 Found 153 hits with Last Name = 'tomek' and Initial = 'p'
Found 153 hits with Last Name = 'tomek' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50235921

(CHEMBL584991)Show InChI InChI=1S/C9H7ClFN5O2/c10-5-3-4(1-2-6(5)11)13-9(14-17)7-8(12)16-18-15-7/h1-3,17H,(H2,12,16)(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Compound was tested in a cell-free SLe-polyacrylamide glycoconjugate binding assay (assay B) in Selectin E |
Eur J Med Chem 126: 983-996 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.029
BindingDB Entry DOI: 10.7270/Q2RX9F9K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50126144

(CHEMBL3629569 | US10155972, Compound NewLink 1 | U...)Show InChI InChI=1S/C18H22N2O/c21-18(13-6-2-1-3-7-13)10-16-14-8-4-5-9-15(14)17-11-19-12-20(16)17/h4-5,8-9,11-13,16,18,21H,1-3,6-7,10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116160
BindingDB Entry DOI: 10.7270/Q2DF6W0V |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50126143

(Epacadostat | INCB-024360)Show SMILES NS(=O)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C11H13BrFN7O4S/c12-7-5-6(1-2-8(7)13)17-11(18-21)9-10(20-24-19-9)15-3-4-16-25(14,22)23/h1-2,5,16,21H,3-4H2,(H,15,20)(H,17,18)(H2,14,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116160
BindingDB Entry DOI: 10.7270/Q2DF6W0V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50126143

(Epacadostat | INCB-024360)Show SMILES NS(=O)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C11H13BrFN7O4S/c12-7-5-6(1-2-8(7)13)17-11(18-21)9-10(20-24-19-9)15-3-4-16-25(14,22)23/h1-2,5,16,21H,3-4H2,(H,15,20)(H,17,18)(H2,14,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116160
BindingDB Entry DOI: 10.7270/Q2DF6W0V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tryptophan 2,3-dioxygenase
(Rattus norvegicus) | BDBM50289137

(6-Fluoro-3-((E)-2-pyridin-3-yl-vinyl)-1H-indole | ...)Show InChI InChI=1S/C15H11FN2/c16-13-5-6-14-12(10-18-15(14)8-13)4-3-11-2-1-7-17-9-11/h1-10,18H/b4-3+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of TDO2 in rat liver homogenate assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate incubated for 1 hr |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116160
BindingDB Entry DOI: 10.7270/Q2DF6W0V |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50126144

(CHEMBL3629569 | US10155972, Compound NewLink 1 | U...)Show InChI InChI=1S/C18H22N2O/c21-18(13-6-2-1-3-7-13)10-16-14-8-4-5-9-15(14)17-11-19-12-20(16)17/h4-5,8-9,11-13,16,18,21H,1-3,6-7,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human IDO1 assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate incubated for 45 mins by m... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116160
BindingDB Entry DOI: 10.7270/Q2DF6W0V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50235921

(CHEMBL584991)Show InChI InChI=1S/C9H7ClFN5O2/c10-5-3-4(1-2-6(5)11)13-9(14-17)7-8(12)16-18-15-7/h1-3,17H,(H2,12,16)(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 expressed in bacterial expression system using L-Tryptophan as substrate after 25 mins in absence of GSH and pre... |
Eur J Med Chem 126: 983-996 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.029
BindingDB Entry DOI: 10.7270/Q2RX9F9K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50126144

(CHEMBL3629569 | US10155972, Compound NewLink 1 | U...)Show InChI InChI=1S/C18H22N2O/c21-18(13-6-2-1-3-7-13)10-16-14-8-4-5-9-15(14)17-11-19-12-20(16)17/h4-5,8-9,11-13,16,18,21H,1-3,6-7,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 expressed in bacterial expression system using L-Tryptophan as substrate after 25 mins in absence of GSH and pre... |
Eur J Med Chem 126: 983-996 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.029
BindingDB Entry DOI: 10.7270/Q2RX9F9K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50235921

(CHEMBL584991)Show InChI InChI=1S/C9H7ClFN5O2/c10-5-3-4(1-2-6(5)11)13-9(14-17)7-8(12)16-18-15-7/h1-3,17H,(H2,12,16)(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116160
BindingDB Entry DOI: 10.7270/Q2DF6W0V |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50126144

