cc5OCOc5cc34)c2cc1OC Show InChI InChI=1S/C21H18NO4/c1-22-10-13-7-17(23-2)18(24-3)8-15(13)14-5-4-12-6-19-20(26-11-25-19)9-16(12)21(14)22/h4-10H,11H2,1-3H3/q+1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.43E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT |
J Nat Prod 54: 143-54
Article DOI: 10.1021/np50073a012
BindingDB Entry DOI: 10.7270/Q2NK3HTG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50368500
(CHEMBL1744153)Show SMILES Oc1cc(cc2cc(cc(NC(=O)CCCCCCCCC([O-])=O)c12)S([O-])(=O)=O)S([O-])(=O)=O Show InChI InChI=1S/C20H25NO10S2/c22-17-12-15(33(29,30)31)10-13-9-14(32(26,27)28)11-16(20(13)17)21-18(23)7-5-3-1-2-4-6-8-19(24)25/h9-12,22H,1-8H2,(H,21,23)(H,24,25)(H,26,27,28)(H,29,30,31)/p-3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universityof Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-2 reverse transcriptase |
J Med Chem 35: 4846-53 (1992)
BindingDB Entry DOI: 10.7270/Q2M0461N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50368500

(CHEMBL1744153)Show SMILES Oc1cc(cc2cc(cc(NC(=O)CCCCCCCCC([O-])=O)c12)S([O-])(=O)=O)S([O-])(=O)=O Show InChI InChI=1S/C20H25NO10S2/c22-17-12-15(33(29,30)31)10-13-9-14(32(26,27)28)11-16(20(13)17)21-18(23)7-5-3-1-2-4-6-8-19(24)25/h9-12,22H,1-8H2,(H,21,23)(H,24,25)(H,26,27,28)(H,29,30,31)/p-3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universityof Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-2 reverse transcriptase |
J Med Chem 35: 4846-53 (1992)
BindingDB Entry DOI: 10.7270/Q2M0461N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50226664
(2-hydroxy-3,9,10-trimethoxy-5,6-dihydroisoquino[3,...)Show InChI InChI=1S/C20H19NO4/c1-23-18-5-4-12-8-16-14-10-17(22)19(24-2)9-13(14)6-7-21(16)11-15(12)20(18)25-3/h4-5,8-11H,6-7H2,1-3H3/p+1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.71E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT |
J Nat Prod 54: 143-54
Article DOI: 10.1021/np50073a012
BindingDB Entry DOI: 10.7270/Q2NK3HTG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50030257
(CHEBI:67862 | COPTISINE)Show InChI InChI=1S/C19H14NO4/c1-2-16-19(24-10-21-16)14-8-20-4-3-12-6-17-18(23-9-22-17)7-13(12)15(20)5-11(1)14/h1-2,5-8H,3-4,9-10H2/q+1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.74E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT |
J Nat Prod 54: 143-54
Article DOI: 10.1021/np50073a012
BindingDB Entry DOI: 10.7270/Q2NK3HTG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50480307
