

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aurora kinase A (Homo sapiens (Human)) | BDBM50004205 (MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128747 BindingDB Entry DOI: 10.7270/Q2FF3XDT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50600442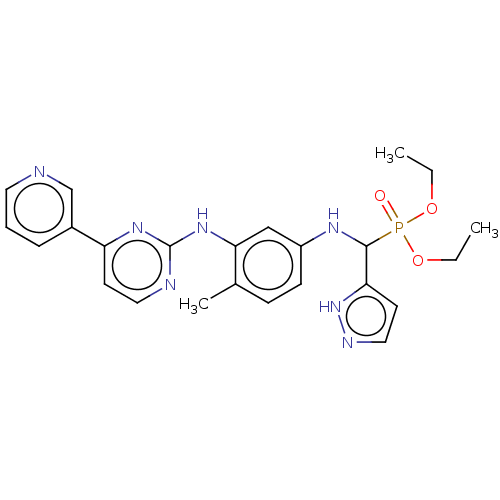 (CHEMBL5200042) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128747 BindingDB Entry DOI: 10.7270/Q2FF3XDT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50600442 (CHEMBL5200042) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128747 BindingDB Entry DOI: 10.7270/Q2FF3XDT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50600441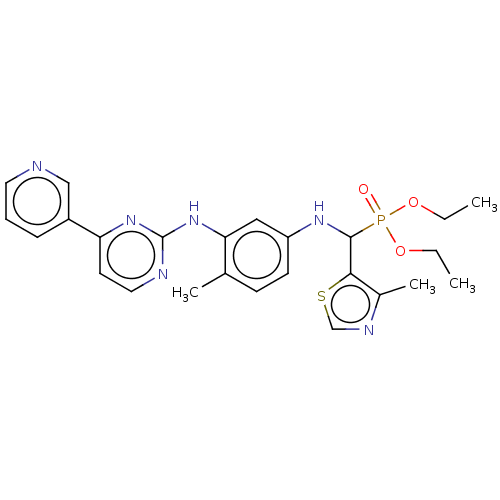 (CHEMBL5180210) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128747 BindingDB Entry DOI: 10.7270/Q2FF3XDT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50004205 (MK-045 | MK-0457 | TOZASERTIB | US9249124, VX680 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128747 BindingDB Entry DOI: 10.7270/Q2FF3XDT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50600443 (CHEMBL5173220) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128747 BindingDB Entry DOI: 10.7270/Q2FF3XDT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50600441 (CHEMBL5180210) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128747 BindingDB Entry DOI: 10.7270/Q2FF3XDT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50600443 (CHEMBL5173220) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128747 BindingDB Entry DOI: 10.7270/Q2FF3XDT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116385 BindingDB