(CHEMBL3629569 | US10155972, Compound NewLink 1 | U...)Show InChI InChI=1S/C18H22N2O/c21-18(13-6-2-1-3-7-13)10-16-14-8-4-5-9-15(14)17-11-19-12-20(16)17/h4-5,8-9,11-13,16,18,21H,1-3,6-7,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116160
BindingDB Entry DOI: 10.7270/Q2DF6W0V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50289137

(6-Fluoro-3-((E)-2-pyridin-3-yl-vinyl)-1H-indole | ...)Show InChI InChI=1S/C15H11FN2/c16-13-5-6-14-12(10-18-15(14)8-13)4-3-11-2-1-7-17-9-11/h1-10,18H/b4-3+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116160
BindingDB Entry DOI: 10.7270/Q2DF6W0V |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50126144

(CHEMBL3629569 | US10155972, Compound NewLink 1 | U...)Show InChI InChI=1S/C18H22N2O/c21-18(13-6-2-1-3-7-13)10-16-14-8-4-5-9-15(14)17-11-19-12-20(16)17/h4-5,8-9,11-13,16,18,21H,1-3,6-7,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116160
BindingDB Entry DOI: 10.7270/Q2DF6W0V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50235921

(CHEMBL584991)Show InChI InChI=1S/C9H7ClFN5O2/c10-5-3-4(1-2-6(5)11)13-9(14-17)7-8(12)16-18-15-7/h1-3,17H,(H2,12,16)(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116160
BindingDB Entry DOI: 10.7270/Q2DF6W0V |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50126144

(CHEMBL3629569 | US10155972, Compound NewLink 1 | U...)Show InChI InChI=1S/C18H22N2O/c21-18(13-6-2-1-3-7-13)10-16-14-8-4-5-9-15(14)17-11-19-12-20(16)17/h4-5,8-9,11-13,16,18,21H,1-3,6-7,10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116160
BindingDB Entry DOI: 10.7270/Q2DF6W0V |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Mus musculus) | BDBM50444455
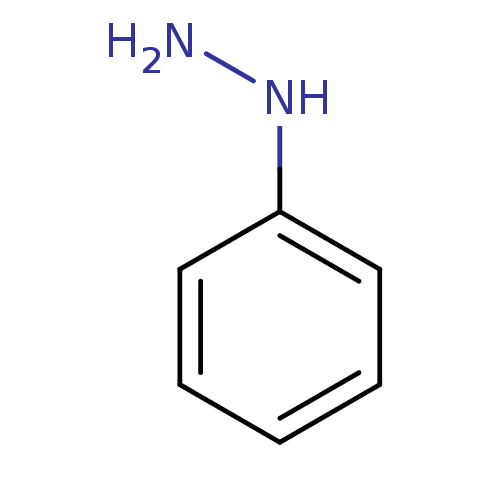
(CHEBI:27924 | Phenylhydrazine)Show InChI InChI=1S/C6H8N2/c7-8-6-4-2-1-3-5-6/h1-5,8H,7H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of mouse IDO1 expressed in mouse LLTC cells using L-tryptophan as substrate after 24 hrs |
Bioorg Med Chem 21: 7595-603 (2013)
Article DOI: 10.1016/j.bmc.2013.10.037
BindingDB Entry DOI: 10.7270/Q2377B6F |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50235867
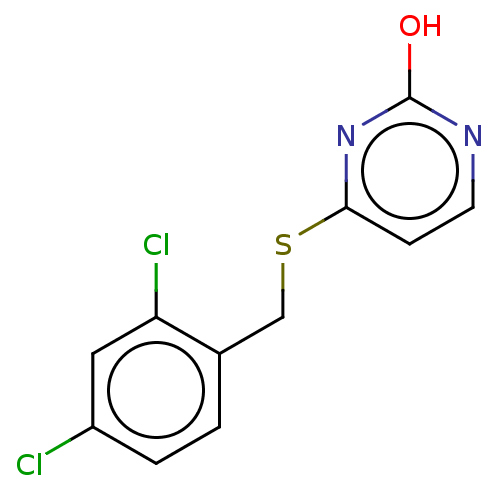
(CHEMBL1351007)Show InChI InChI=1S/C11H8Cl2N2OS/c12-8-2-1-7(9(13)5-8)6-17-10-3-4-14-11(16)15-10/h1-5H,6H2,(H,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged IDO1 expressed in mouse LLTC cells using L-tryptophan as substrate after 24 hrs in presence of... |
Eur J Med Chem 126: 983-996 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.029
BindingDB Entry DOI: 10.7270/Q2RX9F9K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50444455