(Didemnin | Didemnin B)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)C(=O)[C@@H](OC(=O)C[C@H](O)[C@]([H])(NC(=O)[C@@H](NC(=O)[C@@H](CC(C)C)N(C)C(=O)[C@@H]1CCCN1C(=O)C(C)O)[C@@H](C)OC(=O)[C@H](Cc1ccc(OC)cc1)N(C)C2=O)[C@@H](C)CC)C(C)C Show InChI InChI=1S/C57H89N7O15/c1-15-33(8)46-44(66)29-45(67)79-49(32(6)7)48(68)34(9)50(69)58-39(26-30(2)3)54(73)64-25-17-19-41(64)56(75)62(13)43(28-37-20-22-38(77-14)23-21-37)57(76)78-36(11)47(52(71)59-46)60-51(70)42(27-31(4)5)61(12)55(74)40-18-16-24-63(40)53(72)35(10)65/h20-23,30-36,39-44,46-47,49,65-66H,15-19,24-29H2,1-14H3,(H,58,69)(H,59,71)(H,60,70)/t33-,34-,35?,36+,39-,40-,41-,42+,43-,44-,46+,47-,49-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.79E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT |
J Nat Prod 54: 143-54
Article DOI: 10.1021/np50073a012
BindingDB Entry DOI: 10.7270/Q2NK3HTG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50317541
(CHEMBL501515 | Ginsenoside Rb1 | ginsenoside-Rb1)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6][C@]([#6])([#8]-[#6@@H]-1-[#8]-[#6@H](-[#6]-[#8]-[#6@@H]-2-[#8]-[#6@H](-[#6]-[#8])-[#6@@H](-[#8])-[#6@H](-[#8])-[#6@H]-2-[#8])-[#6@@H](-[#8])-[#6@H](-[#8])-[#6@H]-1-[#8])[#6@H]-1-[#6]-[#6][C@]2([#6])[#6@@H]-1-[#6@H](-[#8])-[#6]-[#6@@H]1[C@@]3([#6])[#6]-[#6]-[#6@H](-[#8]-[#6@@H]-4-[#8]-[#6@H](-[#6]-[#8])-[#6@@H](-[#8])-[#6@H](-[#8])-[#6@H]-4-[#8]-[#6@@H]-4-[#8]-[#6@H](-[#6]-[#8])-[#6@@H](-[#8])-[#6@H](-[#8])-[#6@H]-4-[#8])C([#6])([#6])[#6@@H]3-[#6]-[#6][C@@]21[#6] |r| Show InChI InChI=1S/C54H92O23/c1-23(2)10-9-14-54(8,77-48-44(69)40(65)37(62)29(74-48)22-70-46-42(67)38(63)34(59)26(19-55)71-46)24-11-16-53(7)33(24)25(58)18-31-51(5)15-13-32(50(3,4)30(51)12-17-52(31,53)6)75-49-45(41(66)36(61)28(21-57)73-49)76-47-43(68)39(64)35(60)27(20-56)72-47/h10,24-49,55-69H,9,11-22H2,1-8H3/t24-,25+,26+,27+,28+,29+,30-,31+,32-,33-,34+,35+,36+,37+,38-,39-,40-,41-,42+,43+,44+,45+,46+,47-,48-,49-,51-,52+,53+,54-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT |
J Nat Prod 54: 143-54
Article DOI: 10.1021/np50073a012
BindingDB Entry DOI: 10.7270/Q2NK3HTG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50480323

(CHEBI:5188 | Fulvoplumierin)Show SMILES COC(=O)c1coc(=O)c2\C(=C\C=C\C)C=Cc12 |c:15| Show InChI InChI=1S/C14H12O4/c1-3-4-5-9-6-7-10-11(13(15)17-2)8-18-14(16)12(9)10/h3-8H,1-2H3/b4-3+,9-5+ | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.84E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT |
J Nat Prod 54: 143-54
Article DOI: 10.1021/np50073a012
BindingDB Entry DOI: 10.7270/Q2NK3HTG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50480251