Entry DOI: 10.7270/Q28W3J18 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trypsin (Homo sapiens (Human)) | BDBM50491950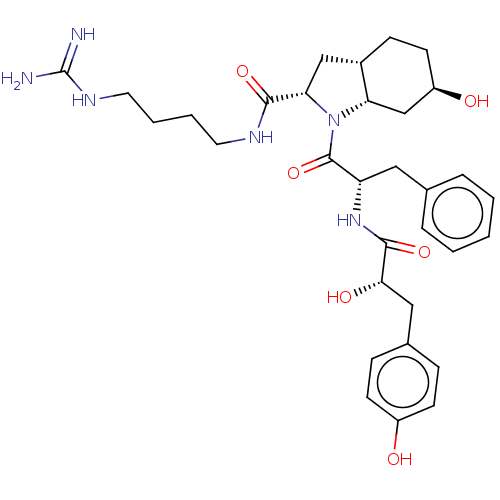 (CHEMBL2386692) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 821 | n/a | n/a | n/a | n/a | n/a | n/a |
Tel-Aviv University Curated by ChEMBL | Assay Description Inhibition of trypsin (unknown origin) | J Nat Prod 76: 1187-90 (2013) Article DOI: 10.1021/np4001152 BindingDB Entry DOI: 10.7270/Q2X069ZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50567300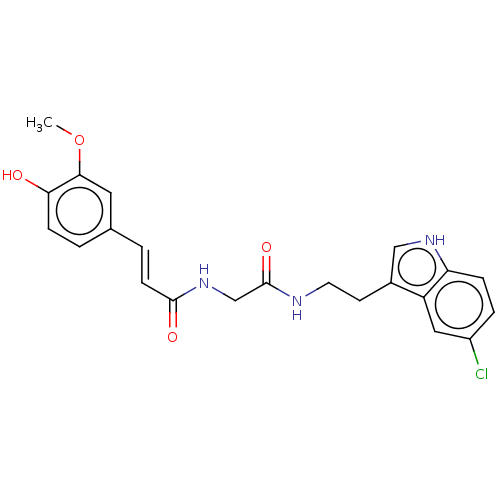 (CHEMBL4866457) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116385 BindingDB Entry DOI: 10.7270/Q28W3J18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM22984 ((8S,10S)-10-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Prof John Barnabas Post Graduate School of Biological Studies Curated by ChEMBL | Assay Description Inhibition of human DNA topoisomerase 2 catalytic activity using supercoiled pRYG DNA as substrate measured after 45 mins in presence of ATP by agaro... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127246 BindingDB Entry DOI: 10.7270/Q2WQ07CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50567299 (CHEMBL4869703) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116385 BindingDB Entry DOI: 10.7270/Q28W3J18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50543963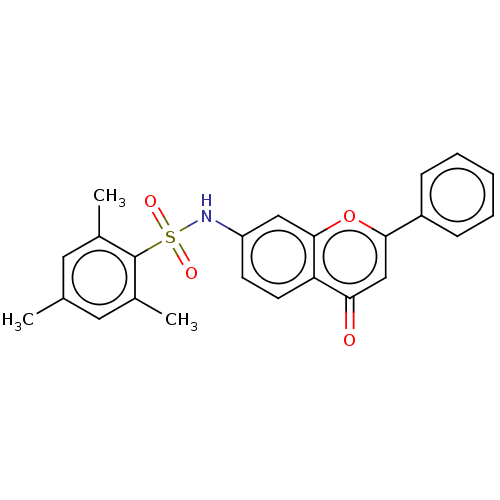 (CHEMBL4636680) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Prof John Barnabas Post Graduate School of Biological Studies Curated by ChEMBL | Assay Description Inhibition of human DNA topoisomerase 2 catalytic activity using supercoiled pRYG DNA as substrate measured after 45 mins in presence of ATP by agaro... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127246 BindingDB Entry DOI: 10.7270/Q2WQ07CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM22984 ((8S,10S)-10-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human topoisomerase-2 alpha in human A498 cells assessed as decrease in relaxation of supercoiled DNA at 10 uM | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127916 BindingDB Entry DOI: 10.7270/Q2VQ36GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50242053 (6,8-didec-(1Z)-enyl-5,7-dimethyl-2,3-dihydro-1H-in...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]gp-120 from human CCR5 receptor expressed in CHO cells | J Nat Prod 67: 1036-8 (2004) Article DOI: 10.1021/np049974l BindingDB Entry DOI: 10.7270/Q29Z94P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50567299 (CHEMBL4869703) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116385 BindingDB Entry DOI: 10.7270/Q28W3J18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50567300 (CHEMBL4866457) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116385 BindingDB Entry DOI: 10.7270/Q28W3J18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50567298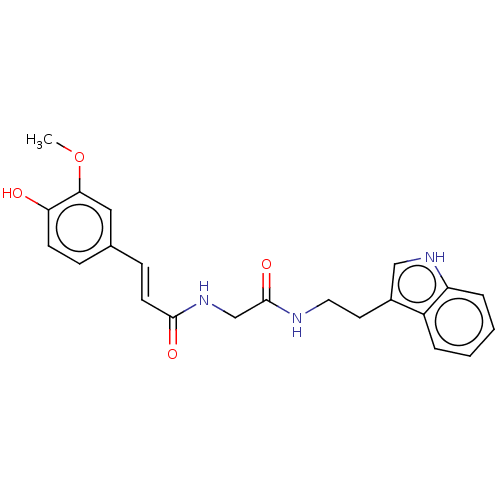 (CHEMBL4854322) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116385 BindingDB Entry DOI: 10.7270/Q28W3J18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50543962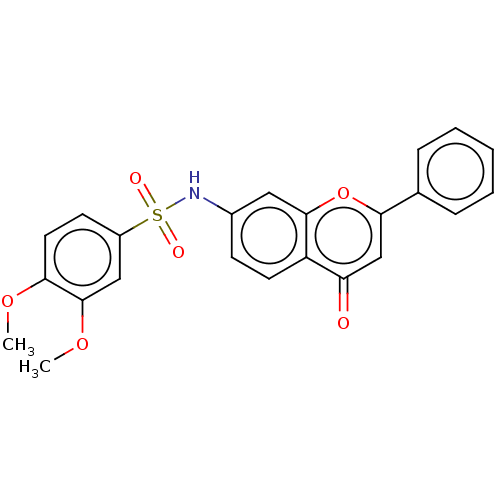 (CHEMBL4642846) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Prof John Barnabas Post Graduate School of Biological Studies Curated by ChEMBL | Assay Description Inhibition of human DNA topoisomerase 2 catalytic activity using supercoiled pRYG DNA as substrate measured after 45 mins in presence of ATP by agaro... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127246 BindingDB Entry DOI: 10.7270/Q2WQ07CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116385 BindingDB Entry DOI: 10.7270/Q28W3J18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50567296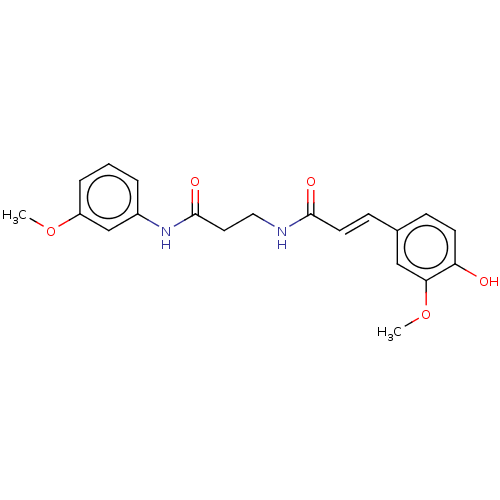 (CHEMBL4848548) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116385 BindingDB Entry DOI: 10.7270/Q28W3J18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin (Homo sapiens (Human)) | BDBM50491951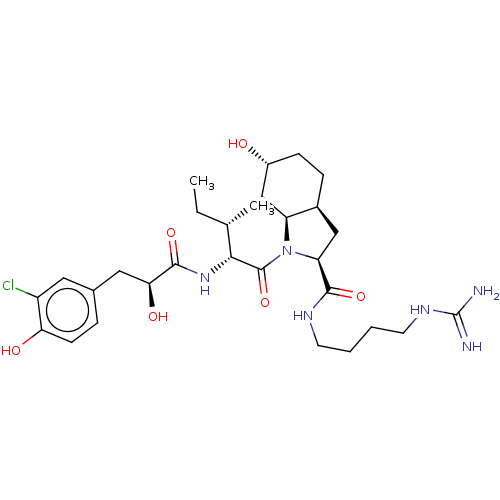 (CHEMBL2386691) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tel-Aviv University Curated by ChEMBL | Assay Description Inhibition of trypsin (unknown origin) | J Nat Prod 76: 1187-90 (2013) Article DOI: 10.1021/np4001152 BindingDB Entry DOI: 10.7270/Q2X069ZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50567298 (CHEMBL4854322) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116385 BindingDB Entry DOI: 10.7270/Q28W3J18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin (Homo sapiens (Human)) | BDBM50491948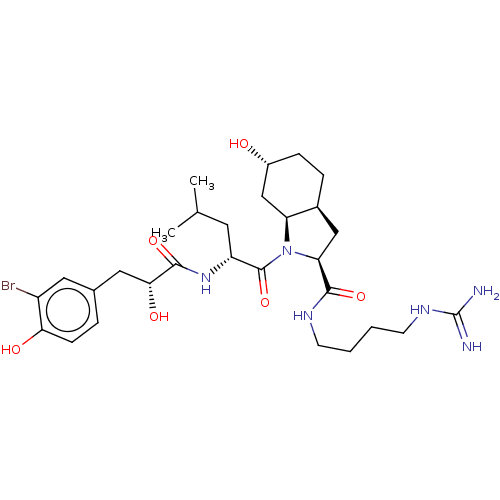 (CHEMBL2386693) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tel-Aviv University Curated by ChEMBL | Assay Description Inhibition of trypsin (unknown origin) | J Nat Prod 76: 1187-90 (2013) Article DOI: 