 Found 222 hits with Last Name = 'thacher' and Initial = 's'
Found 222 hits with Last Name = 'thacher' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM14712
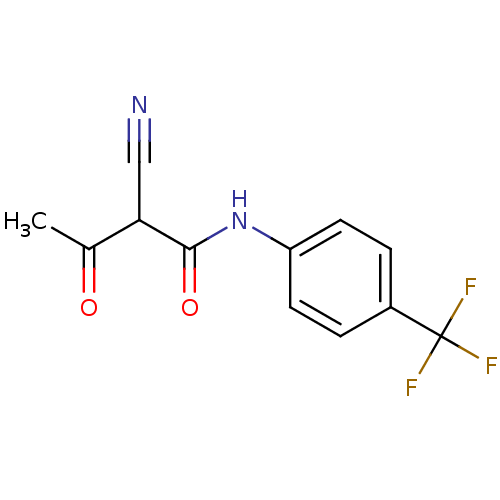
((2Z)-2-cyano-3-hydroxy-N-[4-(trifluoromethyl)pheny...)Show InChI InChI=1S/C12H9F3N2O2/c1-7(18)10(6-16)11(19)17-9-4-2-8(3-5-9)12(13,14)15/h2-5,10H,1H3,(H,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Inhibition of dihydroorotate dehydrogenase |
J Med Chem 44: 281-97 (2001)
BindingDB Entry DOI: 10.7270/Q2NP2541 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50153597
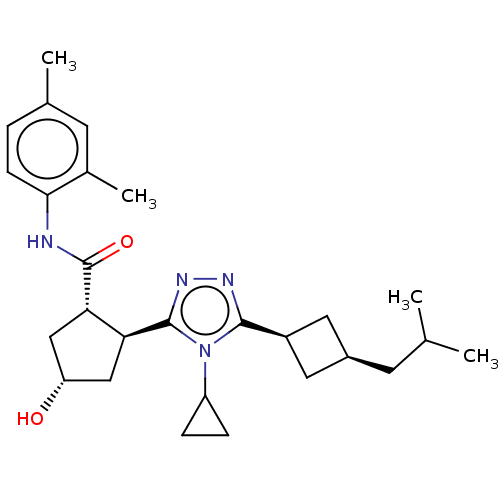
(CHEMBL3775807)Show SMILES CC(C)C[C@H]1C[C@H](C1)c1nnc([C@H]2C[C@H](O)C[C@@H]2C(=O)Nc2ccc(C)cc2C)n1C1CC1 |r,wU:4.3,6.8,12.12,wD:17.19,14.15,(2.36,7.33,;2.68,6.14,;1.81,5.27,;4.17,5.74,;4.56,4.26,;5.9,3.48,;5.11,2.21,;3.79,2.92,;5.62,.75,;7.1,.32,;7.14,-1.22,;5.7,-1.71,;5.38,-3.22,;6.42,-4.36,;5.65,-5.7,;6.15,-6.82,;4.14,-5.38,;4,-3.86,;2.66,-3.08,;2.67,-1.85,;1.33,-3.85,;-.01,-3.08,;0,-1.54,;-1.33,-.76,;-2.67,-1.53,;-3.73,-.91,;-2.67,-3.07,;-1.34,-3.84,;-1.35,-5.07,;4.75,-.52,;3.21,-.57,;1.93,-1.26,;2.03,.28,)| Show InChI InChI=1S/C27H38N4O2/c1-15(2)9-18-11-19(12-18)25-29-30-26(31(25)20-6-7-20)22-13-21(32)14-23(22)27(33)28-24-8-5-16(3)10-17(24)4/h5,8,10,15,18-23,32H,6-7,9,11-14H2,1-4H3,(H,28,33)/t18-,19+,21-,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated with substrate for 5 mins followed by NADPH addition measure... |
ACS Med Chem Lett 7: 23-7 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00253
BindingDB Entry DOI: 10.7270/Q2N58P7P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50153596
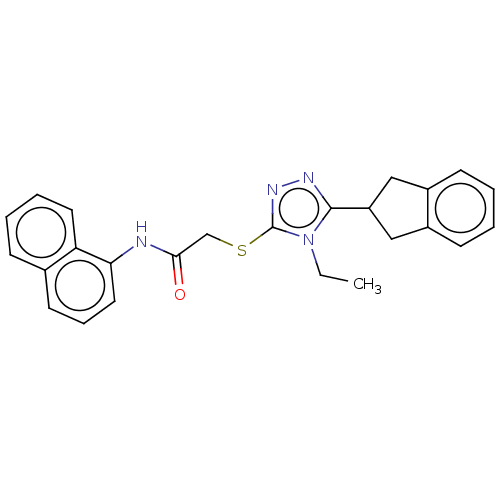
(CHEMBL3775765)Show SMILES CCn1c(SCC(=O)Nc2cccc3ccccc23)nnc1C1Cc2ccccc2C1 Show InChI InChI=1S/C25H24N4OS/c1-2-29-24(20-14-18-9-3-4-10-19(18)15-20)27-28-25(29)31-16-23(30)26-22-13-7-11-17-8-5-6-12-21(17)22/h3-13,20H,2,14-16H2,1H3,(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
ACS Med Chem Lett 7: 23-7 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00253
BindingDB Entry DOI: 10.7270/Q2N58P7P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50153598
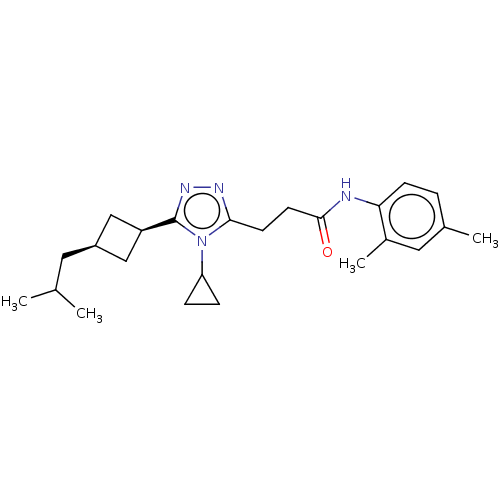
(CHEMBL3775828)Show SMILES CC(C)C[C@H]1C[C@H](C1)c1nnc(CCC(=O)Nc2ccc(C)cc2C)n1C1CC1 |r,wD:4.3,6.8,(9.15,-15.02,;8.56,-13.94,;7.33,-13.91,;9.36,-12.63,;8.62,-11.28,;9.06,-9.8,;7.6,-9.42,;7.15,-10.85,;6.98,-8.01,;7.75,-6.68,;6.72,-5.53,;5.33,-6.17,;4,-5.4,;4,-3.86,;2.66,-3.08,;1.6,-3.7,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;-2.4,1.39,;,1.54,;1.33,.77,;2.4,1.39,;5.47,-7.69,;4.32,-8.72,;2.91,-9.1,;4.04,-10.15,)| Show InChI InChI=1S/C24H34N4O/c1-15(2)11-18-13-19(14-18)24-27-26-22(28(24)20-6-7-20)9-10-23(29)25-21-8-5-16(3)12-17(21)4/h5,8,12,15,18-20H,6-7,9-11,13-14H2,1-4H3,(H,25,29)/t18-,19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated with substrate for 5 mins followed by NADPH addition measure... |
ACS Med Chem Lett 7: 23-7 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00253
BindingDB Entry DOI: 10.7270/Q2N58P7P |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4313
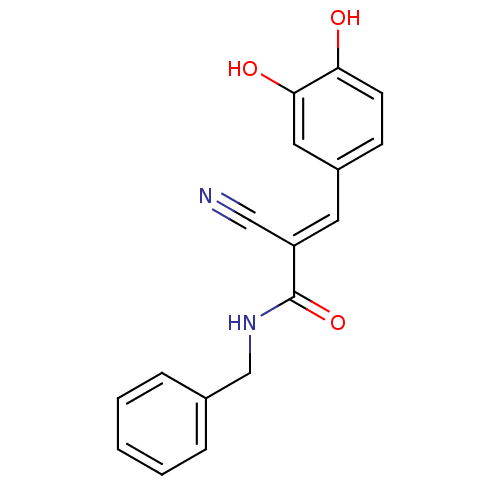
((2E)-N-benzyl-2-cyano-3-(3,4-dihydroxyphenyl)prop-...)Show InChI InChI=1S/C17H14N2O3/c18-10-14(8-13-6-7-15(20)16(21)9-13)17(22)19-11-12-4-2-1-3-5-12/h1-9,20-21H,11H2,(H,19,22)/b14-8+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EGF-dependent proliferation of human and guinea pig keratinocytes; range 7-15 uM |
J Med Chem 44: 281-97 (2001)
BindingDB Entry DOI: 10.7270/Q2NP2541 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4363
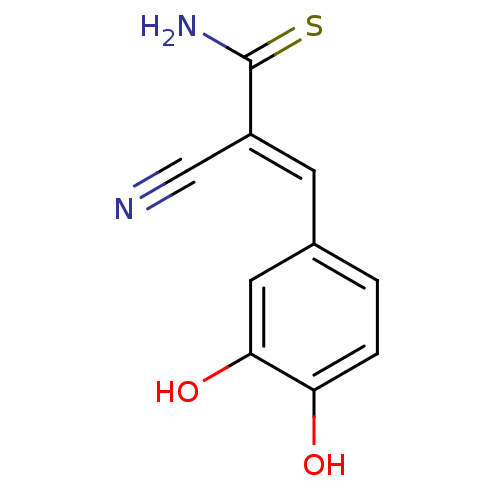
((2E)-2-cyano-3-(3,4-dihydroxyphenyl)prop-2-enethio...)Show InChI InChI=1S/C10H8N2O2S/c11-5-7(10(12)15)3-6-1-2-8(13)9(14)4-6/h1-4,13-14H,(H2,12,15)/b7-3+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EGF-dependent proliferation of human and guinea pig keratinocytes; range 7-15 uM |
J Med Chem 44: 281-97 (2001)
BindingDB Entry DOI: 10.7270/Q2NP2541 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50153595
(CHEMBL3775930)Show InChI InChI=1S/C23H34N4O/c1-4-27-21(13-11-19-8-6-5-7-9-19)25-26-22(27)14-15-23(28)24-20-12-10-17(2)16-18(20)3/h10,12,16,19H,4-9,11,13-15H2,1-3H3,(H,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
ACS Med Chem Lett 7: 23-7 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00253
BindingDB Entry DOI: 10.7270/Q2N58P7P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50537147