(Heparin Sodium | S01XA14 | Sodium Heparin)Show SMILES CC(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](COS(O)(=O)=O)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@@H]2[C@@H](O)O[C@H](O[C@H]3[C@H](O)[C@@H](OS(O)(=O)=O)C(O)O[C@H]3C(O)=O)[C@H](OS(O)(=O)=O)[C@H]2CS(O)(=O)=O)O[C@@H]1C(O)=O |r| Show InChI InChI=1S/C26H41NO34S4/c1-4(28)27-7-9(30)8(29)6(2-52-63(43,44)45)53-24(7)56-15-10(31)11(32)25(58-19(15)21(36)37)55-13-5(3-62(40,41)42)14(60-64(46,47)48)26(59-22(13)38)57-16-12(33)17(61-65(49,50)51)23(39)54-18(16)20(34)35/h5-19,22-26,29-33,38-39H,2-3H2,1H3,(H,27,28)(H,34,35)(H,36,37)(H,40,41,42)(H,43,44,45)(H,46,47,48)(H,49,50,51)/t5-,6+,7+,8+,9+,10+,11+,12-,13-,14+,15-,16-,17+,18+,19-,22-,23?,24+,25+,26-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.92E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT |
J Nat Prod 54: 143-54
Article DOI: 10.1021/np50073a012
BindingDB Entry DOI: 10.7270/Q2NK3HTG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50480249
(CHEBI:9630 | Tomatine)Show SMILES [H][C@]12C[C@@]3([H])[C@]4([H])CC[C@@]5([H])C[C@H](CC[C@]5(C)[C@@]4([H])CC[C@]3(C)[C@@]1([H])[C@H](C)[C@]1(CC[C@H](C)CN1)O2)O[C@]1([H])O[C@H](CO)[C@]([H])(O[C@@H]2O[C@H](CO)[C@@H](O)[C@]([H])(O[C@@H]3OC[C@@H](O)[C@H](O)[C@H]3O)[C@@]2([H])O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C50H83NO21/c1-20-7-12-50(51-15-20)21(2)32-28(72-50)14-26-24-6-5-22-13-23(8-10-48(22,3)25(24)9-11-49(26,32)4)65-45-40(63)37(60)41(31(18-54)68-45)69-47-43(71-46-39(62)36(59)34(57)29(16-52)66-46)42(35(58)30(17-53)67-47)70-44-38(61)33(56)27(55)19-64-44/h20-47,51-63H,5-19H2,1-4H3/t20-,21-,22-,23-,24+,25-,26-,27+,28-,29+,30+,31+,32-,33-,34+,35+,36-,37+,38+,39+,40+,41-,42-,43+,44-,45+,46-,47-,48-,49-,50-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.93E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT |
J Nat Prod 54: 143-54
Article DOI: 10.1021/np50073a012
BindingDB Entry DOI: 10.7270/Q2NK3HTG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50480328
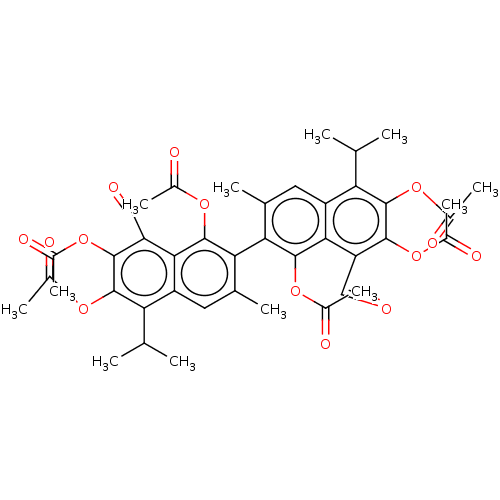
(Gossypol acetic acid)Show SMILES CC(C)c1c(OC(C)=O)c(OC(C)=O)c(C=O)c2c(OC(C)=O)c(c(C)cc12)-c1c(C)cc2c(C(C)C)c(OC(C)=O)c(OC(C)=O)c(C=O)c2c1OC(C)=O |(6.96,-3.81,;7.73,-5.15,;9.27,-5.15,;6.96,-6.48,;7.73,-7.82,;9.27,-7.82,;10.04,-9.14,;9.27,-10.48,;11.57,-9.14,;6.96,-9.14,;7.73,-10.48,;6.96,-11.8,;7.73,-13.14,;5.42,-11.8,;5.42,-9.14,;4.65,-10.48,;3.12,-10.48,;4.65,-7.82,;3.12,-7.82,;2.43,-9.69,;.99,-10.22,;-.35,-9.45,;.85,-11.75,;2.35,-6.48,;3.12,-5.15,;2.35,-3.81,;4.65,-5.15,;5.42,-6.48,;.38,-6.13,;-.39,-7.47,;.38,-8.8,;-1.93,-7.47,;-2.7,-6.13,;-4.24,-6.13,;-5.01,-7.47,;-4.13,-8.73,;-6.52,-7.73,;-5.01,-4.8,;-7.01,-4.8,;-8.29,-6.33,;-9.78,-5.93,;-8.03,-7.85,;-4.24,-3.47,;-5.01,-2.13,;-6.54,-2.13,;-7.32,-3.47,;-7.32,-.81,;-2.7,-3.47,;-2.17,-2.02,;-3.16,-.85,;-1.93,-4.8,;-.39,-4.8,;.6,-3.07,;-.07,-1.19,;1.01,-.1,;-1.52,-.66,)| Show InChI InChI=1S/C42H42O14/c1-17(2)31-27-13-19(5)33(39(53-23(9)47)35(27)29(15-43)37(51-21(7)45)41(31)55-25(11)49)34-20(6)14-28-32(18(3)4)42(56-26(12)50)38(52-22(8)46)30(16-44)36(28)40(34)54-24(10)48/h13-18H,1-12H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1.94E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT |
J Nat Prod 54: 143-54
Article DOI: 10.1021/np50073a012
BindingDB Entry DOI: 10.7270/Q2NK3HTG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50143421

(CHEMBL425511 | Di sodium; 4,5-Dihydroxy-7-sulfo-na...)Show SMILES Oc1cc(cc2cc(cc(O)c12)S([O-])(=O)=O)S([O-])(=O)=O Show InChI InChI=1S/C10H8O8S2/c11-8-3-6(19(13,14)15)1-5-2-7(20(16,17)18)4-9(12)10(5)8/h1-4,11-12H,(H,13,14,15)(H,16,17,18)/p-2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universityof Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-2 reverse transcriptase |
J Med Chem 35: 4846-53 (1992)
BindingDB Entry DOI: 10.7270/Q2M0461N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50226670
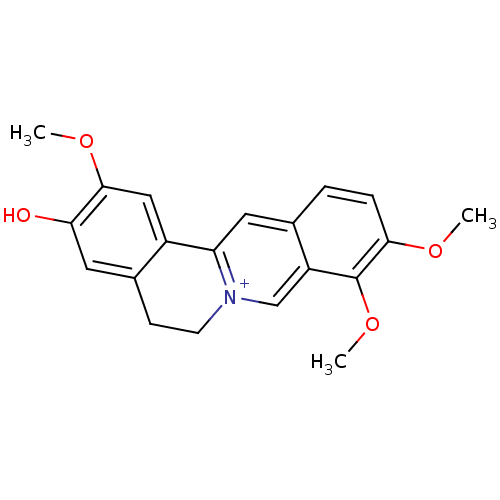
(CHEMBL251055 | jatrorrhizine)Show SMILES COc1cc-2c(CC[n+]3cc4c(OC)c(OC)ccc4cc-23)cc1O Show InChI InChI=1S/C20H19NO4/c1-23-18-5-4-12-8-16-14-10-19(24-2)17(22)9-13(14)6-7-21(16)11-15(12)20(18)25-3/h4-5,8-11H,6-7H2,1-3H3/p+1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT |
J Nat Prod 54: 143-54
Article DOI: 10.1021/np50073a012
BindingDB Entry DOI: 10.7270/Q2NK3HTG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50378779
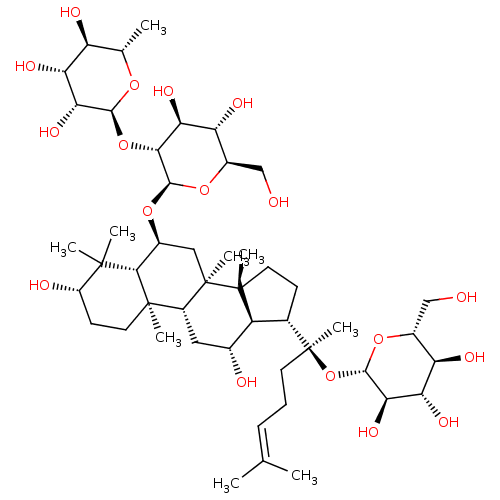
(CHEMBL510095)Show SMILES [#6]-[#6@@H]-1-[#8]-[#6@@H](-[#8]-[#6@@H]-2-[#6@@H](-[#8])-[#6@H](-[#8])-[#6@@H](-[#6]-[#8])-[#8]-[#6@H]-2-[#8]-[#6@H]-2-[#6][C@]3([#6])[#6@H](-[#6]-[#6@@H](-[#8])-[#6@@H]4-[#6@H](-[#6]-[#6][C@@]34[#6])[C@]([#6])([#6]-[#6]\[#6]=[#6](\[#6])-[#6])[#8]-[#6@@H]-3-[#8]-[#6@H](-[#6]-[#8])-[#6@@H](-[#8])-[#6@H](-[#8])-[#6@H]-3-[#8])[C@@]3([#6])[#6]-[#6]-[#6@H](-[#8])C([#6])([#6])[#6@H]-23)-[#6@H](-[#8])-[#6@H](-[#8])-[#6@H]-1-[#8] |r| Show InChI InChI=1S/C48H82O18/c1-21(2)11-10-14-48(9,66-42-38(60)35(57)32(54)26(19-49)63-42)23-12-16-46(7)30(23)24(51)17-28-45(6)15-13-29(52)44(4,5)40(45)25(18-47(28,46)8)62-43-39(36(58)33(55)27(20-50)64-43)65-41-37(59)34(56)31(53)22(3)61-41/h11,22-43,49-60H,10,12-20H2,1-9H3/t22-,23-,24+,25-,26+,27+,28+,29-,30-,31-,32+,33+,34+,35-,36-,37+,38+,39+,40-,41-,42-,43+,45+,46+,47+,48-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT |
J Nat Prod 54: 143-54
Article DOI: 10.1021/np50073a012
BindingDB Entry DOI: 10.7270/Q2NK3HTG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50368501