(CHEBI:27924 | Phenylhydrazine)Show InChI InChI=1S/C6H8N2/c7-8-6-4-2-1-3-5-6/h1-5,8H,7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 using L-tryptophan as substrate after 1 hr |
Bioorg Med Chem 21: 7595-603 (2013)
Article DOI: 10.1016/j.bmc.2013.10.037
BindingDB Entry DOI: 10.7270/Q2377B6F |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50289137

(6-Fluoro-3-((E)-2-pyridin-3-yl-vinyl)-1H-indole | ...)Show InChI InChI=1S/C15H11FN2/c16-13-5-6-14-12(10-18-15(14)8-13)4-3-11-2-1-7-17-9-11/h1-10,18H/b4-3+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116160
BindingDB Entry DOI: 10.7270/Q2DF6W0V |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM24776
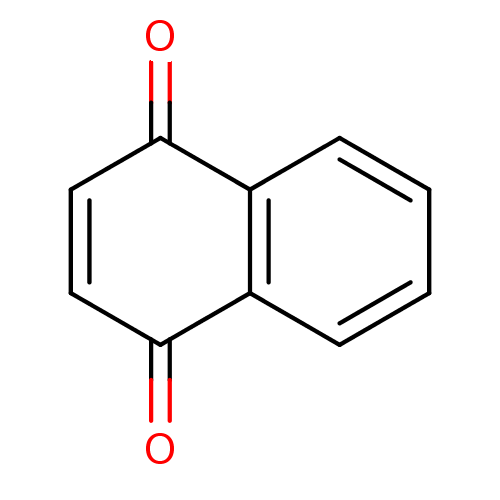
(1,4-Naphthoquinone | 1,4-Naphthoquinone (5a) | 1,4...)Show InChI InChI=1S/C10H6O2/c11-9-5-6-10(12)8-4-2-1-3-7(8)9/h1-6H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Compound was tested in a cell-free SLe-polyacrylamide glycoconjugate binding assay (assay B) in Selectin E |
Eur J Med Chem 126: 983-996 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.029
BindingDB Entry DOI: 10.7270/Q2RX9F9K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50235856

(CHEMBL1976581)Show InChI InChI=1S/C15H11NO2/c1-10-6-2-3-7-11(10)14-15(17)12-8-4-5-9-13(12)16(14)18/h2-9H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin D |
Eur J Med Chem 126: 983-996 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.029
BindingDB Entry DOI: 10.7270/Q2RX9F9K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50126144

(CHEMBL3629569 | US10155972, Compound NewLink 1 | U...)Show InChI InChI=1S/C18H22N2O/c21-18(13-6-2-1-3-7-13)10-16-14-8-4-5-9-15(14)17-11-19-12-20(16)17/h4-5,8-9,11-13,16,18,21H,1-3,6-7,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged IDO1 expressed in mouse LLTC cells using L-tryptophan as substrate after 24 hrs |
Eur J Med Chem 126: 983-996 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.029
BindingDB Entry DOI: 10.7270/Q2RX9F9K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50235867

(CHEMBL1351007)Show InChI InChI=1S/C11H8Cl2N2OS/c12-8-2-1-7(9(13)5-8)6-17-10-3-4-14-11(16)15-10/h1-5H,6H2,(H,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 expressed in bacterial expression system using L-Tryptophan as substrate after 25 mins in presence of GSH and tw... |
Eur J Med Chem 126: 983-996 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.029
BindingDB Entry DOI: 10.7270/Q2RX9F9K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50235877

(CHEMBL4105391)Show InChI InChI=1S/C13H11ClN2O2S/c14-10-1-7-13(8-2-10)19-9-15-11-3-5-12(6-4-11)16(17)18/h1-8,15H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Compound was tested in a cell-free SLe-polyacrylamide glycoconjugate binding assay (assay B) in Selectin E |
Eur J Med Chem 126: 983-996 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.029
BindingDB Entry DOI: 10.7270/Q2RX9F9K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM64800