(CHEMBL4537625)Show SMILES [Na;v0+].[#6]C([#6])([#6])[#6]-c1cc(no1)-c1onc(-[#6@@H](-[#6]-[#6]-[#6](-[#8-])=O)-[#6]-[#6](=O)-[#7]-c2ccc(Cl)cc2Cl)c1-[#6]-1-[#6]-[#6]-1 |r| Show InChI InChI=1S/C26H29Cl2N3O5.Na/c1-26(2,3)13-17-12-20(30-35-17)25-23(14-4-5-14)24(31-36-25)15(6-9-22(33)34)10-21(32)29-19-8-7-16(27)11-18(19)28;/h7-8,11-12,14-15H,4-6,9-10,13H2,1-3H3,(H,29,32)(H,33,34);/q;+1/p-1/t15-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Time-dependent inhibition of human CYP2C9 |
J Med Chem 62: 2837-2842 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01567
BindingDB Entry DOI: 10.7270/Q20R9SWS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50537147

(CHEMBL4537625)Show SMILES [Na;v0+].[#6]C([#6])([#6])[#6]-c1cc(no1)-c1onc(-[#6@@H](-[#6]-[#6]-[#6](-[#8-])=O)-[#6]-[#6](=O)-[#7]-c2ccc(Cl)cc2Cl)c1-[#6]-1-[#6]-[#6]-1 |r| Show InChI InChI=1S/C26H29Cl2N3O5.Na/c1-26(2,3)13-17-12-20(30-35-17)25-23(14-4-5-14)24(31-36-25)15(6-9-22(33)34)10-21(32)29-19-8-7-16(27)11-18(19)28;/h7-8,11-12,14-15H,4-6,9-10,13H2,1-3H3,(H,29,32)(H,33,34);/q;+1/p-1/t15-;/m0./s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Time-dependent inhibition of human CYP2D6 |
J Med Chem 62: 2837-2842 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01567
BindingDB Entry DOI: 10.7270/Q20R9SWS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50537147

(CHEMBL4537625)Show SMILES [Na;v0+].[#6]C([#6])([#6])[#6]-c1cc(no1)-c1onc(-[#6@@H](-[#6]-[#6]-[#6](-[#8-])=O)-[#6]-[#6](=O)-[#7]-c2ccc(Cl)cc2Cl)c1-[#6]-1-[#6]-[#6]-1 |r| Show InChI InChI=1S/C26H29Cl2N3O5.Na/c1-26(2,3)13-17-12-20(30-35-17)25-23(14-4-5-14)24(31-36-25)15(6-9-22(33)34)10-21(32)29-19-8-7-16(27)11-18(19)28;/h7-8,11-12,14-15H,4-6,9-10,13H2,1-3H3,(H,29,32)(H,33,34);/q;+1/p-1/t15-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Time-dependent inhibition of human CYP2C19 |
J Med Chem 62: 2837-2842 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01567
BindingDB Entry DOI: 10.7270/Q20R9SWS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50537147