(CHEMBL1744155)Show SMILES [O-]S(=O)(=O)c1cc(NC(=O)CCCCCCCCCCC(=O)Nc2cc(cc3cc(cc(c23)S([O-])(=O)=O)S([O-])(=O)=O)S([O-])(=O)=O)c2c(cc(cc2c1)S([O-])(=O)=O)S([O-])(=O)=O Show InChI InChI=1S/C32H36N2O20S6/c35-29(33-25-15-21(55(37,38)39)11-19-13-23(57(43,44)45)17-27(31(19)25)59(49,50)51)9-7-5-3-1-2-4-6-8-10-30(36)34-26-16-22(56(40,41)42)12-20-14-24(58(46,47)48)18-28(32(20)26)60(52,53)54/h11-18H,1-10H2,(H,33,35)(H,34,36)(H,37,38,39)(H,40,41,42)(H,43,44,45)(H,46,47,48)(H,49,50,51)(H,52,53,54)/p-6 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universityof Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-2 reverse transcriptase |
J Med Chem 35: 4846-53 (1992)
BindingDB Entry DOI: 10.7270/Q2M0461N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50368501

(CHEMBL1744155)Show SMILES [O-]S(=O)(=O)c1cc(NC(=O)CCCCCCCCCCC(=O)Nc2cc(cc3cc(cc(c23)S([O-])(=O)=O)S([O-])(=O)=O)S([O-])(=O)=O)c2c(cc(cc2c1)S([O-])(=O)=O)S([O-])(=O)=O Show InChI InChI=1S/C32H36N2O20S6/c35-29(33-25-15-21(55(37,38)39)11-19-13-23(57(43,44)45)17-27(31(19)25)59(49,50)51)9-7-5-3-1-2-4-6-8-10-30(36)34-26-16-22(56(40,41)42)12-20-14-24(58(46,47)48)18-28(32(20)26)60(52,53)54/h11-18H,1-10H2,(H,33,35)(H,34,36)(H,37,38,39)(H,40,41,42)(H,43,44,45)(H,46,47,48)(H,49,50,51)(H,52,53,54)/p-6 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universityof Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-2 reverse transcriptase |
J Med Chem 35: 4846-53 (1992)
BindingDB Entry DOI: 10.7270/Q2M0461N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50480288
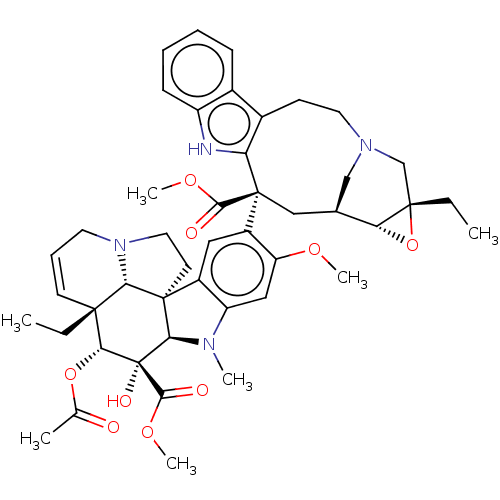
(32645 | Leurosine Sulfate | Vinleurosine Sulfate)Show SMILES OS(O)(=O)=O.[H][C@]12O[C@@]1(CC)CN1C[C@]2([H])C[C@@](C(=O)OC)(c2[nH]c3ccccc3c2CC1)c1cc2c(cc1OC)N(C)[C@]1([H])[C@]22CCN3CC=C[C@@](CC)([C@@H](OC(C)=O)[C@]1(O)C(=O)OC)[C@@]23[H] |r,c:56| Show InChI InChI=1S/C46H56N4O9.H2O4S/c1-8-42-16-12-18-50-20-17-44(37(42)50)30-21-31(34(55-5)22-33(30)48(4)38(44)46(54,41(53)57-7)39(42)58-26(3)51)45(40(52)56-6)23-27-24-49(25-43(9-2)36(27)59-43)19-15-29-28-13-10-11-14-32(28)47-35(29)45;1-5(2,3)4/h10-14,16,21-22,27,36-39,47,54H,8-9,15,17-20,23-25H2,1-7H3;(H2,1,2,3,4)/t27-,36+,37-,38+,39+,42+,43-,44+,45-,46-;/m0./s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT |
J Nat Prod 54: 143-54
Article DOI: 10.1021/np50073a012
BindingDB Entry DOI: 10.7270/Q2NK3HTG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50001839