(1-oxidanidyl-2-phenyl-indol-1-ium-3-one | 1-oxido-...)Show InChI InChI=1S/C14H9NO2/c16-14-11-8-4-5-9-12(11)15(17)13(14)10-6-2-1-3-7-10/h1-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 expressed in bacterial expression system using L-Tryptophan as substrate after 25 mins in absence of GSH and pre... |
Eur J Med Chem 126: 983-996 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.029
BindingDB Entry DOI: 10.7270/Q2RX9F9K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM24663
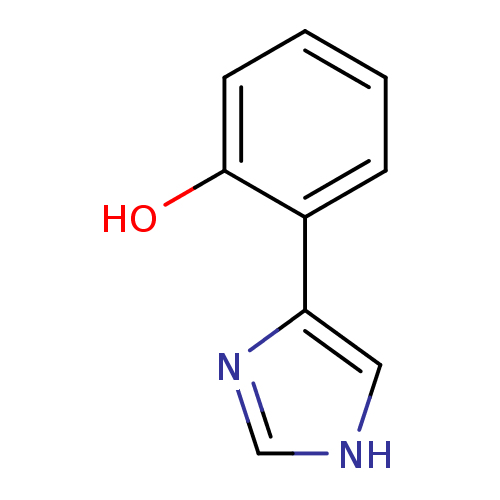
(2-(1H-imidazol-4-yl)phenol | 2-(1H-imidazol-4-yl)p...)Show InChI InChI=1S/C9H8N2O/c12-9-4-2-1-3-7(9)8-5-10-6-11-8/h1-6,12H,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 expressed in bacterial expression system using L-Tryptophan as substrate after 25 mins in absence of GSH and pre... |
Eur J Med Chem 126: 983-996 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.029
BindingDB Entry DOI: 10.7270/Q2RX9F9K |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50444455

(CHEBI:27924 | Phenylhydrazine)Show InChI InChI=1S/C6H8N2/c7-8-6-4-2-1-3-5-6/h1-5,8H,7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human IDO1 expressed in mouse LLTC cells using L-tryptophan as substrate after 24 hrs |
Bioorg Med Chem 21: 7595-603 (2013)
Article DOI: 10.1016/j.bmc.2013.10.037
BindingDB Entry DOI: 10.7270/Q2377B6F |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50013793

(CHEMBL3192687)Show InChI InChI=1S/C15H11NO3S2/c17-14-8-7-11-4-1-2-5-12(11)13(14)10-16-21(18,19)15-6-3-9-20-15/h1-10,17H/b16-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116160
BindingDB Entry DOI: 10.7270/Q2DF6W0V |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50444457

(CHEMBL3092383)Show InChI InChI=1S/C7H6ClN3S/c8-4-1-2-6-5(3-4)10-7(11-9)12-6/h1-3H,9H2,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 using L-tryptophan as substrate after 1 hr |
Bioorg Med Chem 21: 7595-603 (2013)
Article DOI: 10.1016/j.bmc.2013.10.037
BindingDB Entry DOI: 10.7270/Q2377B6F |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50235919

