

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001609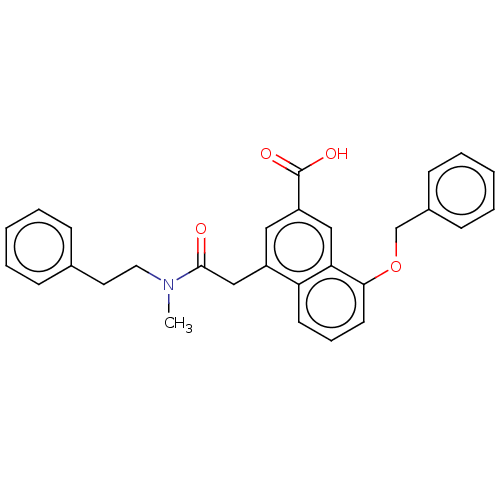 (8-Benzyloxy-4-[(methyl-phenethyl-carbamoyl)-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Antagonistic activity of the compound against LTB4 receptor using guinea pig (GP) spleen cell membrane | J Med Chem 35: 4253-5 (1992) BindingDB Entry DOI: 10.7270/Q208648W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001609 (8-Benzyloxy-4-[(methyl-phenethyl-carbamoyl)-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Antagonistic activity of the compound against monkey neutrophil LTB4 receptor 2 min after an iv dose of 3 mg/kg . | J Med Chem 35: 4253-5 (1992) BindingDB Entry DOI: 10.7270/Q208648W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001609 (8-Benzyloxy-4-[(methyl-phenethyl-carbamoyl)-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Affinity of the compound for human PMN LTB-4 receptors. | J Med Chem 35: 4253-5 (1992) BindingDB Entry DOI: 10.7270/Q208648W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001609 (8-Benzyloxy-4-[(methyl-phenethyl-carbamoyl)-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Concentration of the compound inhibiting 1 nM LTB4-induced aggregation in GP polymorphonuclear (PMN) leukocytes. | J Med Chem 35: 4253-5 (1992) BindingDB Entry DOI: 10.7270/Q208648W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001609 (8-Benzyloxy-4-[(methyl-phenethyl-carbamoyl)-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Concentration of the compound inhibiting the binding of [3H]-LTB4 to human whole cell neutrophils | J Med Chem 35: 4253-5 (1992) BindingDB Entry DOI: 10.7270/Q208648W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001609 (8-Benzyloxy-4-[(methyl-phenethyl-carbamoyl)-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Affinity of the compound for guinea pig PMN LTB-4 receptors. | J Med Chem 35: 4253-5 (1992) BindingDB Entry DOI: 10.7270/Q208648W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001611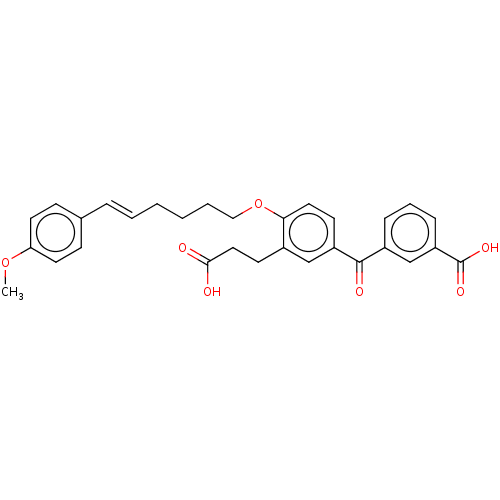 ((E)-3-{3-(2-Carboxy-ethyl)-4-[6-(4-methoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Antagonistic activity of the compound against human polymorphonuclear (PMN) LTB4 receptor | J Med Chem 35: 4253-5 (1992) BindingDB Entry DOI: 10.7270/Q208648W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001610 (7-[3-(4-Acetyl-3-methoxy-2-propyl-phenoxy)-propoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Concentration of the compound inhibiting the binding of [3H]-LTB4 to human neutrophils | J Med Chem 35: 4253-5 (1992) BindingDB Entry DOI: 10.7270/Q208648W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001612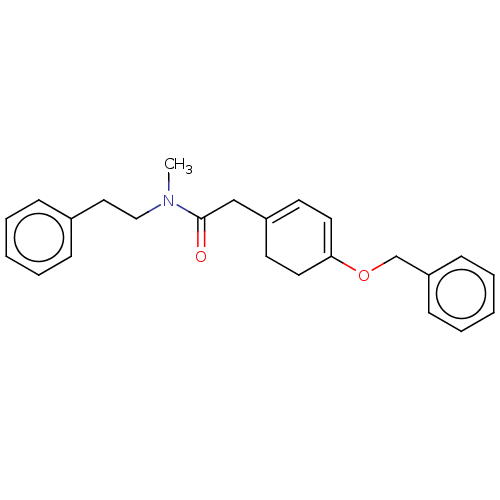 (2-(4-Benzyloxy-cyclohexa-1,3-dienyl)-N-methyl-N-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Antagonistic activity of the compound in human polymorphonuclear (PMN) LTB4 receptor | J Med Chem 35: 4253-5 (1992) BindingDB Entry DOI: 10.7270/Q208648W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||