(CHEMBL4537625)Show SMILES [Na;v0+].[#6]C([#6])([#6])[#6]-c1cc(no1)-c1onc(-[#6@@H](-[#6]-[#6]-[#6](-[#8-])=O)-[#6]-[#6](=O)-[#7]-c2ccc(Cl)cc2Cl)c1-[#6]-1-[#6]-[#6]-1 |r| Show InChI InChI=1S/C26H29Cl2N3O5.Na/c1-26(2,3)13-17-12-20(30-35-17)25-23(14-4-5-14)24(31-36-25)15(6-9-22(33)34)10-21(32)29-19-8-7-16(27)11-18(19)28;/h7-8,11-12,14-15H,4-6,9-10,13H2,1-3H3,(H,29,32)(H,33,34);/q;+1/p-1/t15-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Time-dependent inhibition of human CYP1A2 |
J Med Chem 62: 2837-2842 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01567
BindingDB Entry DOI: 10.7270/Q20R9SWS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50537147

(CHEMBL4537625)Show SMILES [Na;v0+].[#6]C([#6])([#6])[#6]-c1cc(no1)-c1onc(-[#6@@H](-[#6]-[#6]-[#6](-[#8-])=O)-[#6]-[#6](=O)-[#7]-c2ccc(Cl)cc2Cl)c1-[#6]-1-[#6]-[#6]-1 |r| Show InChI InChI=1S/C26H29Cl2N3O5.Na/c1-26(2,3)13-17-12-20(30-35-17)25-23(14-4-5-14)24(31-36-25)15(6-9-22(33)34)10-21(32)29-19-8-7-16(27)11-18(19)28;/h7-8,11-12,14-15H,4-6,9-10,13H2,1-3H3,(H,29,32)(H,33,34);/q;+1/p-1/t15-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Time-dependent inhibition of human CYP3A4 using midazolam as substrate |
J Med Chem 62: 2837-2842 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01567
BindingDB Entry DOI: 10.7270/Q20R9SWS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50153594
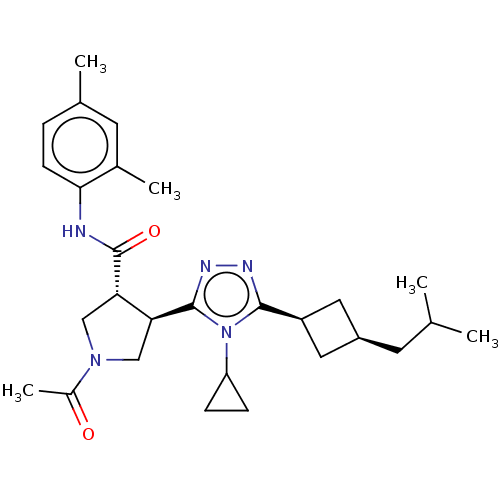
(CHEMBL3774855)Show SMILES CC(C)C[C@H]1C[C@H](C1)c1nnc([C@H]2CN(C[C@@H]2C(=O)Nc2ccc(C)cc2C)C(C)=O)n1C1CC1 |r,wU:4.3,6.8,12.12,wD:16.18,(2.36,7.33,;2.68,6.14,;1.81,5.27,;4.17,5.74,;4.56,4.26,;5.9,3.48,;5.11,2.21,;3.79,2.92,;5.62,.75,;7.1,.32,;7.14,-1.22,;5.7,-1.71,;5.38,-3.22,;6.42,-4.36,;5.65,-5.7,;4.14,-5.38,;4,-3.86,;2.66,-3.08,;2.67,-1.85,;1.33,-3.85,;-.01,-3.08,;0,-1.54,;-1.33,-.76,;-2.67,-1.53,;-3.73,-.91,;-2.67,-3.07,;-1.34,-3.84,;-1.35,-5.07,;6.27,-7.1,;5.55,-8.1,;7.5,-7.23,;4.75,-.52,;3.21,-.57,;1.93,-1.26,;2.03,.28,)| Show InChI InChI=1S/C28H39N5O2/c1-16(2)10-20-12-21(13-20)26-30-31-27(33(26)22-7-8-22)23-14-32(19(5)34)15-24(23)28(35)29-25-9-6-17(3)11-18(25)4/h6,9,11,16,20-24H,7-8,10,12-15H2,1-5H3,(H,29,35)/t20-,21+,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated with substrate for 5 mins followed by NADPH addition measure... |
ACS Med Chem Lett 7: 23-7 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00253
BindingDB Entry DOI: 10.7270/Q2N58P7P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50153594

(CHEMBL3774855)Show SMILES CC(C)C[C@H]1C[C@H](C1)c1nnc([C@H]2CN(C[C@@H]2C(=O)Nc2ccc(C)cc2C)C(C)=O)n1C1CC1 |r,wU:4.3,6.8,12.12,wD:16.18,(2.36,7.33,;2.68,6.14,;1.81,5.27,;4.17,5.74,;4.56,4.26,;5.9,3.48,;5.11,2.21,;3.79,2.92,;5.62,.75,;7.1,.32,;7.14,-1.22,;5.7,-1.71,;5.38,-3.22,;6.42,-4.36,;5.65,-5.7,;4.14,-5.38,;4,-3.86,;2.66,-3.08,;2.67,-1.85,;1.33,-3.85,;-.01,-3.08,;0,-1.54,;-1.33,-.76,;-2.67,-1.53,;-3.73,-.91,;-2.67,-3.07,;-1.34,-3.84,;-1.35,-5.07,;6.27,-7.1,;5.55,-8.1,;7.5,-7.23,;4.75,-.52,;3.21,-.57,;1.93,-1.26,;2.03,.28,)| Show InChI InChI=1S/C28H39N5O2/c1-16(2)10-20-12-21(13-20)26-30-31-27(33(26)22-7-8-22)23-14-32(19(5)34)15-24(23)28(35)29-25-9-6-17(3)11-18(25)4/h6,9,11,16,20-24H,7-8,10,12-15H2,1-5H3,(H,29,35)/t20-,21+,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate preincubated with substrate for 5 mins followed by NADPH addition measur... |
ACS Med Chem Lett 7: 23-7 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00253
BindingDB Entry DOI: 10.7270/Q2N58P7P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50153594