(CHEMBL577939)Show SMILES COc1cc2C(=O)C(c3ccccc3)=[N+]([O-])c2cc1OC |t:14| Show InChI InChI=1S/C16H13NO4/c1-20-13-8-11-12(9-14(13)21-2)17(19)15(16(11)18)10-6-4-3-5-7-10/h3-9H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 expressed in bacterial expression system using L-Tryptophan as substrate after 25 mins in presence of GSH and tw... |
Eur J Med Chem 126: 983-996 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.029
BindingDB Entry DOI: 10.7270/Q2RX9F9K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50235869
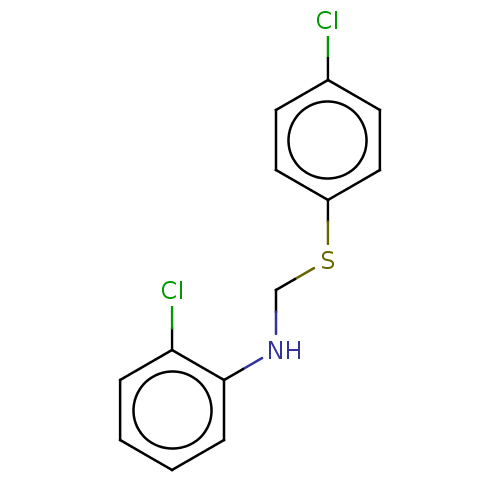
(CHEMBL1600105)Show InChI InChI=1S/C13H11Cl2NS/c14-10-5-7-11(8-6-10)17-9-16-13-4-2-1-3-12(13)15/h1-8,16H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 expressed in bacterial expression system using L-Tryptophan as substrate after 25 mins in presence of GSH and tw... |
Eur J Med Chem 126: 983-996 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.029
BindingDB Entry DOI: 10.7270/Q2RX9F9K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50235857
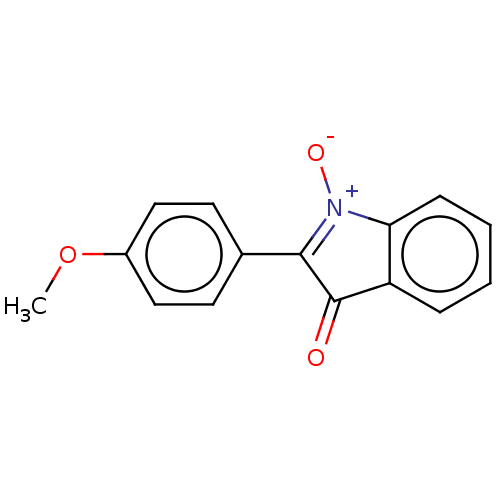
(CHEMBL581422 | TCMDC-123940)Show SMILES COc1ccc(cc1)C1=[N+]([O-])c2ccccc2C1=O |c:9| Show InChI InChI=1S/C15H11NO3/c1-19-11-8-6-10(7-9-11)14-15(17)12-4-2-3-5-13(12)16(14)18/h2-9H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 expressed in bacterial expression system using L-Tryptophan as substrate after 25 mins in absence of GSH and pre... |
Eur J Med Chem 126: 983-996 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.029
BindingDB Entry DOI: 10.7270/Q2RX9F9K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50235857

(CHEMBL581422 | TCMDC-123940)Show SMILES COc1ccc(cc1)C1=[N+]([O-])c2ccccc2C1=O |c:9| Show InChI InChI=1S/C15H11NO3/c1-19-11-8-6-10(7-9-11)14-15(17)12-4-2-3-5-13(12)16(14)18/h2-9H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasmepsin-2 from Plasmodium falciparum. |
Eur J Med Chem 126: 983-996 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.029
BindingDB Entry DOI: 10.7270/Q2RX9F9K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50568051
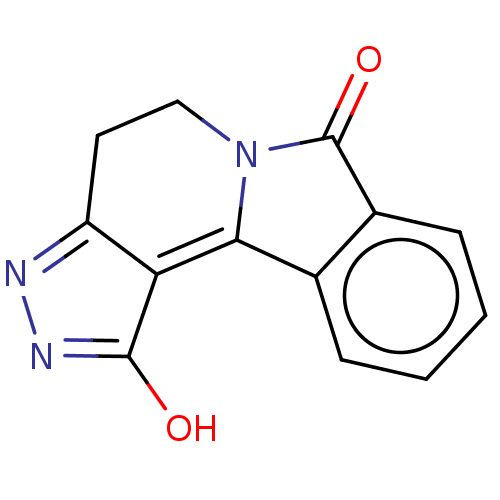
(CHEMBL4092918)Show SMILES OC1=NN=C2CCN3C(=O)c4ccccc4C3=C12 |t:1,3,18| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116160
BindingDB Entry DOI: 10.7270/Q2DF6W0V |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50235883
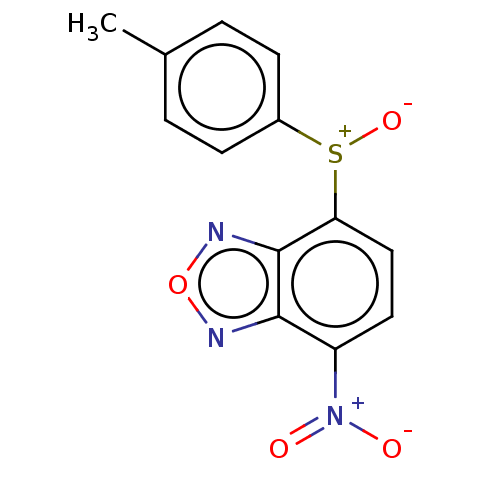
(CHEMBL1702248)Show SMILES Cc1ccc(cc1)[S+]([O-])c1ccc([N+]([O-])=O)c2nonc12 Show InChI InChI=1S/C13H9N3O4S/c1-8-2-4-9(5-3-8)21(19)11-7-6-10(16(17)18)12-13(11)15-20-14-12/h2-7H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 expressed in bacterial expression system using L-Tryptophan as substrate after 25 mins in presence of GSH and tw... |
Eur J Med Chem 126: 983-996 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.029
BindingDB Entry DOI: 10.7270/Q2RX9F9K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50126143