10.1021/np4001152 BindingDB Entry DOI: 10.7270/Q2X069ZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin (Homo sapiens (Human)) | BDBM50491949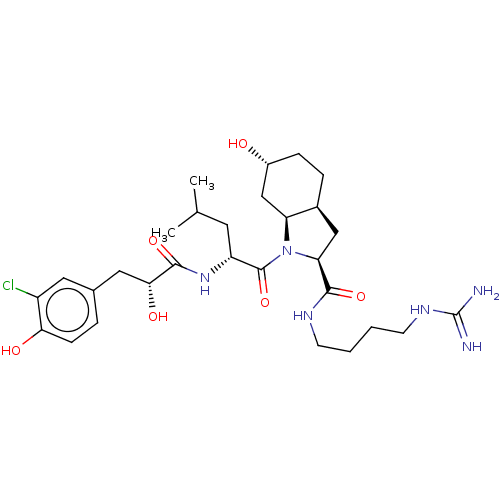 (CHEMBL2386694) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tel-Aviv University Curated by ChEMBL | Assay Description Inhibition of trypsin (unknown origin) | J Nat Prod 76: 1187-90 (2013) Article DOI: 10.1021/np4001152 BindingDB Entry DOI: 10.7270/Q2X069ZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50567297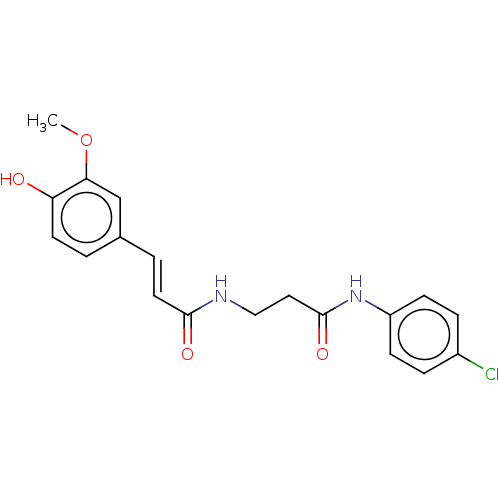 (CHEMBL4856181) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116385 BindingDB Entry DOI: 10.7270/Q28W3J18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50564501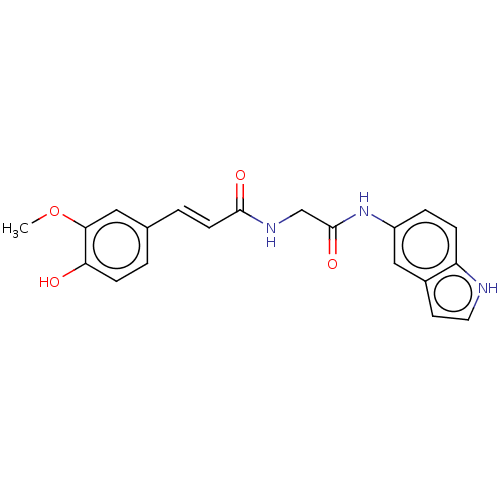 (CHEMBL4799941) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116385 BindingDB Entry DOI: 10.7270/Q28W3J18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50567297 (CHEMBL4856181) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116385 BindingDB Entry DOI: 10.7270/Q28W3J18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50543960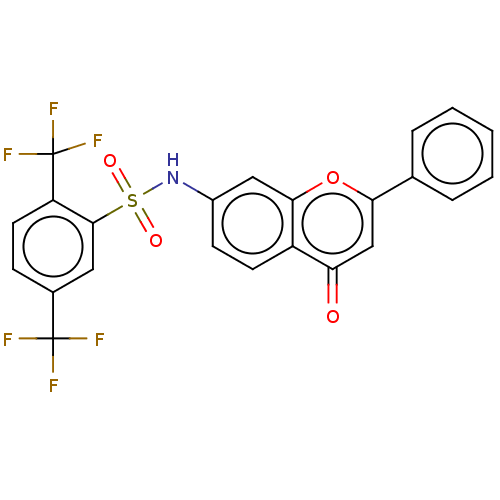 (CHEMBL4633308) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Prof John Barnabas Post Graduate School of Biological Studies Curated by ChEMBL | Assay Description Inhibition of human DNA topoisomerase 2 catalytic activity using supercoiled pRYG DNA as substrate measured after 45 mins in presence of ATP by agaro... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127246 BindingDB Entry DOI: 10.7270/Q2WQ07CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50564504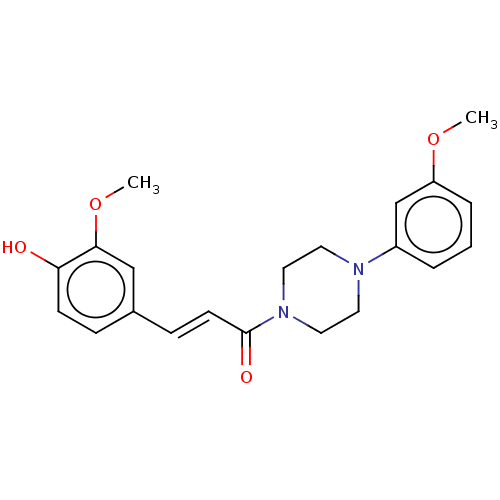 (CHEMBL4785400) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116385 BindingDB Entry DOI: 10.7270/Q28W3J18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50570427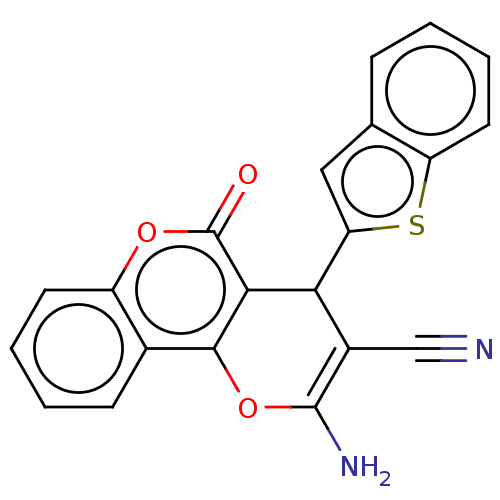 (CHEMBL4864715) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human topoisomerase-2 alpha in human A498 cells assessed as decrease in relaxation of supercoiled DNA at 10 uM | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127916 BindingDB Entry DOI: 10.7270/Q2VQ36GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50567296 (CHEMBL4848548) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116385 BindingDB Entry DOI: 10.7270/Q28W3J18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50543961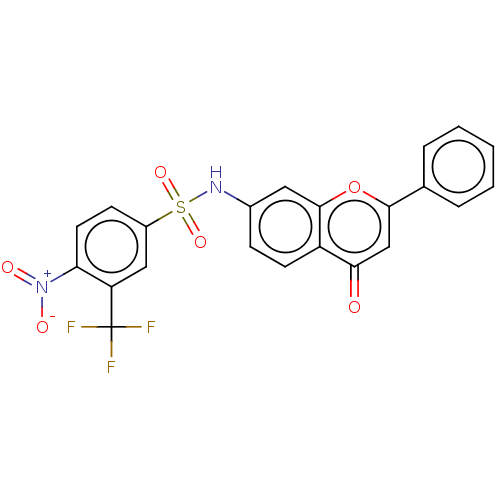 (CHEMBL4647427) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Prof John Barnabas Post Graduate School of Biological Studies Curated by ChEMBL | Assay Description Inhibition of human DNA topoisomerase 2 catalytic activity using supercoiled pRYG DNA as substrate measured after 45 mins in presence of ATP by agaro... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127246 BindingDB Entry DOI: 10.7270/Q2WQ07CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50570429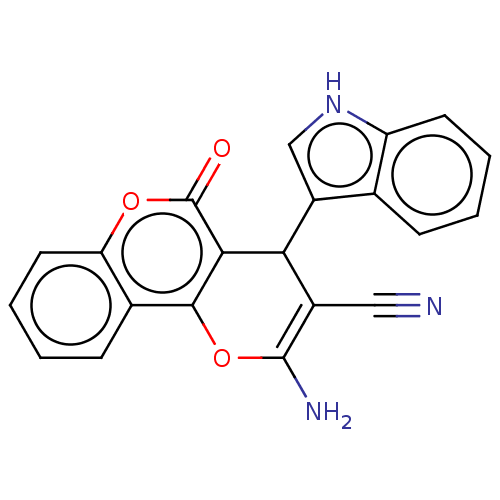 (CHEMBL4878037) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human topoisomerase-2 alpha in human A498 cells assessed as decrease in relaxation of supercoiled DNA at 10 uM | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127916 BindingDB Entry