(CHEMBL428647 | PACLITAXEL | taxol)Show SMILES CC(=O)O[C@@H]1C2=C(C)[C@H](C[C@@](O)([C@@H](OC(=O)c3ccccc3)[C@@H]3[C@@]4(CO[C@@H]4C[C@H](O)[C@@]3(C)C1=O)OC(C)=O)C2(C)C)OC(=O)[C@H](O)[C@@H](NC(=O)c1ccccc1)c1ccccc1 |r,wU:4.3,28.30,26.26,10.10,22.23,30.33,wD:23.37,8.45,44.49,46.51,12.12,c:5,(23.85,-5.1,;25.19,-4.32,;25.18,-2.78,;26.52,-5.08,;26.53,-6.61,;23.91,-8.14,;22.57,-8.89,;21.25,-8.11,;22.55,-10.43,;23.89,-11.22,;25.21,-10.45,;25.2,-11.99,;26.54,-11.23,;26.53,-12.77,;25.19,-13.53,;23.87,-12.75,;25.19,-15.05,;23.85,-15.82,;23.84,-17.36,;25.17,-18.14,;26.51,-17.37,;26.52,-15.82,;27.88,-10.47,;29.2,-11.23,;29.96,-12.57,;31.31,-11.81,;30.55,-10.47,;30.55,-8.91,;29.2,-8.13,;30.52,-7.35,;27.88,-8.91,;26.78,-10,;27.87,-7.37,;29.19,-6.59,;28.1,-12.3,;28.1,-13.84,;29.43,-14.62,;26.76,-14.61,;25.23,-8.91,;25.98,-7.57,;26.76,-8.91,;21.21,-11.18,;19.89,-10.4,;19.91,-8.87,;18.55,-11.16,;18.54,-12.7,;17.22,-10.38,;17.24,-8.84,;15.92,-8.06,;14.57,-8.82,;15.93,-6.52,;17.27,-5.78,;17.28,-4.24,;15.95,-3.46,;14.61,-4.23,;14.6,-5.76,;15.89,-11.14,;14.57,-10.36,;13.23,-11.11,;13.21,-12.65,;14.56,-13.43,;15.89,-12.67,)| Show InChI InChI=1S/C47H51NO14/c1-25-31(60-43(56)36(52)35(28-16-10-7-11-17-28)48-41(54)29-18-12-8-13-19-29)23-47(57)40(61-42(55)30-20-14-9-15-21-30)38-45(6,32(51)22-33-46(38,24-58-33)62-27(3)50)39(53)37(59-26(2)49)34(25)44(47,4)5/h7-21,31-33,35-38,40,51-52,57H,22-24H2,1-6H3,(H,48,54)/t31-,32-,33+,35-,36+,37+,38-,40-,45+,46-,47+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.34E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT |
J Nat Prod 54: 143-54
Article DOI: 10.1021/np50073a012
BindingDB Entry DOI: 10.7270/Q2NK3HTG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50368352

(Cerubidine | DAUNORUBICIN)Show SMILES COc1cccc2C(=O)c3c(O)c4C[C@](O)(C[C@H](O[C@H]5C[C@H](N)[C@H](O)[C@H](C)O5)c4c(O)c3C(=O)c12)C(C)=O |r| Show InChI InChI=1S/C27H29NO10/c1-10-22(30)14(28)7-17(37-10)38-16-9-27(35,11(2)29)8-13-19(16)26(34)21-20(24(13)32)23(31)12-5-4-6-15(36-3)18(12)25(21)33/h4-6,10,14,16-17,22,30,32,34-35H,7-9,28H2,1-3H3/t10-,14-,16-,17-,22+,27-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT |
J Nat Prod 54: 143-54
Article DOI: 10.1021/np50073a012
BindingDB Entry DOI: 10.7270/Q2NK3HTG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50139772
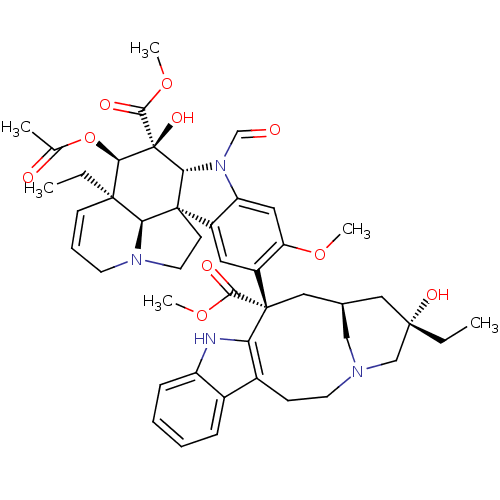
(22-oxovincaleukoblastine | leurocristine | vincris...)Show SMILES CC[C@]1(O)C[C@H]2CN(C1)CCc1c([nH]c3ccccc13)[C@@](C2)(C(=O)OC)c1cc2c(cc1OC)N(C=O)[C@@H]1[C@]22CCN3CC=C[C@](CC)([C@@H]23)[C@@H](OC(C)=O)[C@]1(O)C(=O)OC |r,c:49| Show InChI InChI=1S/C46H56N4O10/c1-7-42(55)22-28-23-45(40(53)58-5,36-30(14-18-48(24-28)25-42)29-12-9-10-13-33(29)47-36)32-20-31-34(21-35(32)57-4)50(26-51)38-44(31)16-19-49-17-11-15-43(8-2,37(44)49)39(60-27(3)52)46(38,56)41(54)59-6/h9-13,15,20-21,26,28,37-39,47,55-56H,7-8,14,16-19,22-25H2,1-6H3/t28-,37+,38-,39-,42+,43-,44-,45+,46+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT |
J Nat Prod 54: 143-54
Article DOI: 10.1021/np50073a012