(CHEMBL3774855)Show SMILES CC(C)C[C@H]1C[C@H](C1)c1nnc([C@H]2CN(C[C@@H]2C(=O)Nc2ccc(C)cc2C)C(C)=O)n1C1CC1 |r,wU:4.3,6.8,12.12,wD:16.18,(2.36,7.33,;2.68,6.14,;1.81,5.27,;4.17,5.74,;4.56,4.26,;5.9,3.48,;5.11,2.21,;3.79,2.92,;5.62,.75,;7.1,.32,;7.14,-1.22,;5.7,-1.71,;5.38,-3.22,;6.42,-4.36,;5.65,-5.7,;4.14,-5.38,;4,-3.86,;2.66,-3.08,;2.67,-1.85,;1.33,-3.85,;-.01,-3.08,;0,-1.54,;-1.33,-.76,;-2.67,-1.53,;-3.73,-.91,;-2.67,-3.07,;-1.34,-3.84,;-1.35,-5.07,;6.27,-7.1,;5.55,-8.1,;7.5,-7.23,;4.75,-.52,;3.21,-.57,;1.93,-1.26,;2.03,.28,)| Show InChI InChI=1S/C28H39N5O2/c1-16(2)10-20-12-21(13-20)26-30-31-27(33(26)22-7-8-22)23-14-32(19(5)34)15-24(23)28(35)29-25-9-6-17(3)11-18(25)4/h6,9,11,16,20-24H,7-8,10,12-15H2,1-5H3,(H,29,35)/t20-,21+,23-,24-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes using bufuralol as substrate preincubated with substrate for 5 mins followed by NADPH addition measure... |
ACS Med Chem Lett 7: 23-7 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00253
BindingDB Entry DOI: 10.7270/Q2N58P7P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50153594

(CHEMBL3774855)Show SMILES CC(C)C[C@H]1C[C@H](C1)c1nnc([C@H]2CN(C[C@@H]2C(=O)Nc2ccc(C)cc2C)C(C)=O)n1C1CC1 |r,wU:4.3,6.8,12.12,wD:16.18,(2.36,7.33,;2.68,6.14,;1.81,5.27,;4.17,5.74,;4.56,4.26,;5.9,3.48,;5.11,2.21,;3.79,2.92,;5.62,.75,;7.1,.32,;7.14,-1.22,;5.7,-1.71,;5.38,-3.22,;6.42,-4.36,;5.65,-5.7,;4.14,-5.38,;4,-3.86,;2.66,-3.08,;2.67,-1.85,;1.33,-3.85,;-.01,-3.08,;0,-1.54,;-1.33,-.76,;-2.67,-1.53,;-3.73,-.91,;-2.67,-3.07,;-1.34,-3.84,;-1.35,-5.07,;6.27,-7.1,;5.55,-8.1,;7.5,-7.23,;4.75,-.52,;3.21,-.57,;1.93,-1.26,;2.03,.28,)| Show InChI InChI=1S/C28H39N5O2/c1-16(2)10-20-12-21(13-20)26-30-31-27(33(26)22-7-8-22)23-14-32(19(5)34)15-24(23)28(35)29-25-9-6-17(3)11-18(25)4/h6,9,11,16,20-24H,7-8,10,12-15H2,1-5H3,(H,29,35)/t20-,21+,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using ethoxyresorufin as substrate preincubated with substrate for 5 mins followed by NADPH addition m... |
ACS Med Chem Lett 7: 23-7 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00253
BindingDB Entry DOI: 10.7270/Q2N58P7P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50537147

(CHEMBL4537625)Show SMILES [Na;v0+].[#6]C([#6])([#6])[#6]-c1cc(no1)-c1onc(-[#6@@H](-[#6]-[#6]-[#6](-[#8-])=O)-[#6]-[#6](=O)-[#7]-c2ccc(Cl)cc2Cl)c1-[#6]-1-[#6]-[#6]-1 |r| Show InChI InChI=1S/C26H29Cl2N3O5.Na/c1-26(2,3)13-17-12-20(30-35-17)25-23(14-4-5-14)24(31-36-25)15(6-9-22(33)34)10-21(32)29-19-8-7-16(27)11-18(19)28;/h7-8,11-12,14-15H,4-6,9-10,13H2,1-3H3,(H,29,32)(H,33,34);/q;+1/p-1/t15-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Time-dependent inhibition of human CYP3A4 using testosterone as substrate |
J Med Chem 62: 2837-2842 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01567
BindingDB Entry DOI: 10.7270/Q20R9SWS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50153594

(CHEMBL3774855)Show SMILES CC(C)C[C@H]1C[C@H](C1)c1nnc([C@H]2CN(C[C@@H]2C(=O)Nc2ccc(C)cc2C)C(C)=O)n1C1CC1 |r,wU:4.3,6.8,12.12,wD:16.18,(2.36,7.33,;2.68,6.14,;1.81,5.27,;4.17,5.74,;4.56,4.26,;5.9,3.48,;5.11,2.21,;3.79,2.92,;5.62,.75,;7.1,.32,;7.14,-1.22,;5.7,-1.71,;5.38,-3.22,;6.42,-4.36,;5.65,-5.7,;4.14,-5.38,;4,-3.86,;2.66,-3.08,;2.67,-1.85,;1.33,-3.85,;-.01,-3.08,;0,-1.54,;-1.33,-.76,;-2.67,-1.53,;-3.73,-.91,;-2.67,-3.07,;-1.34,-3.84,;-1.35,-5.07,;6.27,-7.1,;5.55,-8.1,;7.5,-7.23,;4.75,-.52,;3.21,-.57,;1.93,-1.26,;2.03,.28,)| Show InChI InChI=1S/C28H39N5O2/c1-16(2)10-20-12-21(13-20)26-30-31-27(33(26)22-7-8-22)23-14-32(19(5)34)15-24(23)28(35)29-25-9-6-17(3)11-18(25)4/h6,9,11,16,20-24H,7-8,10,12-15H2,1-5H3,(H,29,35)/t20-,21+,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes using S-mephenytoin as substrate preincubated with substrate for 5 mins followed by NADPH addition me... |
ACS Med Chem Lett 7: 23-7 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00253
BindingDB Entry DOI: 10.7270/Q2N58P7P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50153594