DOI: 10.7270/Q2VQ36GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50567293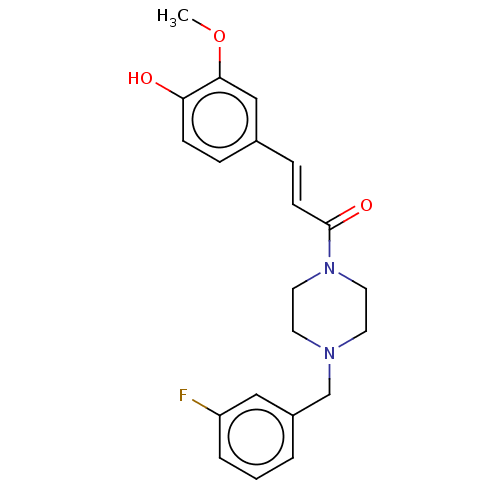 (CHEMBL4873165) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116385 BindingDB Entry DOI: 10.7270/Q28W3J18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50567289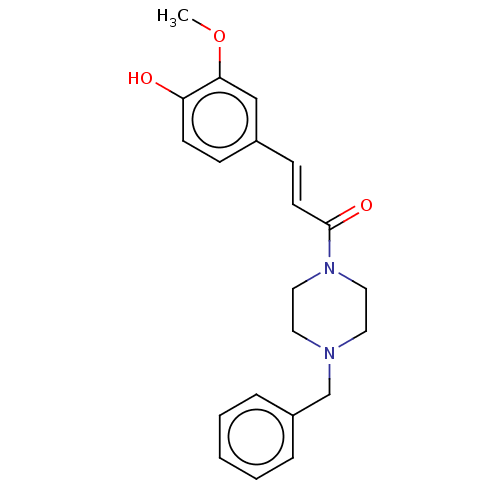 (CHEMBL4848801) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116385 BindingDB Entry DOI: 10.7270/Q28W3J18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50567293 (CHEMBL4873165) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116385 BindingDB Entry DOI: 10.7270/Q28W3J18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50564501 (CHEMBL4799941) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116385 BindingDB Entry DOI: 10.7270/Q28W3J18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50567294 (CHEMBL4864801) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116385 BindingDB Entry DOI: 10.7270/Q28W3J18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50567310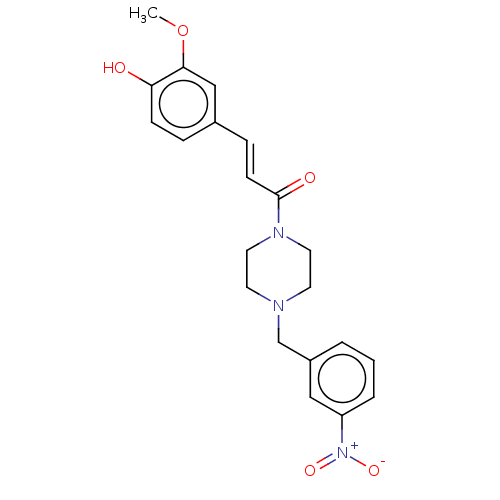 (CHEMBL4863669) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116385 BindingDB Entry DOI: 10.7270/Q28W3J18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50567302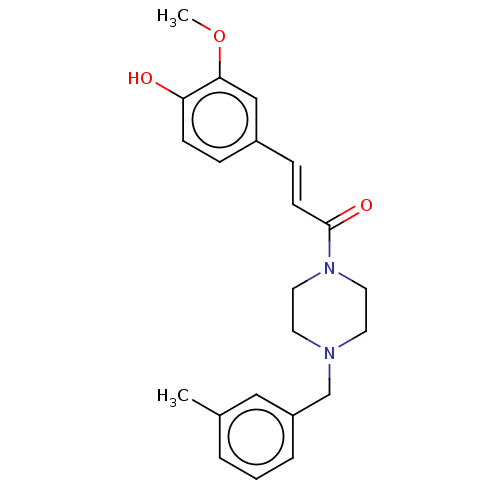 (CHEMBL4871018) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116385 BindingDB Entry DOI: 10.7270/Q28W3J18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50567308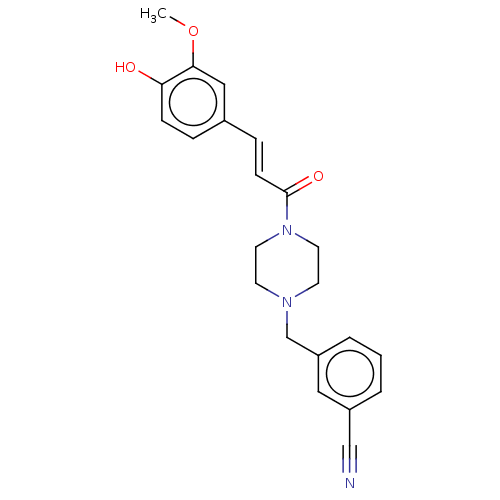 (CHEMBL4865179) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116385 BindingDB Entry DOI: 