(Epacadostat | INCB-024360)Show SMILES NS(=O)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C11H13BrFN7O4S/c12-7-5-6(1-2-8(7)13)17-11(18-21)9-10(20-24-19-9)15-3-4-16-25(14,22)23/h1-2,5,16,21H,3-4H2,(H,15,20)(H,17,18)(H2,14,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116160
BindingDB Entry DOI: 10.7270/Q2DF6W0V |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM64800

(1-oxidanidyl-2-phenyl-indol-1-ium-3-one | 1-oxido-...)Show InChI InChI=1S/C14H9NO2/c16-14-11-8-4-5-9-12(11)15(17)13(14)10-6-2-1-3-7-10/h1-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Compound was tested in a cell-free SLe-polyacrylamide glycoconjugate binding assay (assay B) in P-selectin |
Eur J Med Chem 126: 983-996 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.029
BindingDB Entry DOI: 10.7270/Q2RX9F9K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50568048

(CHEMBL1981840) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116160
BindingDB Entry DOI: 10.7270/Q2DF6W0V |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM58106
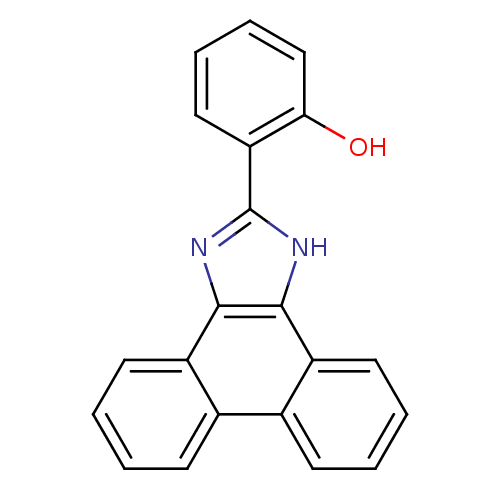
(6-(1,3-dihydrophenanthr[9,10-d]imidazol-2-ylidene)...)Show InChI InChI=1S/C21H14N2O/c24-18-12-6-5-11-17(18)21-22-19-15-9-3-1-7-13(15)14-8-2-4-10-16(14)20(19)23-21/h1-12,24H,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 expressed in bacterial expression system using L-Tryptophan as substrate after 25 mins in absence of GSH and pre... |
Eur J Med Chem 126: 983-996 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.029
BindingDB Entry DOI: 10.7270/Q2RX9F9K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50126143

(Epacadostat | INCB-024360)Show SMILES NS(=O)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C11H13BrFN7O4S/c12-7-5-6(1-2-8(7)13)17-11(18-21)9-10(20-24-19-9)15-3-4-16-25(14,22)23/h1-2,5,16,21H,3-4H2,(H,15,20)(H,17,18)(H2,14,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116160
BindingDB Entry DOI: 10.7270/Q2DF6W0V |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50235921

(CHEMBL584991)Show InChI InChI=1S/C9H7ClFN5O2/c10-5-3-4(1-2-6(5)11)13-9(14-17)7-8(12)16-18-15-7/h1-3,17H,(H2,12,16)(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116160
BindingDB Entry DOI: 10.7270/Q2DF6W0V |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50235921