(CHEMBL3774855)Show SMILES CC(C)C[C@H]1C[C@H](C1)c1nnc([C@H]2CN(C[C@@H]2C(=O)Nc2ccc(C)cc2C)C(C)=O)n1C1CC1 |r,wU:4.3,6.8,12.12,wD:16.18,(2.36,7.33,;2.68,6.14,;1.81,5.27,;4.17,5.74,;4.56,4.26,;5.9,3.48,;5.11,2.21,;3.79,2.92,;5.62,.75,;7.1,.32,;7.14,-1.22,;5.7,-1.71,;5.38,-3.22,;6.42,-4.36,;5.65,-5.7,;4.14,-5.38,;4,-3.86,;2.66,-3.08,;2.67,-1.85,;1.33,-3.85,;-.01,-3.08,;0,-1.54,;-1.33,-.76,;-2.67,-1.53,;-3.73,-.91,;-2.67,-3.07,;-1.34,-3.84,;-1.35,-5.07,;6.27,-7.1,;5.55,-8.1,;7.5,-7.23,;4.75,-.52,;3.21,-.57,;1.93,-1.26,;2.03,.28,)| Show InChI InChI=1S/C28H39N5O2/c1-16(2)10-20-12-21(13-20)26-30-31-27(33(26)22-7-8-22)23-14-32(19(5)34)15-24(23)28(35)29-25-9-6-17(3)11-18(25)4/h6,9,11,16,20-24H,7-8,10,12-15H2,1-5H3,(H,29,35)/t20-,21+,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of CYP2A6 in human liver microsomes using coumarin as substrate preincubated with substrate for 5 mins followed by NADPH addition measured... |
ACS Med Chem Lett 7: 23-7 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00253
BindingDB Entry DOI: 10.7270/Q2N58P7P |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM50290178
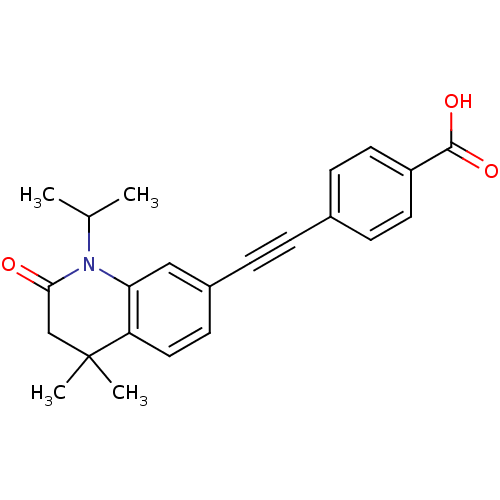
(4-(1-Isopropyl-4,4-dimethyl-2-oxo-1,2,3,4-tetrahyd...)Show SMILES CC(C)N1C(=O)CC(C)(C)c2ccc(cc12)C#Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H23NO3/c1-15(2)24-20-13-17(9-12-19(20)23(3,4)14-21(24)25)6-5-16-7-10-18(11-8-16)22(26)27/h7-13,15H,14H2,1-4H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was determined for Retinoic acid receptor beta |
Bioorg Med Chem Lett 7: 2373-2378 (1997)
Article DOI: 10.1016/S0960-894X(97)00435-6
BindingDB Entry DOI: 10.7270/Q2K35TN8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50290184
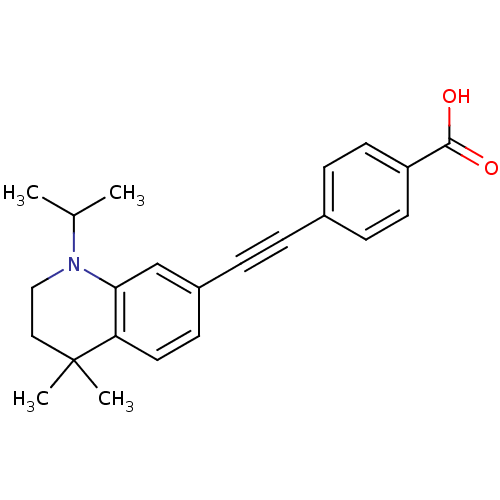
(4-(1-Isopropyl-4,4-dimethyl-1,2,3,4-tetrahydro-qui...)Show SMILES CC(C)N1CCC(C)(C)c2ccc(cc12)C#Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H25NO2/c1-16(2)24-14-13-23(3,4)20-12-9-18(15-21(20)24)6-5-17-7-10-19(11-8-17)22(25)26/h7-12,15-16H,13-14H2,1-4H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transactivation potency of the compound was determined for Retinoic acid receptor alpha; Not active |
Bioorg Med Chem Lett 7: 2373-2378 (1997)
Article DOI: 10.1016/S0960-894X(97)00435-6
BindingDB Entry DOI: 10.7270/Q2K35TN8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM50290184

(4-(1-Isopropyl-4,4-dimethyl-1,2,3,4-tetrahydro-qui...)Show SMILES CC(C)N1CCC(C)(C)c2ccc(cc12)C#Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H25NO2/c1-16(2)24-14-13-23(3,4)20-12-9-18(15-21(20)24)6-5-17-7-10-19(11-8-17)22(25)26/h7-12,15-16H,13-14H2,1-4H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was determined for Retinoic acid receptor beta |
Bioorg Med Chem Lett 7: 2373-2378 (1997)
Article DOI: 10.1016/S0960-894X(97)00435-6
BindingDB Entry DOI: 10.7270/Q2K35TN8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM50290181
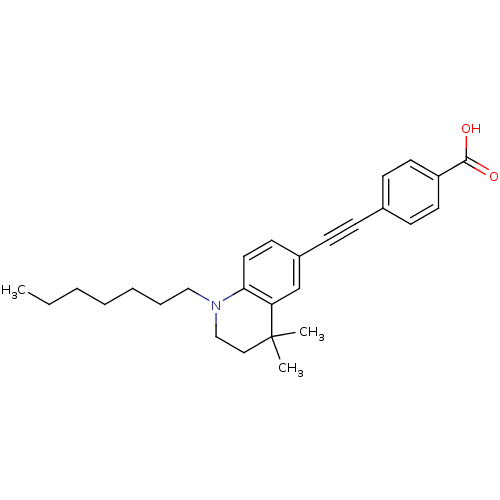
(4-(1-Heptyl-4,4-dimethyl-1,2,3,4-tetrahydro-quinol...)Show SMILES CCCCCCCN1CCC(C)(C)c2cc(ccc12)C#Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C27H33NO2/c1-4-5-6-7-8-18-28-19-17-27(2,3)24-20-22(13-16-25(24)28)10-9-21-11-14-23(15-12-21)26(29)30/h11-16,20H,4-8,17-19H2,1-3H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was determined for Retinoic acid receptor beta |
Bioorg Med Chem Lett 7: 2373-2378 (1997)
Article DOI: 10.1016/S0960-894X(97)00435-6
BindingDB Entry DOI: 10.7270/Q2K35TN8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | 350 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transactivation potency of the compound was determined for Retinoic acid receptor alpha |
Bioorg Med Chem Lett 7: 2373-2378 (1997)
Article DOI: 10.1016/S0960-894X(97)00435-6
BindingDB Entry DOI: 10.7270/Q2K35TN8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50290178

