

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (Homo sapiens (Human)) | BDBM558072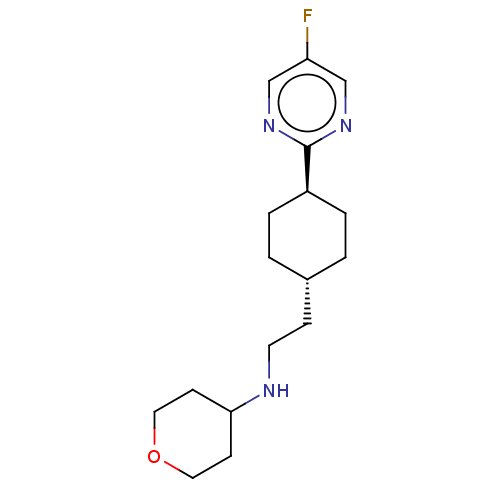 (US11365191, Example 12) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM558095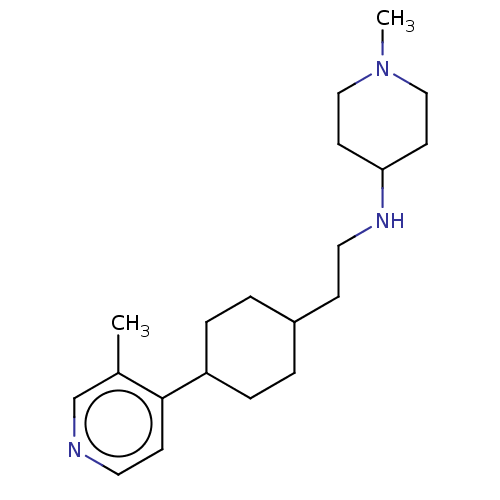 (US11365191, Example 187) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM558074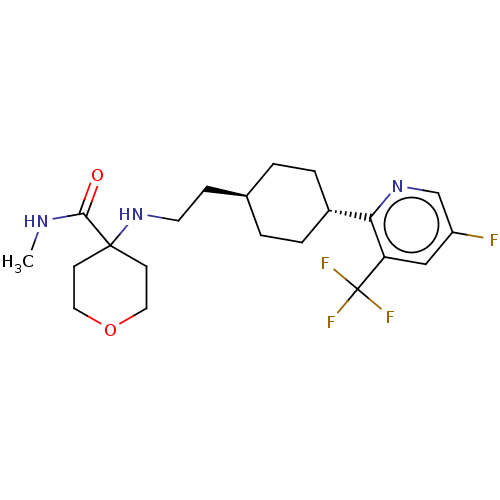 (US11365191, Example 51) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM558075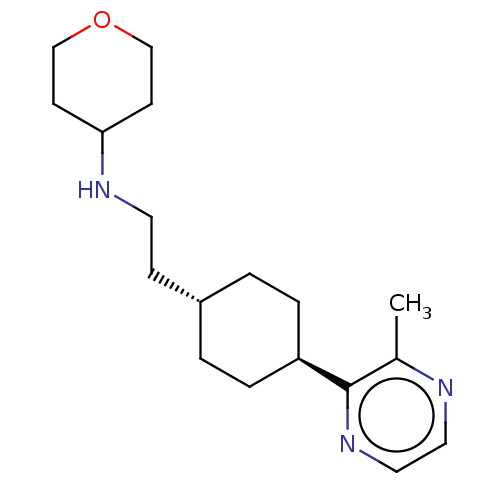 (US11365191, Example 88) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM558076 (US11365191, Example 106) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM558077 (US11365191, Example 111) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM558078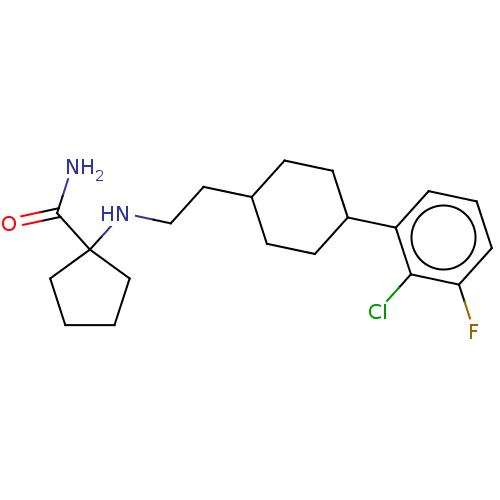 (US11365191, Example 114) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM558079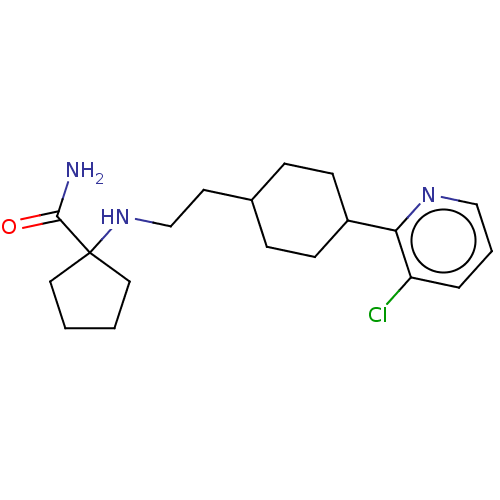 (US11365191, Example 116) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM558080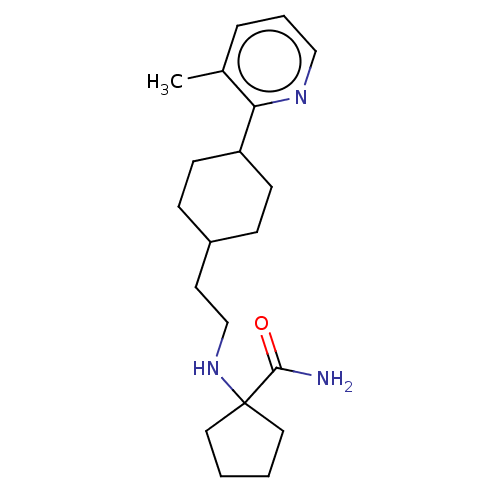 (US11365191, Example 117) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM558081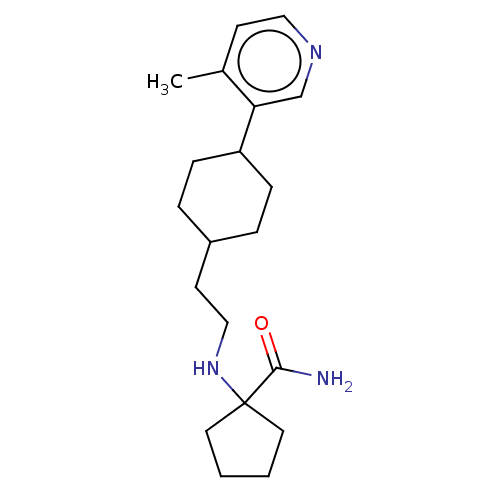 (US11365191, Example 118) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM558082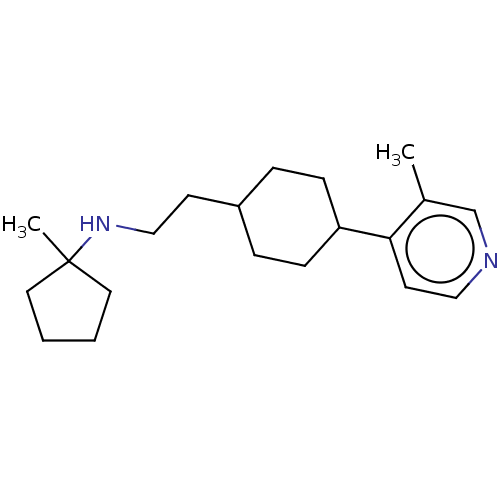 (US11365191, Example 121) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM558083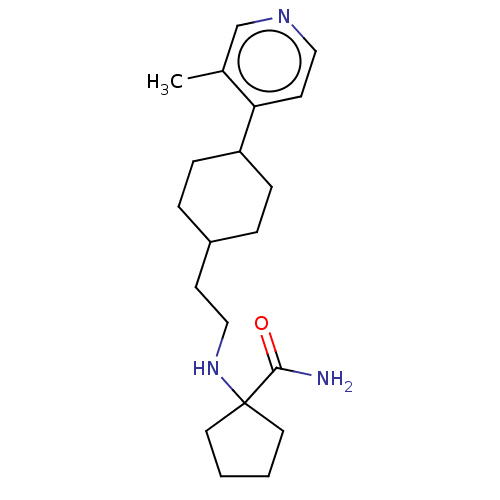 (US11365191, Example 123) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM558084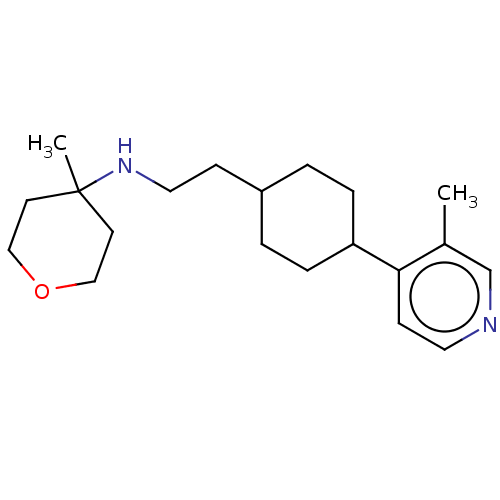 (US11365191, Example 139) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM558085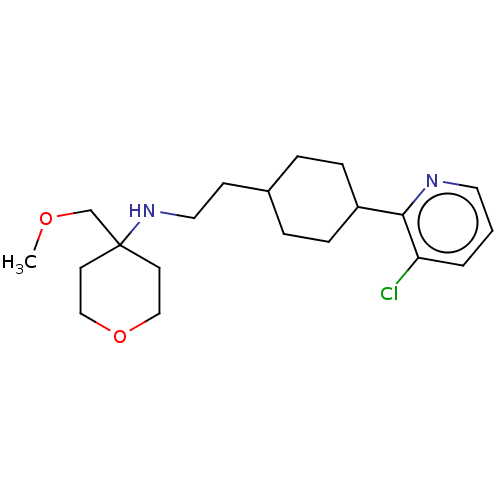 (US11365191, Example 146) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM558086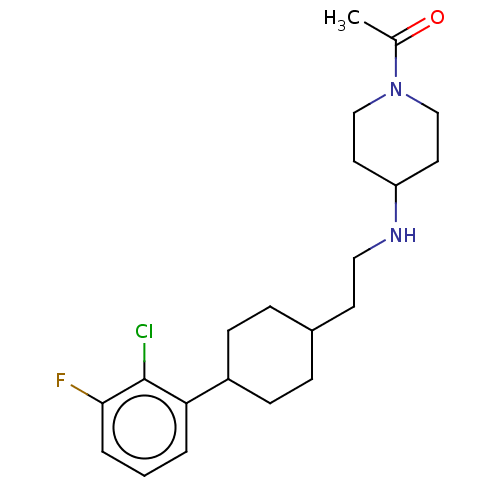 (US11365191, Example 171) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM558087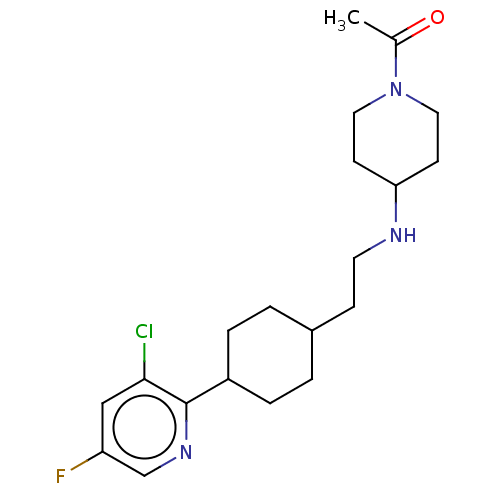 (US11365191, Example 172) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM558088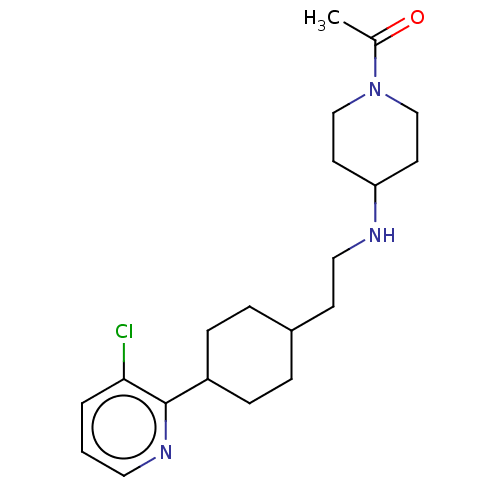 (US11365191, Example 173) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM558089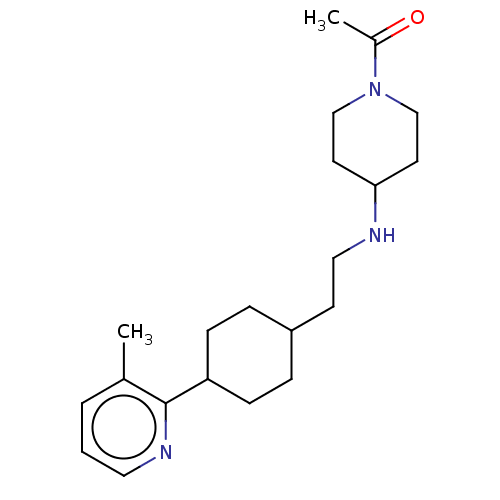 (US11365191, Example 174) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM558090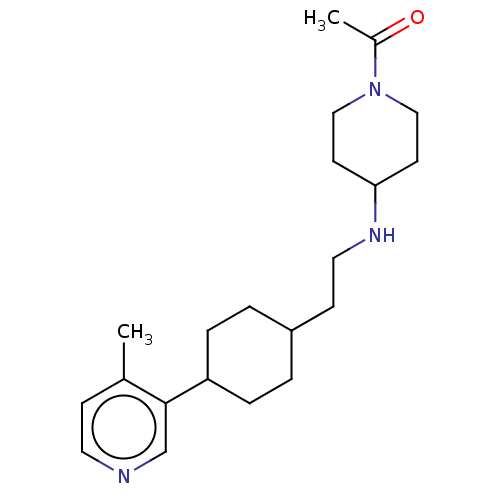 (US11365191, Example 175) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM558091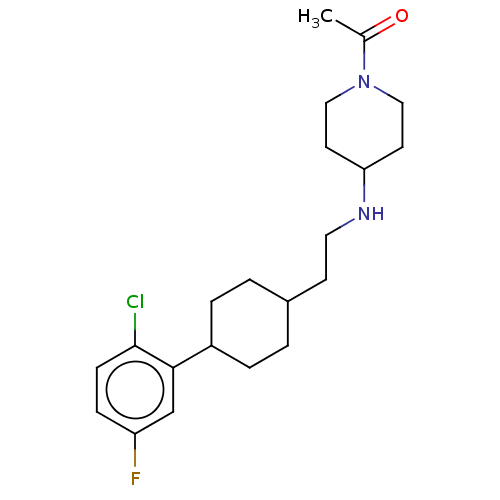 (US11365191, Example 178) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM558092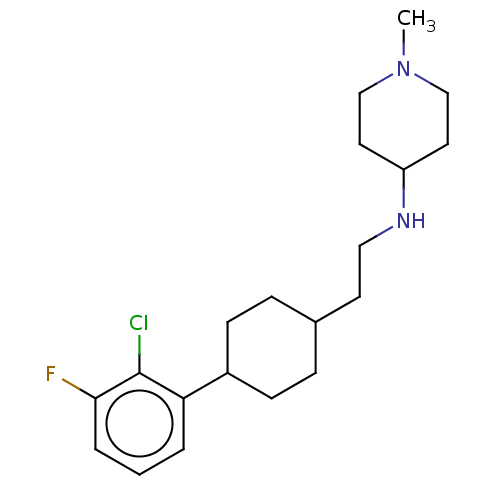 (US11365191, Example 179) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM558093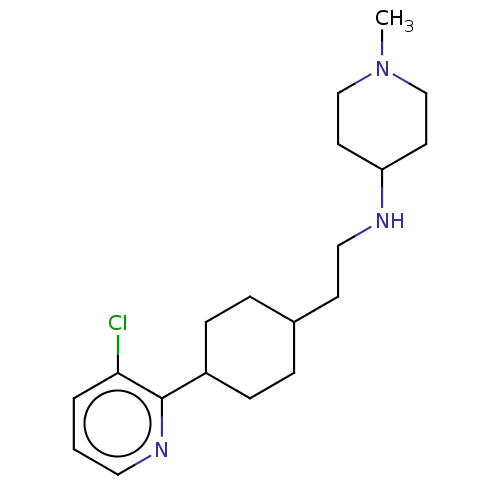 (US11365191, Example 181) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM558094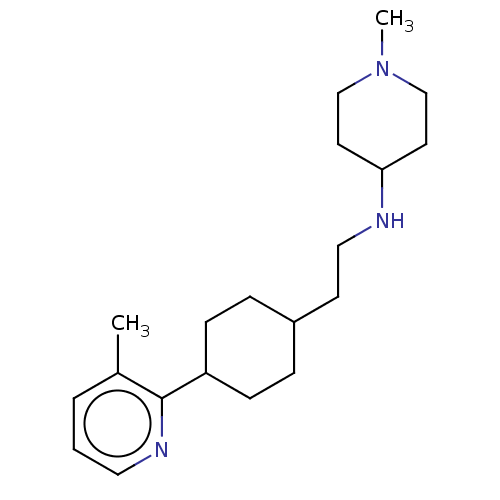 (US11365191, Example 182) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM558073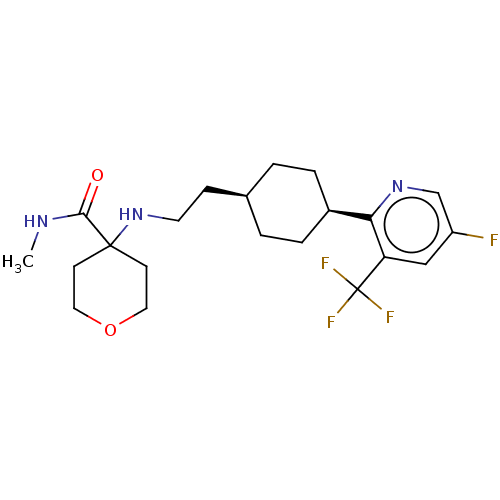 (4-((2-((trans)-4-(5-fluoro-3-(trifluoromethyl)pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50148310 (((S)-1-Formyl-pentyl)-carbamic acid (S)-1-benzyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant human cathepsin K determined in a fluorescence assay using 10 