BindingDB Entry DOI: 10.7270/Q2NK3HTG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50368510
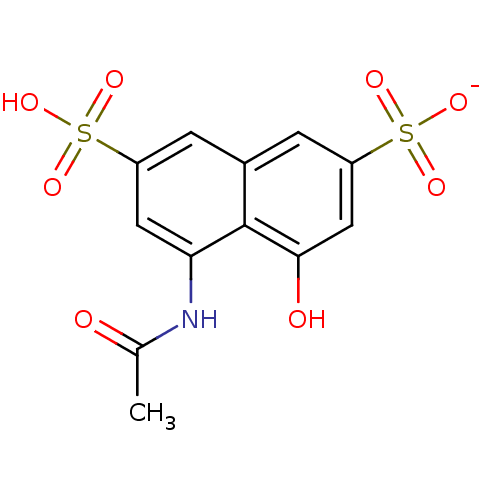
(CHEMBL1788137)Show SMILES CC(=O)Nc1cc(cc2cc(cc(O)c12)S([O-])(=O)=O)S(O)(=O)=O Show InChI InChI=1S/C12H11NO8S2/c1-6(14)13-10-4-8(22(16,17)18)2-7-3-9(23(19,20)21)5-11(15)12(7)10/h2-5,15H,1H3,(H,13,14)(H,16,17,18)(H,19,20,21)/p-1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.43E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universityof Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-2 reverse transcriptase |
J Med Chem 35: 4846-53 (1992)
BindingDB Entry DOI: 10.7270/Q2M0461N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50368510

(CHEMBL1788137)Show SMILES CC(=O)Nc1cc(cc2cc(cc(O)c12)S([O-])(=O)=O)S(O)(=O)=O Show InChI InChI=1S/C12H11NO8S2/c1-6(14)13-10-4-8(22(16,17)18)2-7-3-9(23(19,20)21)5-11(15)12(7)10/h2-5,15H,1H3,(H,13,14)(H,16,17,18)(H,19,20,21)/p-1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.43E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universityof Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-1 reverse transcriptase |
J Med Chem 35: 4846-53 (1992)
BindingDB Entry DOI: 10.7270/Q2M0461N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50012278
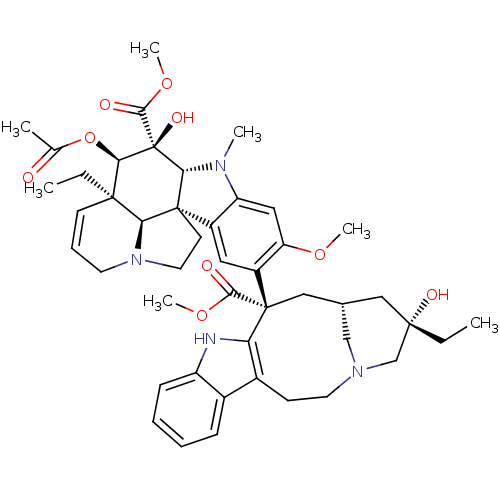
((2ALPHA,2''BETA,3BETA,4ALPHA,5BETA)-VINCALEUKOBLAS...)Show SMILES CC[C@]1(O)C[C@@H]2CN(C1)CCc1c([nH]c3ccccc13)[C@@](C2)(C(=O)OC)c1cc2c(cc1OC)N(C)[C@@H]1[C@]22CCN3CC=C[C@](CC)([C@@H]23)[C@@H](OC(C)=O)[C@]1(O)C(=O)OC |r,c:48| Show InChI InChI=1S/C46H58N4O9/c1-8-42(54)23-28-24-45(40(52)57-6,36-30(15-19-49(25-28)26-42)29-13-10-11-14-33(29)47-36)32-21-31-34(22-35(32)56-5)48(4)38-44(31)17-20-50-18-12-16-43(9-2,37(44)50)39(59-27(3)51)46(38,55)41(53)58-7/h10-14,16,21-22,28,37-39,47,54-55H,8-9,15,17-20,23-26H2,1-7H3/t28-,37-,38+,39+,42-,43+,44+,45-,46-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.46E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT |
J Nat Prod 54: 143-54
Article DOI: 10.1021/np50073a012
BindingDB Entry DOI: 10.7270/Q2NK3HTG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50480312
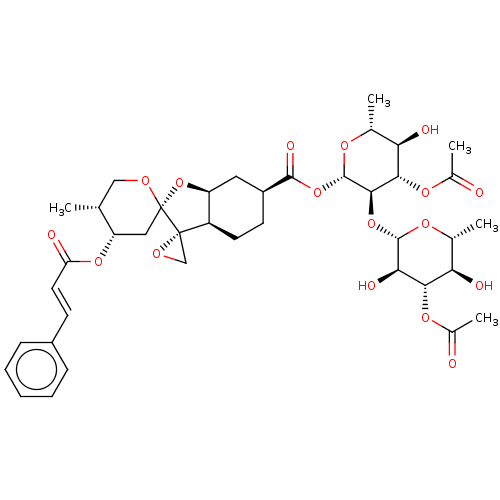
(Phyllanthoside)Show SMILES [H][C@]12C[C@H](CC[C@@]1([H])[C@@]1(CO1)[C@]1(C[C@H](OC(=O)\C=C\c3ccccc3)[C@H](C)CO1)O2)C(=O)O[C@@H]1O[C@H](C)[C@@H](O)[C@H](OC(C)=O)[C@H]1O[C@@H]1O[C@H](C)[C@@H](O)[C@H](OC(C)=O)[C@H]1O |r| Show InChI InChI=1S/C40H52O17/c1-19-17-48-40(16-28(19)54-29(43)14-11-24-9-7-6-8-10-24)39(18-49-39)26-13-12-25(15-27(26)57-40)36(47)56-38-35(34(53-23(5)42)31(45)21(3)51-38)55-37-32(46)33(52-22(4)41)30(44)20(2)50-37/h6-11,14,19-21,25-28,30-35,37-38,44-46H,12-13,15-18H2,1-5H3/b14-11+/t19-,20-,21-,25+,26-,27+,28+,30-,31-,32-,33+,34+,35-,37+,38+,39+,40+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.48E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT |
J Nat Prod 54: 143-54
Article DOI: 10.1021/np50073a012
BindingDB Entry DOI: 10.7270/Q2NK3HTG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50480264
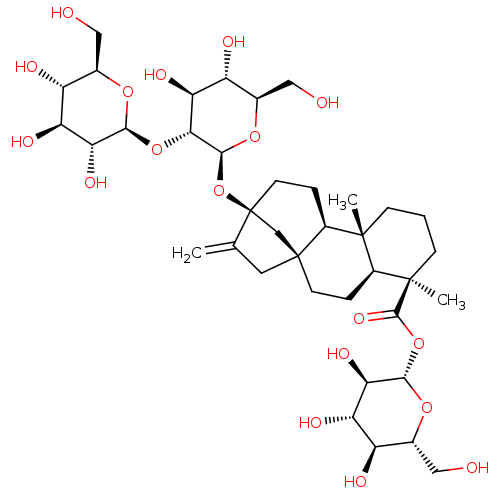
(CHEBI:9271 | Stevioside)Show SMILES [H][C@@]1(O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1O[C@@]12C[C@]3(CC1=C)CC[C@]1([H])[C@@](C)(CCC[C@@]1(C)[C@]3([H])CC2)C(=O)O[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C38H60O18/c1-16-11-37-9-5-20-35(2,7-4-8-36(20,3)34(50)55-32-29(49)26(46)23(43)18(13-40)52-32)21(37)6-10-38(16,15-37)56-33-30(27(47)24(44)19(14-41)53-33)54-31-28(48)25(45)22(42)17(12-39)51-31/h17-33,39-49H,1,4-15H2,2-3H3/t17-,18-,19-,20+,21+,22-,23-,24-,25+,26+,27+,28-,29-,30-,31+,32+,33+,35-,36-,37-,38+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.48E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT |
J Nat Prod 54: 143-54
Article DOI: 10.1021/np50073a012
BindingDB Entry DOI: 10.7270/Q2NK3HTG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50480326
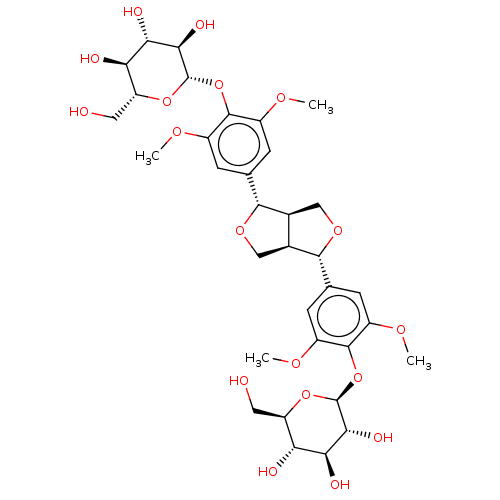
(Liriodendrin)Show SMILES [H][C@]12CO[C@]([H])(c3cc(OC)c(O[C@]4([H])O[C@H](CO)[C@@H](O)[C@H](O)[C@H]4O)c(OC)c3)[C@@]1([H])CO[C@]2([H])c1cc(OC)c(O[C@]2([H])O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)c(OC)c1 |r| Show InChI InChI=1S/C34H46O18/c1-43-17-5-13(6-18(44-2)31(17)51-33-27(41)25(39)23(37)21(9-35)49-33)29-15-11-48-30(16(15)12-47-29)14-7-19(45-3)32(20(8-14)46-4)52-34-28(42)26(40)24(38)22(10-36)50-34/h5-8,15-16,21-30,33-42H,9-12H2,1-4H3/t15-,16-,21+,22+,23+,24+,25-,26-,27+,28+,29+,30+,33-,34-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.69E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT |
J Nat Prod 54: 143-54
Article DOI: 10.1021/np50073a012
BindingDB Entry DOI: 10.7270/Q2NK3HTG |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50480321
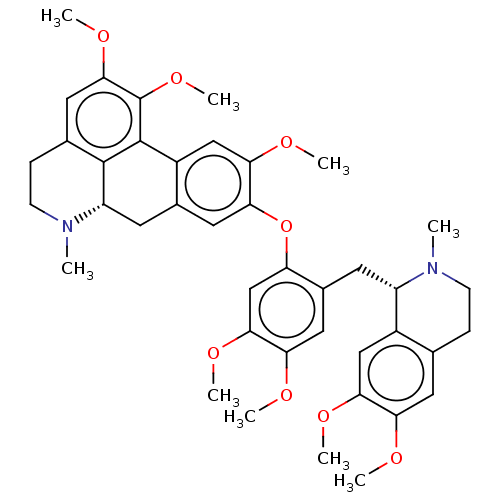
(CHEBI:9509 | Thalicarpine)Show SMILES [H][C@@]1(Cc2cc(OC)c(OC)cc2Oc2cc3C[C@]4([H])N(C)CCc5cc(OC)c(OC)c(-c3cc2OC)c45)N(C)CCc2cc(OC)c(OC)cc12 |r| Show InChI InChI=1S/C41H48N2O8/c1-42-12-10-23-16-32(44-3)34(46-5)20-27(23)29(42)15-26-19-33(45-4)36(48-7)22-31(26)51-37-18-25-14-30-39-24(11-13-43(30)2)17-38(49-8)41(50-9)40(39)28(25)21-35(37)47-6/h16-22,29-30H,10-15H2,1-9H3/t29-,30-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.87E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT |
J Nat Prod 54: 143-54
Article DOI: 10.1021/np50073a012
BindingDB Entry DOI: 10.7270/Q2NK3HTG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data