(4-(1-Isopropyl-4,4-dimethyl-2-oxo-1,2,3,4-tetrahyd...)Show SMILES CC(C)N1C(=O)CC(C)(C)c2ccc(cc12)C#Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H23NO3/c1-15(2)24-20-13-17(9-12-19(20)23(3,4)14-21(24)25)6-5-16-7-10-18(11-8-16)22(26)27/h7-13,15H,14H2,1-4H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transactivation potency of the compound was determined for Retinoic acid receptor gamma |
Bioorg Med Chem Lett 7: 2373-2378 (1997)
Article DOI: 10.1016/S0960-894X(97)00435-6
BindingDB Entry DOI: 10.7270/Q2K35TN8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM50290180
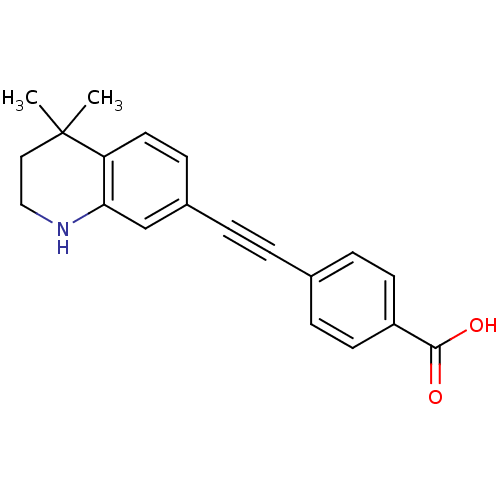
(4-(4,4-Dimethyl-1,2,3,4-tetrahydro-quinolin-7-ylet...)Show InChI InChI=1S/C20H19NO2/c1-20(2)11-12-21-18-13-15(7-10-17(18)20)4-3-14-5-8-16(9-6-14)19(22)23/h5-10,13,21H,11-12H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 36 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transactivation potency of the compound was determined for Retinoic acid receptor beta |
Bioorg Med Chem Lett 7: 2373-2378 (1997)
Article DOI: 10.1016/S0960-894X(97)00435-6
BindingDB Entry DOI: 10.7270/Q2K35TN8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50290185

(4-(1-Isopropyl-4,4-dimethyl-1,2,3,4-tetrahydro-qui...)Show SMILES CC(C)N1CCC(C)(C)c2cc(ccc12)C#Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H25NO2/c1-16(2)24-14-13-23(3,4)20-15-18(9-12-21(20)24)6-5-17-7-10-19(11-8-17)22(25)26/h7-12,15-16H,13-14H2,1-4H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transactivation potency of the compound was determined for Retinoic acid receptor gamma |
Bioorg Med Chem Lett 7: 2373-2378 (1997)
Article DOI: 10.1016/S0960-894X(97)00435-6
BindingDB Entry DOI: 10.7270/Q2K35TN8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50290179

(4-(1-Isopropyl-4,4-dimethyl-2-oxo-1,2,3,4-tetrahyd...)Show SMILES CC(C)N1C(=O)CC(C)(C)c2cc(ccc12)C#Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H23NO3/c1-15(2)24-20-12-9-17(13-19(20)23(3,4)14-21(24)25)6-5-16-7-10-18(11-8-16)22(26)27/h7-13,15H,14H2,1-4H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transactivation potency of the compound was determined for Retinoic acid receptor gamma |
Bioorg Med Chem Lett 7: 2373-2378 (1997)
Article DOI: 10.1016/S0960-894X(97)00435-6
BindingDB Entry DOI: 10.7270/Q2K35TN8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM50290182
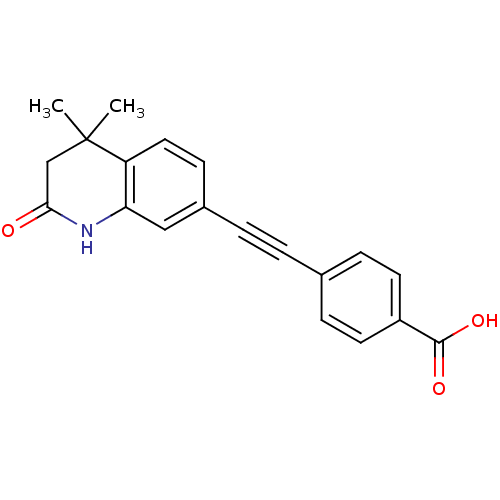
(4-(4,4-Dimethyl-2-oxo-1,2,3,4-tetrahydro-quinolin-...)Show SMILES CC1(C)CC(=O)Nc2cc(ccc12)C#Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C20H17NO3/c1-20(2)12-18(22)21-17-11-14(7-10-16(17)20)4-3-13-5-8-15(9-6-13)19(23)24/h5-11H,12H2,1-2H3,(H,21,22)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transactivation potency of the compound was determined for Retinoic acid receptor beta |
Bioorg Med Chem Lett 7: 2373-2378 (1997)
Article DOI: 10.1016/S0960-894X(97)00435-6
BindingDB Entry DOI: 10.7270/Q2K35TN8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50032219
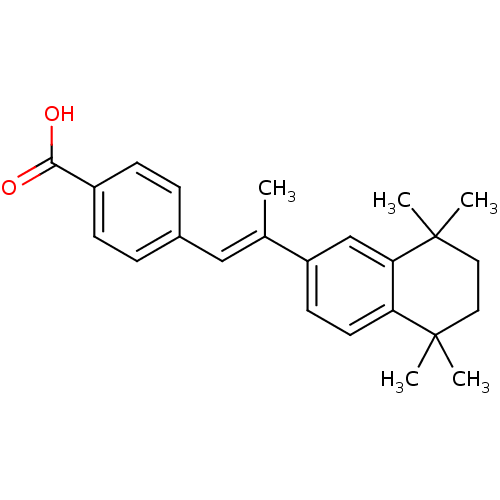
((E)-4-(2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydrona...)Show SMILES C\C(=C/c1ccc(cc1)C(O)=O)c1ccc2c(c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-16(14-17-6-8-18(9-7-17)22(25)26)19-10-11-20-21(15-19)24(4,5)13-12-23(20,2)3/h6-11,14-15H,12-13H2,1-5H3,(H,25,26)/b16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transactivation potency of the compound was determined for Retinoic acid receptor alpha |
Bioorg Med Chem Lett 7: 2373-2378 (1997)
Article DOI: 10.1016/S0960-894X(97)00435-6
BindingDB Entry DOI: 10.7270/Q2K35TN8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50290177