(CHEMBL584991)Show InChI InChI=1S/C9H7ClFN5O2/c10-5-3-4(1-2-6(5)11)13-9(14-17)7-8(12)16-18-15-7/h1-3,17H,(H2,12,16)(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116160
BindingDB Entry DOI: 10.7270/Q2DF6W0V |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50568050
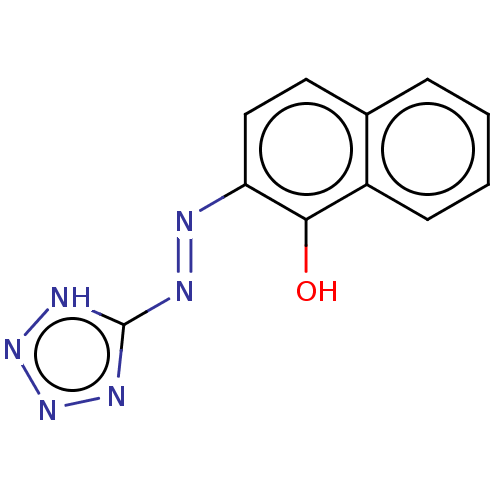
(CHEMBL4296947) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116160
BindingDB Entry DOI: 10.7270/Q2DF6W0V |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50235856

(CHEMBL1976581)Show InChI InChI=1S/C15H11NO2/c1-10-6-2-3-7-11(10)14-15(17)12-8-4-5-9-13(12)16(14)18/h2-9H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Compound was tested in a cell-free SLe-polyacrylamide glycoconjugate binding assay (assay B) in Selectin E |
Eur J Med Chem 126: 983-996 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.029
BindingDB Entry DOI: 10.7270/Q2RX9F9K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50235919

(CHEMBL577939)Show SMILES COc1cc2C(=O)C(c3ccccc3)=[N+]([O-])c2cc1OC |t:14| Show InChI InChI=1S/C16H13NO4/c1-20-13-8-11-12(9-14(13)21-2)17(19)15(16(11)18)10-6-4-3-5-7-10/h3-9H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Compound was tested in a cell-free SLe-polyacrylamide glycoconjugate binding assay (assay B) in P-selectin |
Eur J Med Chem 126: 983-996 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.029
BindingDB Entry DOI: 10.7270/Q2RX9F9K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50235919

(CHEMBL577939)Show SMILES COc1cc2C(=O)C(c3ccccc3)=[N+]([O-])c2cc1OC |t:14| Show InChI InChI=1S/C16H13NO4/c1-20-13-8-11-12(9-14(13)21-2)17(19)15(16(11)18)10-6-4-3-5-7-10/h3-9H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged IDO1 expressed in mouse LLTC cells using L-tryptophan as substrate after 24 hrs |
Eur J Med Chem 126: 983-996 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.029
BindingDB Entry DOI: 10.7270/Q2RX9F9K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50235867

(CHEMBL1351007)Show InChI InChI=1S/C11H8Cl2N2OS/c12-8-2-1-7(9(13)5-8)6-17-10-3-4-14-11(16)15-10/h1-5H,6H2,(H,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 expressed in bacterial expression system using L-Tryptophan as substrate after 25 mins in absence of GSH and twe... |
Eur J Med Chem 126: 983-996 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.029
BindingDB Entry DOI: 10.7270/Q2RX9F9K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50568051

(CHEMBL4092918)Show SMILES OC1=NN=C2CCN3C(=O)c4ccccc4C3=C12 |t:1,3,18| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116160
BindingDB Entry DOI: 10.7270/Q2DF6W0V |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50568049

(CHEMBL1487639) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human IDO1 expressed in Escherichia coli EC538 assessed as reduction in N-formylkynurenine formation using L-tryptophan as ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116160
BindingDB Entry DOI: 10.7270/Q2DF6W0V |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50235888

(CHEMBL2409283)Show InChI InChI=1S/C13H9N3O3S/c1-8-2-4-9(5-3-8)20-11-7-6-10(16(17)18)12-13(11)15-19-14-12/h2-7H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged IDO1 expressed in mouse LLTC cells using L-tryptophan as substrate after 24 hrs |
Eur J Med Chem 126: 983-996 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.029
BindingDB Entry DOI: 10.7270/Q2RX9F9K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50235867

(CHEMBL1351007)Show InChI InChI=1S/C11H8Cl2N2OS/c12-8-2-1-7(9(13)5-8)6-17-10-3-4-14-11(16)15-10/h1-5H,6H2,(H,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged IDO1 expressed in mouse LLTC cells using L-tryptophan as substrate after 24 hrs |
Eur J Med Chem 126: 983-996 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.029
BindingDB Entry DOI: 10.7270/Q2RX9F9K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data