10.7270/Q28W3J18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50567295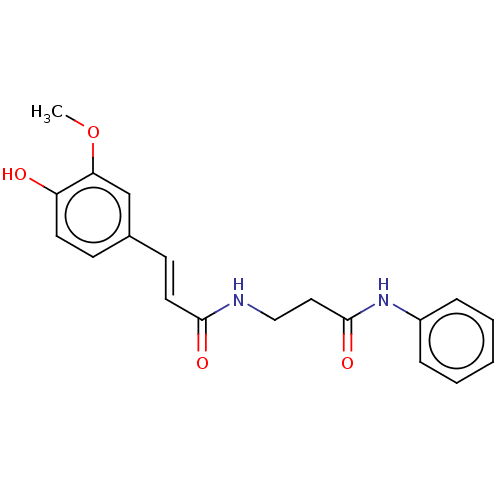 (CHEMBL4860467) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116385 BindingDB Entry DOI: 10.7270/Q28W3J18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50567303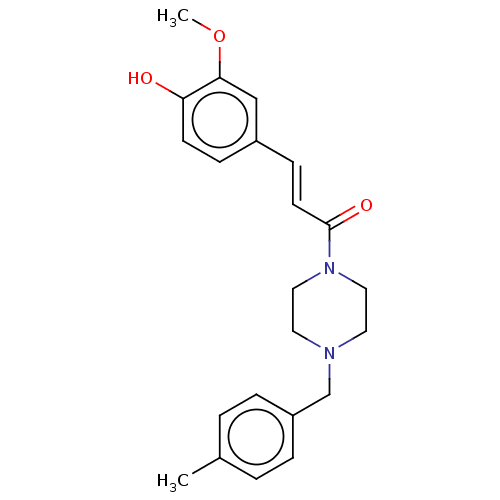 (CHEMBL4876437) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116385 BindingDB Entry DOI: 10.7270/Q28W3J18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50567292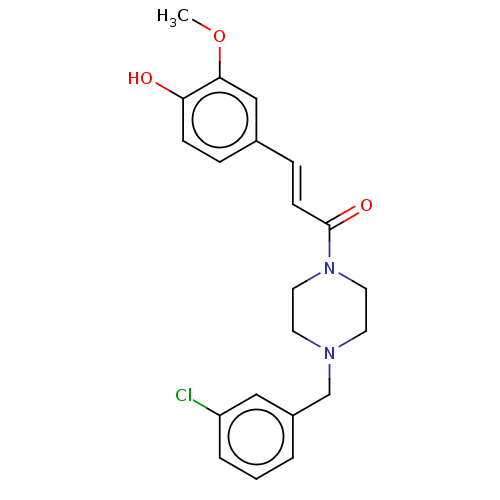 (CHEMBL4868234) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116385 BindingDB Entry DOI: 10.7270/Q28W3J18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50567294 (CHEMBL4864801) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116385 BindingDB Entry DOI: 10.7270/Q28W3J18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50567307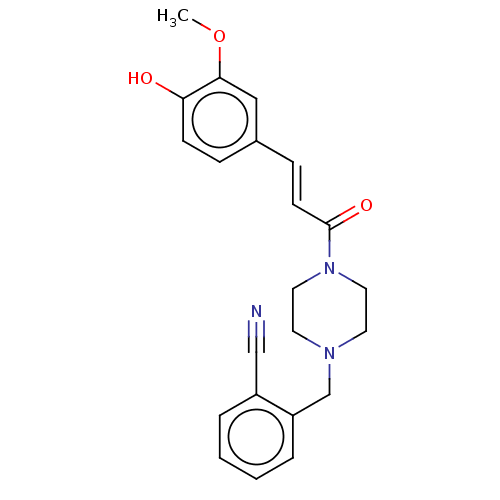 (CHEMBL4863005) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116385 BindingDB Entry DOI: 10.7270/Q28W3J18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50567301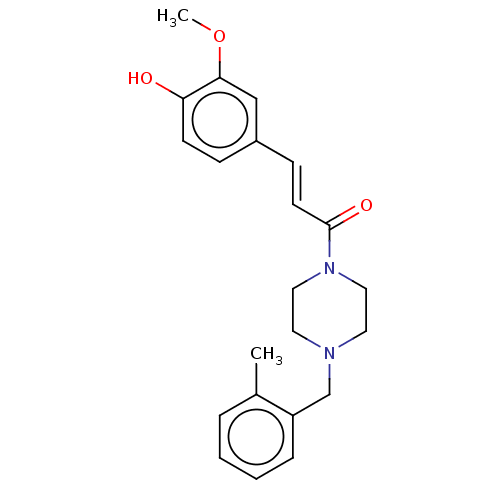 (CHEMBL4860607) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116385 BindingDB Entry DOI: 10.7270/Q28W3J18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50567290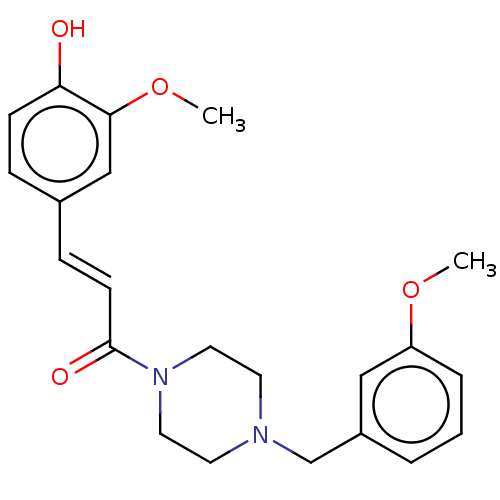 (CHEMBL4851290) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addit... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116385 BindingDB Entry DOI: 10.7270/Q28W3J18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 94 total ) | Next | Last >> |