(4-(4,4-Dimethyl-1,2,3,4-tetrahydro-quinolin-6-ylet...)Show InChI InChI=1S/C20H19NO2/c1-20(2)11-12-21-18-10-7-15(13-17(18)20)4-3-14-5-8-16(9-6-14)19(22)23/h5-10,13,21H,11-12H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transactivation potency of the compound was determined for Retinoic acid receptor alpha |
Bioorg Med Chem Lett 7: 2373-2378 (1997)
Article DOI: 10.1016/S0960-894X(97)00435-6
BindingDB Entry DOI: 10.7270/Q2K35TN8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50290182

(4-(4,4-Dimethyl-2-oxo-1,2,3,4-tetrahydro-quinolin-...)Show SMILES CC1(C)CC(=O)Nc2cc(ccc12)C#Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C20H17NO3/c1-20(2)12-18(22)21-17-11-14(7-10-16(17)20)4-3-13-5-8-15(9-6-13)19(23)24/h5-11H,12H2,1-2H3,(H,21,22)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transactivation potency of the compound was determined for Retinoic acid receptor gamma |
Bioorg Med Chem Lett 7: 2373-2378 (1997)
Article DOI: 10.1016/S0960-894X(97)00435-6
BindingDB Entry DOI: 10.7270/Q2K35TN8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50290180

(4-(4,4-Dimethyl-1,2,3,4-tetrahydro-quinolin-7-ylet...)Show InChI InChI=1S/C20H19NO2/c1-20(2)11-12-21-18-13-15(7-10-17(18)20)4-3-14-5-8-16(9-6-14)19(22)23/h5-10,13,21H,11-12H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 54 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transactivation potency of the compound was determined for Retinoic acid receptor gamma |
Bioorg Med Chem Lett 7: 2373-2378 (1997)
Article DOI: 10.1016/S0960-894X(97)00435-6
BindingDB Entry DOI: 10.7270/Q2K35TN8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM50290179

(4-(1-Isopropyl-4,4-dimethyl-2-oxo-1,2,3,4-tetrahyd...)Show SMILES CC(C)N1C(=O)CC(C)(C)c2cc(ccc12)C#Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H23NO3/c1-15(2)24-20-12-9-17(13-19(20)23(3,4)14-21(24)25)6-5-16-7-10-18(11-8-16)22(26)27/h7-13,15H,14H2,1-4H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was determined for Retinoic acid receptor beta |
Bioorg Med Chem Lett 7: 2373-2378 (1997)
Article DOI: 10.1016/S0960-894X(97)00435-6
BindingDB Entry DOI: 10.7270/Q2K35TN8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
| n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Retinoic acid receptor gamma |
Bioorg Med Chem Lett 7: 2373-2378 (1997)
Article DOI: 10.1016/S0960-894X(97)00435-6
BindingDB Entry DOI: 10.7270/Q2K35TN8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM50290177

(4-(4,4-Dimethyl-1,2,3,4-tetrahydro-quinolin-6-ylet...)Show InChI InChI=1S/C20H19NO2/c1-20(2)11-12-21-18-10-7-15(13-17(18)20)4-3-14-5-8-16(9-6-14)19(22)23/h5-10,13,21H,11-12H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transactivation potency of the compound was determined for Retinoic acid receptor beta |
Bioorg Med Chem Lett 7: 2373-2378 (1997)
Article DOI: 10.1016/S0960-894X(97)00435-6
BindingDB Entry DOI: 10.7270/Q2K35TN8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50290181

(4-(1-Heptyl-4,4-dimethyl-1,2,3,4-tetrahydro-quinol...)Show SMILES CCCCCCCN1CCC(C)(C)c2cc(ccc12)C#Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C27H33NO2/c1-4-5-6-7-8-18-28-19-17-27(2,3)24-20-22(13-16-25(24)28)10-9-21-11-14-23(15-12-21)26(29)30/h11-16,20H,4-8,17-19H2,1-3H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 260 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transactivation potency of the compound was determined for Retinoic acid receptor gamma |
Bioorg Med Chem Lett 7: 2373-2378 (1997)
Article DOI: 10.1016/S0960-894X(97)00435-6
BindingDB Entry DOI: 10.7270/Q2K35TN8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-ATRA binding to baculovirus expressed retinoic acid receptor RAR-beta |
J Med Chem 44: 2298-303 (2001)
BindingDB Entry DOI: 10.7270/Q2P84B41 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR alpha |
J Med Chem 44: 2298-303 (2001)
BindingDB Entry DOI: 10.7270/Q2P84B41 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50101444
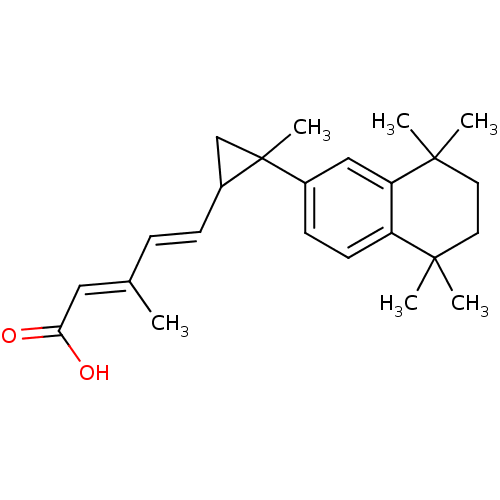
((E)-3-Methyl-5-[2-methyl-2-(5,5,8,8-tetramethyl-5,...)Show SMILES C\C(\C=C\C1CC1(C)c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C24H32O2/c1-16(13-21(25)26)7-8-18-15-24(18,6)17-9-10-19-20(14-17)23(4,5)12-11-22(19,2)3/h7-10,13-14,18H,11-12,15H2,1-6H3,(H,25,26)/b8-7+,16-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-retinoic acid binding to baculovirus expressed retinoic acid receptor RXR-beta |
J Med Chem 44: 2298-303 (2001)
BindingDB Entry DOI: 10.7270/Q2P84B41 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 29 | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR beta |
J Med Chem 44: 2298-303 (2001)
BindingDB Entry DOI: 10.7270/Q2P84B41 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Transcriptional activation in CV-1 cells expressing retinoic acid receptor RAR gamma |
J Med Chem 44: 2298-303 (2001)
BindingDB Entry DOI: 10.7270/Q2P84B41 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-retinoic acid binding to baculovirus expressed retinoic acid receptor RXR-beta |
J Med Chem 44: 2298-303 (2001)
BindingDB Entry DOI: 10.7270/Q2P84B41 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50101445
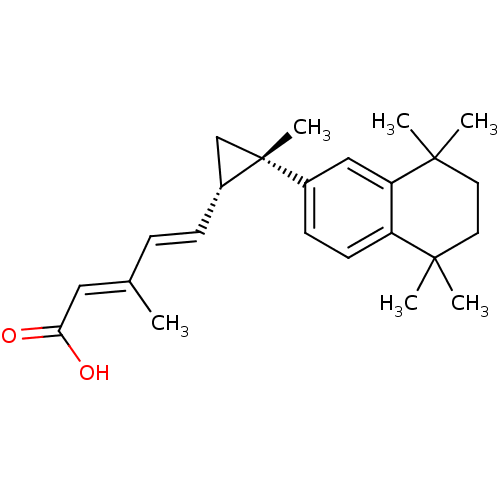
((2E,4E)-3-Methyl-5-[(1S,2S)-2-methyl-2-(5,5,8,8-te...)Show SMILES C\C(\C=C\[C@@H]1C[C@]1(C)c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C24H32O2/c1-16(13-21(25)26)7-8-18-15-24(18,6)17-9-10-19-20(14-17)23(4,5)12-11-22(19,2)3/h7-10,13-14,18H,11-12,15H2,1-6H3,(H,25,26)/b8-7+,16-13+/t18-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR beta |
J Med Chem 44: 2298-303 (2001)
BindingDB Entry DOI: 10.7270/Q2P84B41 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Homo sapiens (Human)) | BDBM50101445