microM Cbz-Phe-Arg-AMC as substrate | Bioorg Med Chem Lett 14: 3425-9 (2004) Article DOI: 10.1016/j.bmcl.2004.04.084 BindingDB Entry DOI: 10.7270/Q2SF2VMD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50148292 (((S)-1-Formyl-pentyl)-carbamic acid 1-benzyl-cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant human cathepsin K determined in a fluorescence assay using 10 microM Cbz-Phe-Arg-AMC as substrate | Bioorg Med Chem Lett 14: 3425-9 (2004) Article DOI: 10.1016/j.bmcl.2004.04.084 BindingDB Entry DOI: 10.7270/Q2SF2VMD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50148298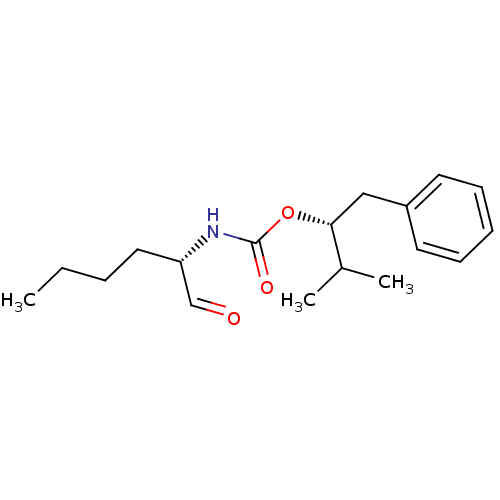 (((S)-1-Formyl-pentyl)-carbamic acid (R)-1-benzyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant human cathepsin K determined in a fluorescence assay using 10 microM Cbz-Phe-Arg-AMC as substrate | Bioorg Med Chem Lett 14: 3425-9 (2004) Article DOI: 10.1016/j.bmcl.2004.04.084 BindingDB Entry DOI: 10.7270/Q2SF2VMD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50148307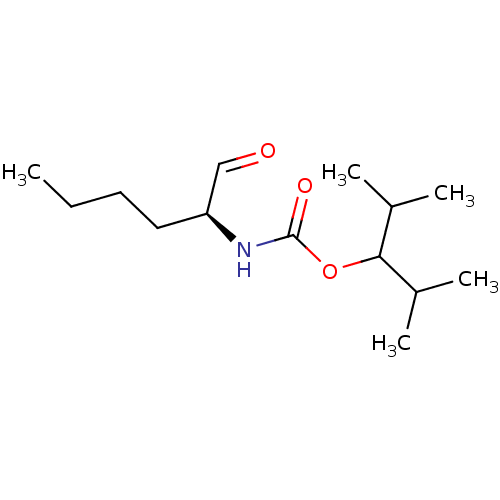 (((S)-1-Formyl-pentyl)-carbamic acid 1-isopropyl-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant human cathepsin K determined in a fluorescence assay using 10 microM Cbz-Phe-Arg-AMC as substrate | Bioorg Med Chem Lett 14: 3425-9 (2004) Article DOI: 10.1016/j.bmcl.2004.04.084 BindingDB Entry DOI: 10.7270/Q2SF2VMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50148300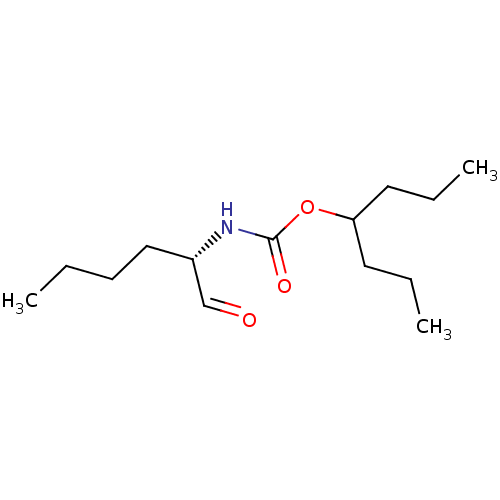 (((S)-1-Formyl-pentyl)-carbamic acid 1-propyl-butyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant human cathepsin K determined in a fluorescence assay using 10 microM Cbz-Phe-Arg-AMC as substrate | Bioorg Med Chem Lett 14: 3425-9 (2004) Article DOI: 10.1016/j.bmcl.2004.04.084 BindingDB Entry DOI: 10.7270/Q2SF2VMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM557916 (US11365191, Example 54) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM557912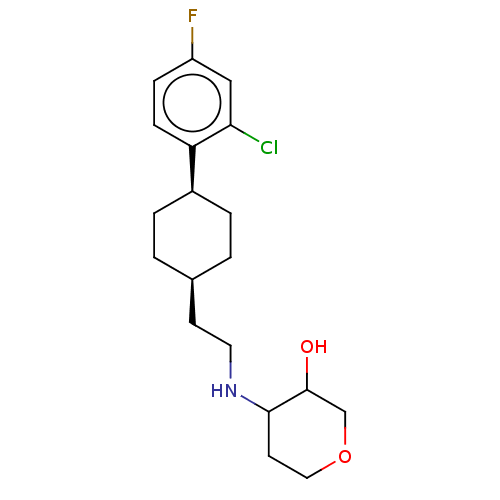 (US11365191, Example 25) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM557913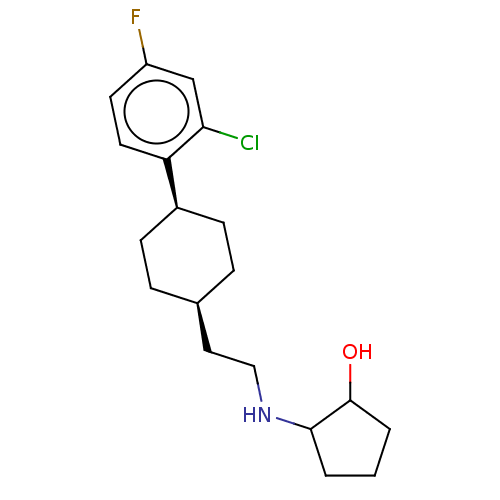 (US11365191, Example 27) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50484379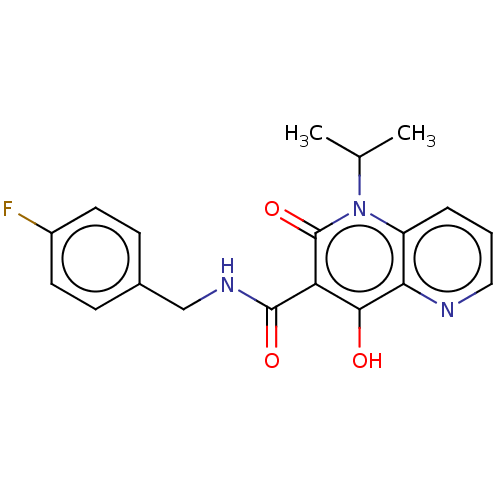 (CHEMBL1917873) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research & Development Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer by biochemical assay | Bioorg Med Chem Lett 21: 6461-4 (2011) Article DOI: 10.1016/j.bmcl.2011.08.082 BindingDB Entry DOI: 10.7270/Q22Z18CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM557915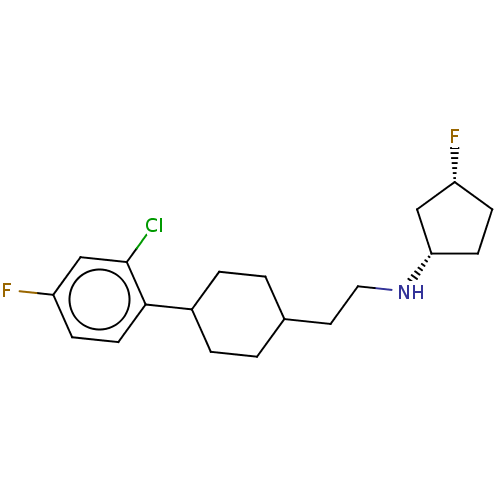 (US11365191, Example 53) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM557911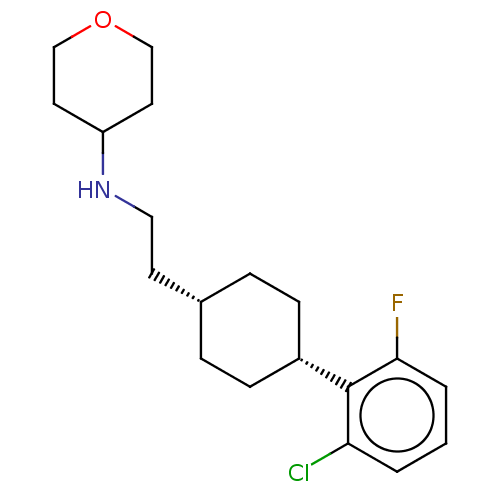 (N-(2-((trans)-4-(2-chloro-6-fluorophenyl)cyclohexy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM557910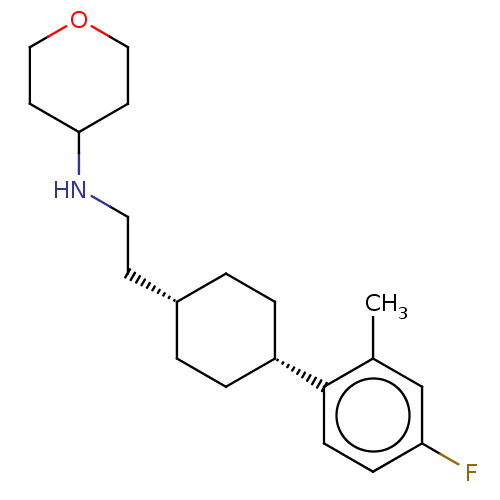 (N-(2-((trans)-4-(4-fluoro-2-methylphenyl)cyclohexy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM557893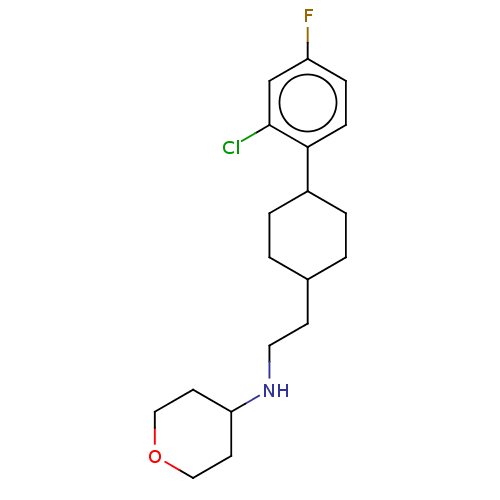 (US11365191, Example 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM557917 (US11365191, Example 55) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM557926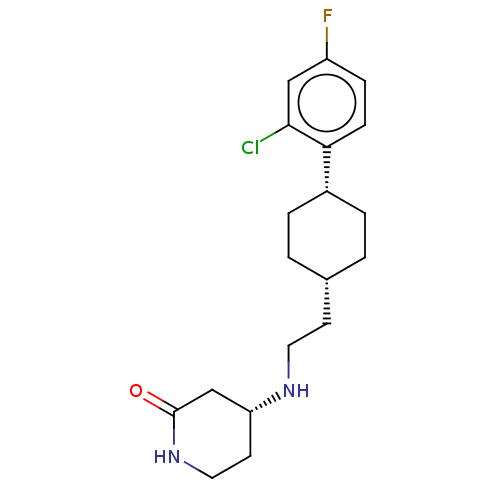 (US11365191, Example 161) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM557925 (US11365191, Example 158) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM557924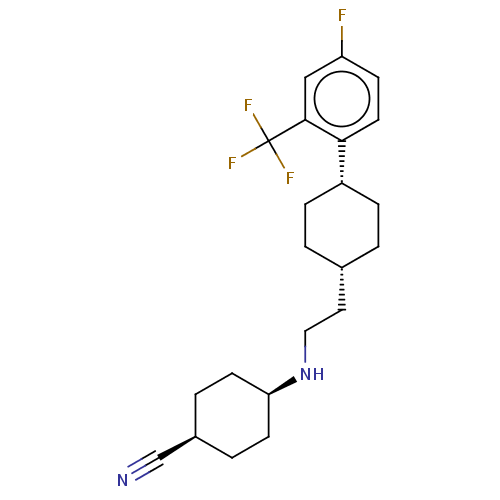 (US11365191, Example 93) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM557923 (N-(2-((trans)-4-(3-chloro-5-fluoropyridin-2-yl)cyc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM557922 (US11365191, Example 76) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM557921 (US11365191, Example 64) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM557920 (US11365191, Example 61) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM557919 (US11365191, Example 57) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM557918 (N-(2-((cis)-4-(2-chloro-4-fluorophenyl)cyclohexyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Formula (I) compounds were tested for antagonism of the nociceptin receptor (NOP) in a cell-based cAMP assay, using the LANCE cAMP Detection Kit, Per... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50148314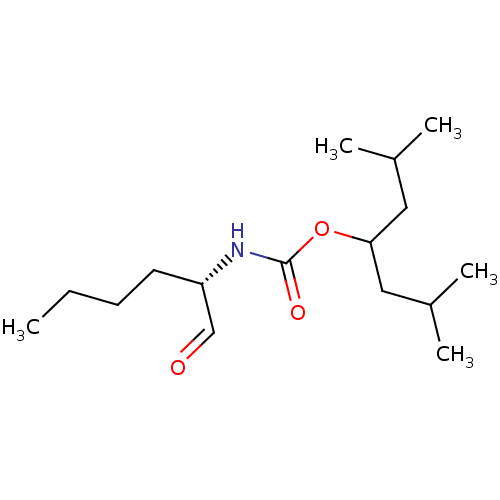 (((S)-1-Formyl-pentyl)-carbamic acid 1-isobutyl-3-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant human cathepsin K determined in a fluorescence assay using 10 microM Cbz-Phe-Arg-AMC as substrate | Bioorg Med Chem Lett 14: 3425-9 (2004) Article DOI: 10.1016/j.bmcl.2004.04.084 BindingDB Entry DOI: 10.7270/Q2SF2VMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054718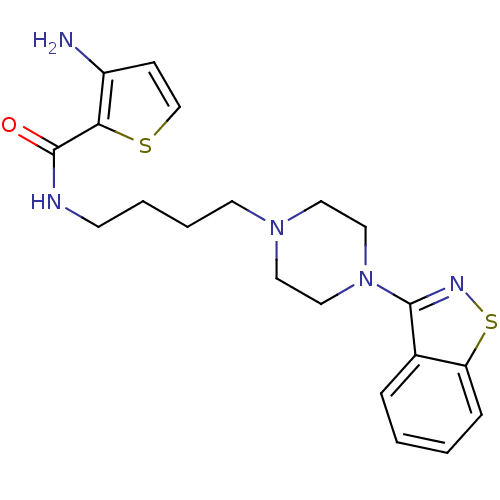 (3-Amino-thiophene-2-carboxylic acid [4-(4-benzo[d]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50148297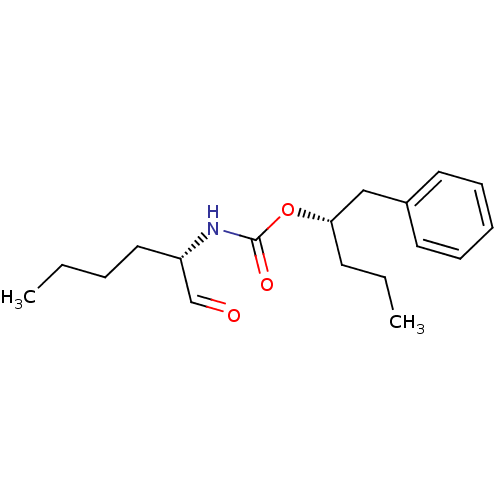 (((S)-1-Formyl-pentyl)-carbamic acid (S)-1-benzyl-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant human cathepsin K determined in a fluorescence assay using 10 microM Cbz-Phe-Arg-AMC as substrate | Bioorg Med Chem Lett 14: 3425-9 (2004) Article DOI: 10.1016/j.bmcl.2004.04.084 BindingDB Entry DOI: 10.7270/Q2SF2VMD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Displayed 1 to 50 (of 525 total ) | Next | Last >> |