

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Homo sapiens (Human)) | BDBM50092532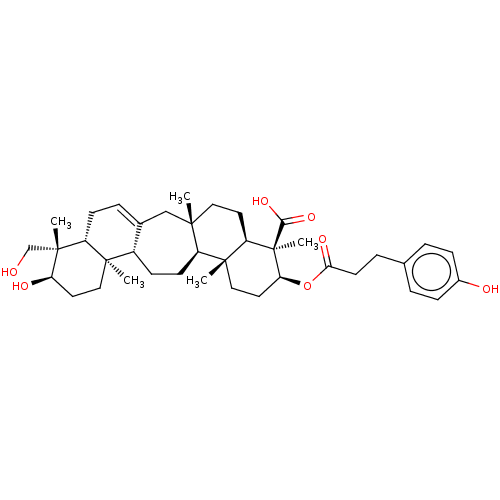 (CHEMBL3586200) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) by Dixon plot analysis | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50092540 (CHEMBL3586207) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) by Dixon plot analysis | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50092531 (CHEMBL3586199) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) by Dixon plot analysis | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50092529 (CHEMBL3586197) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) by Dixon plot analysis | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50092533 (CHEMBL3586201) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) by Dixon plot analysis | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50092532 (CHEMBL3586200) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) by Dixon plot analysis | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM24567 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenyl butyrate as substrate assessed as formation of p-nitrophenol preincubated for 10 mins fol... | Bioorg Med Chem Lett 26: 2788-2794 (2016) Article DOI: 10.1016/j.bmcl.2016.04.066 BindingDB Entry DOI: 10.7270/Q2WD42GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50303006 (CHEMBL517247 | mulberrofuran D) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenyl butyrate as substrate assessed as formation of p-nitrophenol preincubated for 10 mins fol... | Bioorg Med Chem Lett 26: 2788-2794 (2016) Article DOI: 10.1016/j.bmcl.2016.04.066 BindingDB Entry DOI: 10.7270/Q2WD42GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50203126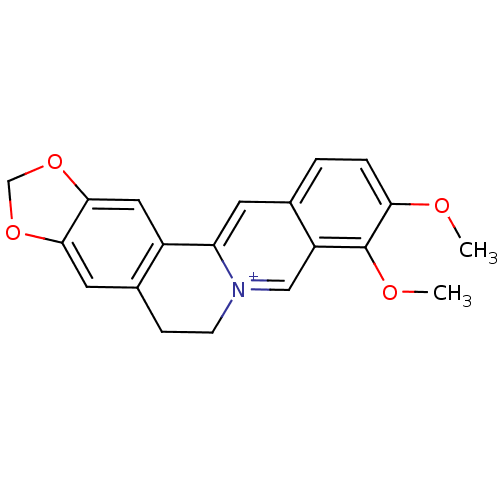 (3,4-dimethoxy-6,7-dihydro-[1,3]dioxolo[4,5-g]pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as substrate hydrolysis by spectrophotometric/Ellman method | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50092540 (CHEMBL3586207) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as substrate hydrolysis by spectrophotometric/Ellman method | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50092529 (CHEMBL3586197) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 by fluorescence resonance energy transfer (FRET) assay | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50092537 (CHEMBL3586204) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 by fluorescence resonance energy transfer (FRET) assay | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50168024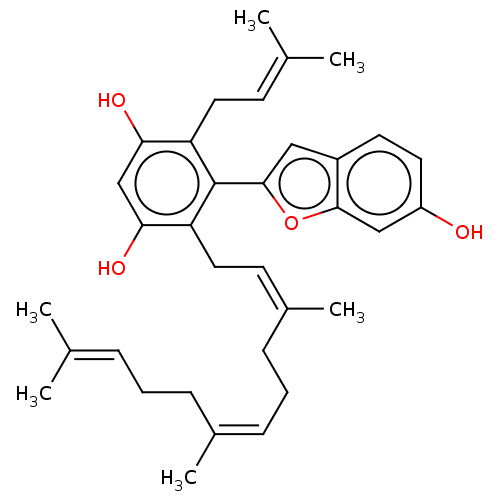 (CHEMBL3800462) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenyl butyrate as substrate assessed as formation of p-nitrophenol preincubated for 10 mins fol... | Bioorg Med Chem Lett 26: 2788-2794 (2016) Article DOI: 10.1016/j.bmcl.2016.04.066 BindingDB Entry DOI: 10.7270/Q2WD42GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50092532 (CHEMBL3586200) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine as substrate assessed as substrate hydrolysis by spectrophotometric/Ellman method | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50168022 (CHEMBL3797266) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenyl butyrate as substrate assessed as formation of p-nitrophenol preincubated for 10 mins fol... | Bioorg Med Chem Lett 26: 2788-2794 (2016) Article DOI: 10.1016/j.bmcl.2016.04.066 BindingDB Entry DOI: 10.7270/Q2WD42GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50168023 (CHEMBL3800628) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenyl butyrate as substrate assessed as formation of p-nitrophenol preincubated for 10 mins fol... | Bioorg Med Chem Lett 26: 2788-2794 (2016) Article DOI: 10.1016/j.bmcl.2016.04.066 BindingDB Entry DOI: 10.7270/Q2WD42GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50303004 (CHEMBL562810 | mulberrofuran W) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenyl butyrate as substrate assessed as formation of p-nitrophenol preincubated for 10 mins fol... | Bioorg Med Chem Lett 26: 2788-2794 (2016) Article DOI: 10.1016/j.bmcl.2016.04.066 BindingDB Entry DOI: 10.7270/Q2WD42GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50168019 (CHEMBL3798170) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenyl butyrate as substrate assessed as formation of p-nitrophenol preincubated for 10 mins fol... | Bioorg Med Chem Lett 26: 2788-2794 (2016) Article DOI: 10.1016/j.bmcl.2016.04.066 BindingDB Entry DOI: 10.7270/Q2WD42GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50168021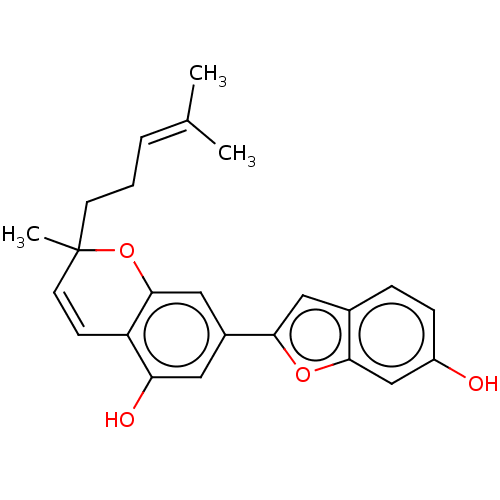 (CHEMBL3800241) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenyl butyrate as substrate assessed as formation of p-nitrophenol preincubated for 10 mins fol... | Bioorg Med Chem Lett 26: 2788-2794 (2016) Article DOI: 10.1016/j.bmcl.2016.04.066 BindingDB Entry DOI: 10.7270/Q2WD42GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50168018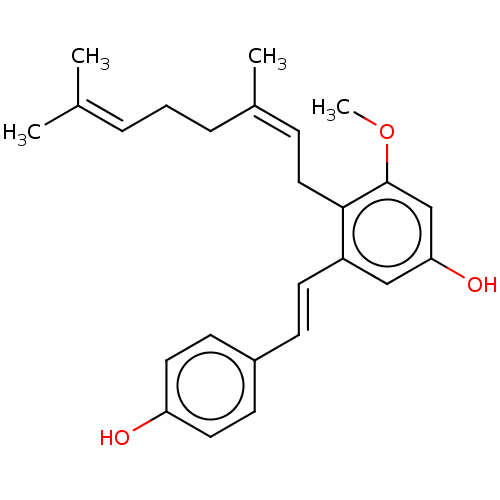 (CHEMBL3798236) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenyl butyrate as substrate assessed as formation of p-nitrophenol preincubated for 10 mins fol... | Bioorg Med Chem Lett 26: 2788-2794 (2016) Article DOI: 10.1016/j.bmcl.2016.04.066 BindingDB Entry DOI: 10.7270/Q2WD42GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50092531 (CHEMBL3586199) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as substrate hydrolysis by spectrophotometric/Ellman method | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pancreatic triacylglycerol lipase (Sus scrofa (Pig)) | BDBM50168020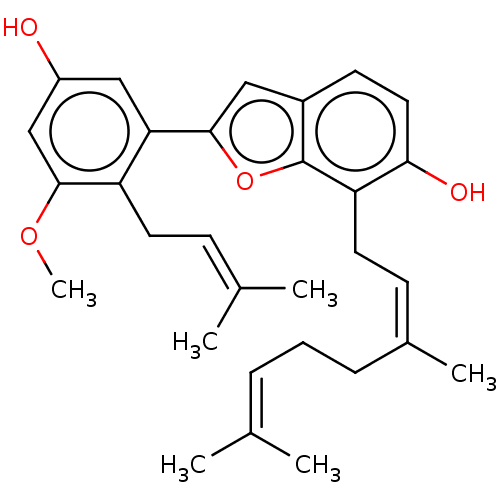 (CHEMBL3797932) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic lipase using p-nitrophenyl butyrate as substrate assessed as formation of p-nitrophenol preincubated for 10 mins fol... | Bioorg Med Chem Lett 26: 2788-2794 (2016) Article DOI: 10.1016/j.bmcl.2016.04.066 BindingDB Entry DOI: 10.7270/Q2WD42GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50092530 (CHEMBL3586198) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 by fluorescence resonance energy transfer (FRET) assay | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50092527 (CHEMBL3586196) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 by fluorescence resonance energy transfer (FRET) assay | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50203126 (3,4-dimethoxy-6,7-dihydro-[1,3]dioxolo[4,5-g]pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine as substrate assessed as substrate hydrolysis by spectrophotometric/Ellman method | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50092533 (CHEMBL3586201) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine as substrate assessed as substrate hydrolysis by spectrophotometric/Ellman method | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50092532 (CHEMBL3586200) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as substrate hydrolysis by spectrophotometric/Ellman method | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 by fluorescence resonance energy transfer (FRET) assay | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50092540 (CHEMBL3586207) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 by fluorescence resonance energy transfer (FRET) assay | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50092530 (CHEMBL3586198) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as substrate hydrolysis by spectrophotometric/Ellman method | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50092532 (CHEMBL3586200) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 by fluorescence resonance energy transfer (FRET) assay | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50092533 (CHEMBL3586201) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 by fluorescence resonance energy transfer (FRET) assay | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50092538 (CHEMBL3586205) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 by fluorescence resonance energy transfer (FRET) assay | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50092534 (CHEMBL3586202) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 by fluorescence resonance energy transfer (FRET) assay | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50092531 (CHEMBL3586199) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 by fluorescence resonance energy transfer (FRET) assay | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50092539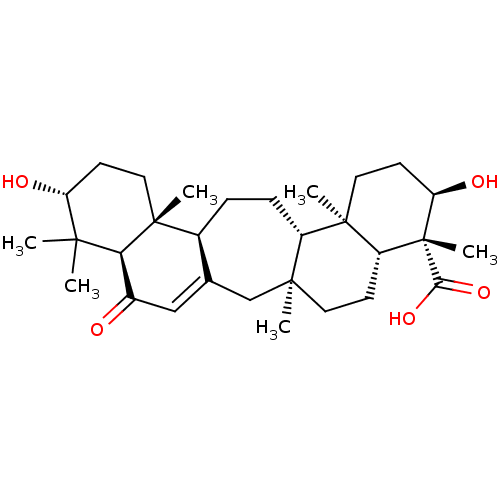 (CHEMBL3586206) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 by fluorescence resonance energy transfer (FRET) assay | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50092536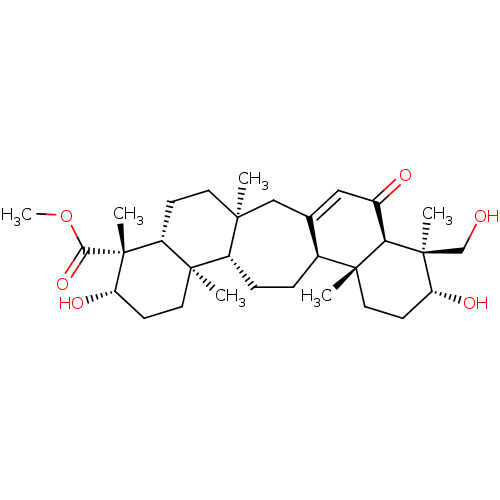 (LYCERNUIC KETONE A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 by fluorescence resonance energy transfer (FRET) assay | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50092535 (CHEMBL3586203) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 by fluorescence resonance energy transfer (FRET) assay | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50092529 (CHEMBL3586197) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as substrate hydrolysis by spectrophotometric/Ellman method | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50092534 (CHEMBL3586202) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as substrate hydrolysis by spectrophotometric/Ellman method | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50092533 (CHEMBL3586201) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as substrate hydrolysis by spectrophotometric/Ellman method | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50092527 (CHEMBL3586196) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as substrate hydrolysis by spectrophotometric/Ellman method | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50092535 (CHEMBL3586203) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as substrate hydrolysis by spectrophotometric/Ellman method | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50092536 (LYCERNUIC KETONE A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as substrate hydrolysis by spectrophotometric/Ellman method | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50092537 (CHEMBL3586204) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as substrate hydrolysis by spectrophotometric/Ellman method | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50092538 (CHEMBL3586205) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as substrate hydrolysis by spectrophotometric/Ellman method | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50092539 (CHEMBL3586206) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as substrate hydrolysis by spectrophotometric/Ellman method | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50092527 (CHEMBL3586196) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine as substrate assessed as substrate hydrolysis by spectrophotometric/Ellman method | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50092529 (CHEMBL3586197) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine as substrate assessed as substrate hydrolysis by spectrophotometric/Ellman method | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50092530 (CHEMBL3586198) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using butyrylthiocholine as substrate assessed as substrate hydrolysis by spectrophotometric/Ellman method | Bioorg Med Chem 23: 3126-34 (2015) Article DOI: 10.1016/j.bmc.2015.04.080 BindingDB Entry DOI: 10.7270/Q24X59JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 58 total ) | Next | Last >> |