((2E,4E)-3-Methyl-5-[(1S,2S)-2-methyl-2-(5,5,8,8-te...)Show SMILES C\C(\C=C\[C@@H]1C[C@]1(C)c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C24H32O2/c1-16(13-21(25)26)7-8-18-15-24(18,6)17-9-10-19-20(14-17)23(4,5)12-11-22(19,2)3/h7-10,13-14,18H,11-12,15H2,1-6H3,(H,25,26)/b8-7+,16-13+/t18-,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR gamma |
J Med Chem 44: 2298-303 (2001)
BindingDB Entry DOI: 10.7270/Q2P84B41 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50101444

((E)-3-Methyl-5-[2-methyl-2-(5,5,8,8-tetramethyl-5,...)Show SMILES C\C(\C=C\C1CC1(C)c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C24H32O2/c1-16(13-21(25)26)7-8-18-15-24(18,6)17-9-10-19-20(14-17)23(4,5)12-11-22(19,2)3/h7-10,13-14,18H,11-12,15H2,1-6H3,(H,25,26)/b8-7+,16-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-retinoic acid binding to baculovirus expressed retinoic acid receptor RXR-alpha |
J Med Chem 44: 2298-303 (2001)
BindingDB Entry DOI: 10.7270/Q2P84B41 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM50101446
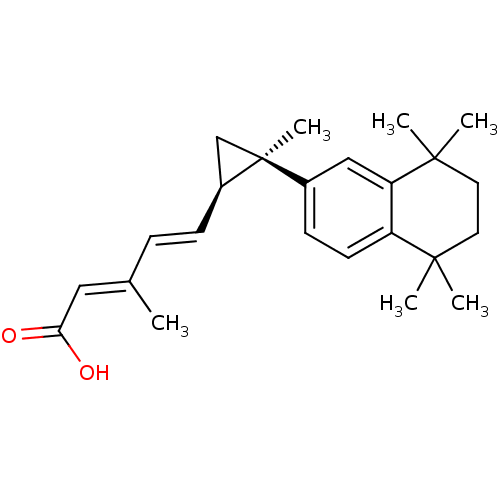
((2E,4E)-3-Methyl-5-[(1R,2R)-2-methyl-2-(5,5,8,8-te...)Show SMILES C\C(\C=C\[C@H]1C[C@@]1(C)c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C24H32O2/c1-16(13-21(25)26)7-8-18-15-24(18,6)17-9-10-19-20(14-17)23(4,5)12-11-22(19,2)3/h7-10,13-14,18H,11-12,15H2,1-6H3,(H,25,26)/b8-7+,16-13+/t18-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-ATRA binding to baculovirus expressed retinoic acid receptor RAR-beta |
J Med Chem 44: 2298-303 (2001)
BindingDB Entry DOI: 10.7270/Q2P84B41 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM50101446

((2E,4E)-3-Methyl-5-[(1R,2R)-2-methyl-2-(5,5,8,8-te...)Show SMILES C\C(\C=C\[C@H]1C[C@@]1(C)c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C24H32O2/c1-16(13-21(25)26)7-8-18-15-24(18,6)17-9-10-19-20(14-17)23(4,5)12-11-22(19,2)3/h7-10,13-14,18H,11-12,15H2,1-6H3,(H,25,26)/b8-7+,16-13+/t18-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >500 | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Transcriptional activation in CV-1 cells expressing retinoic acid receptor RAR beta |
J Med Chem 44: 2298-303 (2001)
BindingDB Entry DOI: 10.7270/Q2P84B41 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Homo sapiens (Human)) | BDBM50101446

((2E,4E)-3-Methyl-5-[(1R,2R)-2-methyl-2-(5,5,8,8-te...)Show SMILES C\C(\C=C\[C@H]1C[C@@]1(C)c1ccc2c(c1)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C24H32O2/c1-16(13-21(25)26)7-8-18-15-24(18,6)17-9-10-19-20(14-17)23(4,5)12-11-22(19,2)3/h7-10,13-14,18H,11-12,15H2,1-6H3,(H,25,26)/b8-7+,16-13+/t18-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-ATRA binding to baculovirus expressed retinoic acid receptor RAR-gamma |
J Med Chem 44: 2298-303 (2001)
BindingDB Entry DOI: 10.7270/Q2P84B41 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a |
Allergan Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-retinoic acid binding to baculovirus expressed retinoic acid receptor RXR-alpha |
J Med Chem 44: 2298-303 (2001)
BindingDB Entry DOI: 10.7270/Q2P84B41 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data