

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50362592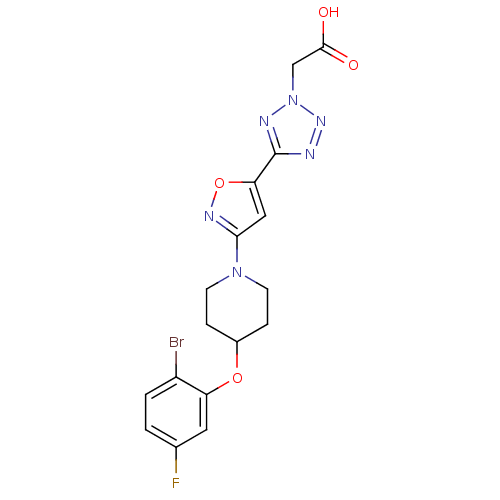 (CHEMBL1938870) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of mouse SCD-1 | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50362586 (CHEMBL1938874) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of mouse SCD-1 | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362586 (CHEMBL1938874) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat liver microsomes assessed as reduction in [1-14C] stearoyl CoA desaturation by scintillation counting | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50362583 (CHEMBL1938871) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of mouse SCD-1 | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50362587 (CHEMBL1938875) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of mouse SCD-1 | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362583 (CHEMBL1938871) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat liver microsomes assessed as reduction in [1-14C] stearoyl CoA desaturation by scintillation counting | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50362583 (CHEMBL1938871) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human SCD-1 | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362587 (CHEMBL1938875) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat liver microsomes assessed as reduction in [1-14C] stearoyl CoA desaturation by scintillation counting | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362588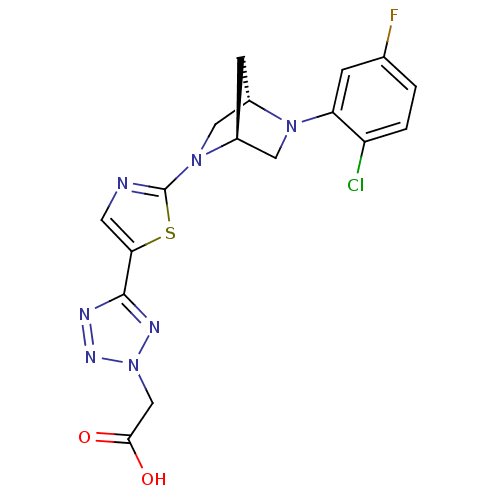 (CHEMBL1938876) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat liver microsomes assessed as reduction in [1-14C] stearoyl CoA desaturation by scintillation counting | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50175297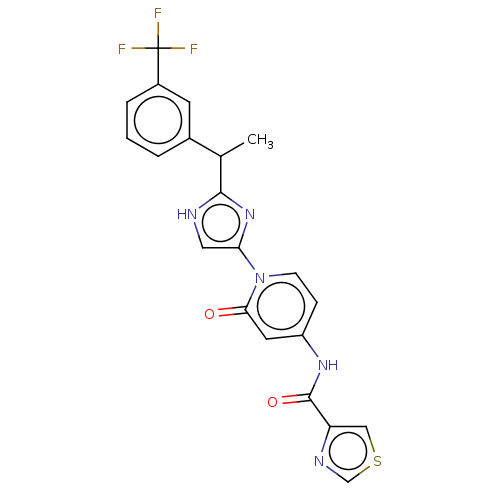 (CHEMBL3810245) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362584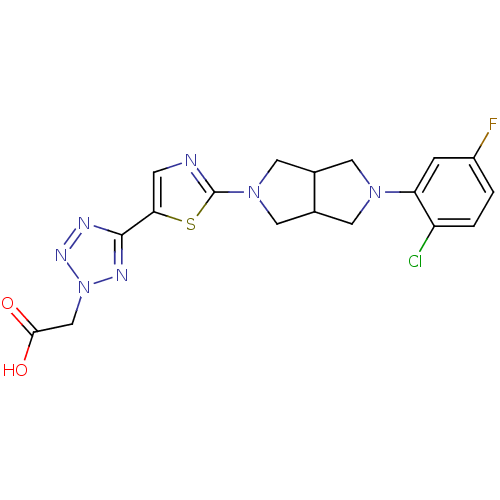 (CHEMBL1938872) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat liver microsomes assessed as reduction in [1-14C] stearoyl CoA desaturation by scintillation counting | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362591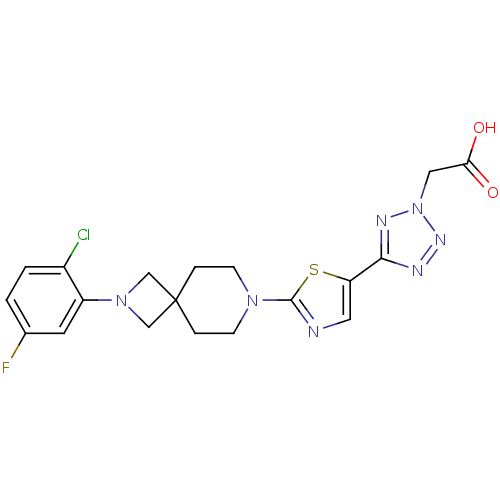 (CHEMBL1938879) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat liver microsomes assessed as reduction in [1-14C] stearoyl CoA desaturation by scintillation counting | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362589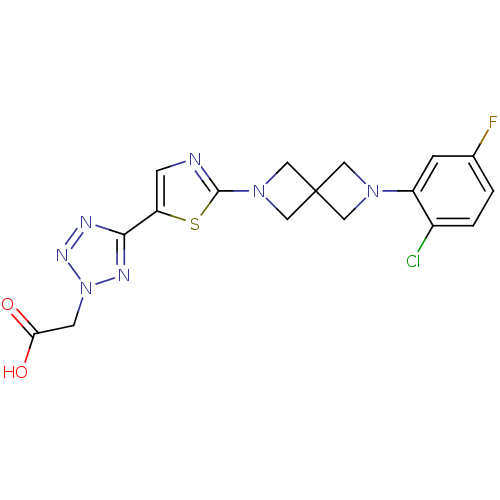 (CHEMBL1938877) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat liver microsomes assessed as reduction in [1-14C] stearoyl CoA desaturation by scintillation counting | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362585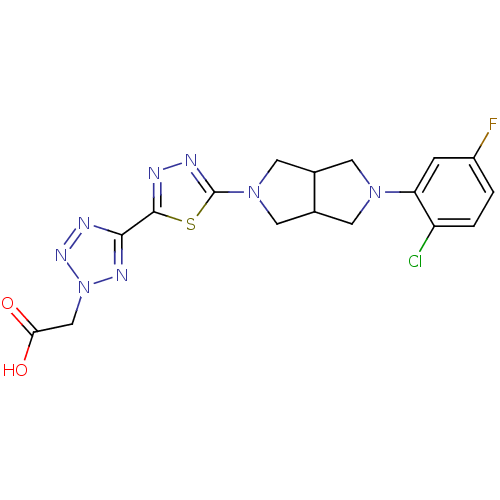 (CHEMBL1938873) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat liver microsomes assessed as reduction in [1-14C] stearoyl CoA desaturation by scintillation counting | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362589 (CHEMBL1938877) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat hepatocytes expressing organic anion transporting polypeptides assessed as reduction in [1-14C] stearoyl CoA desa... | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362586 (CHEMBL1938874) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat hepatocytes expressing organic anion transporting polypeptides assessed as reduction in [1-14C] stearoyl CoA desa... | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362590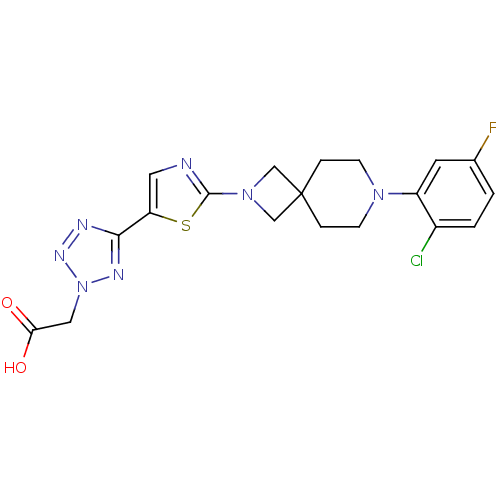 (CHEMBL1938878) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat liver microsomes assessed as reduction in [1-14C] stearoyl CoA desaturation by scintillation counting | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362587 (CHEMBL1938875) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 134 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat hepatocytes expressing organic anion transporting polypeptides assessed as reduction in [1-14C] stearoyl CoA desa... | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50175302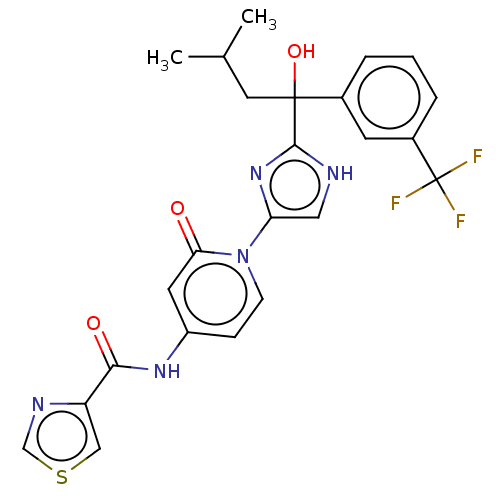 (CHEMBL3808401) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362588 (CHEMBL1938876) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 176 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat hepatocytes expressing organic anion transporting polypeptides assessed as reduction in [1-14C] stearoyl CoA desa... | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50175303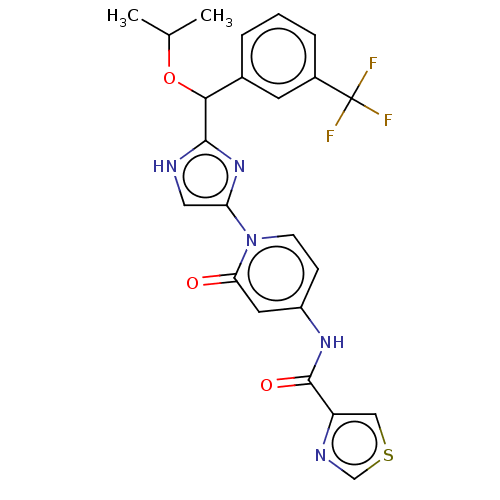 (CHEMBL3810042) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C8 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362583 (CHEMBL1938871) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 198 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat hepatocytes expressing organic anion transporting polypeptides assessed as reduction in [1-14C] stearoyl CoA desa... | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362584 (CHEMBL1938872) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 233 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat hepatocytes expressing organic anion transporting polypeptides assessed as reduction in [1-14C] stearoyl CoA desa... | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase 5 (Homo sapiens (Human)) | BDBM50362583 (CHEMBL1938871) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 242 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human SCD-5 | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362585 (CHEMBL1938873) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat hepatocytes expressing organic anion transporting polypeptides assessed as reduction in [1-14C] stearoyl CoA desa... | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362591 (CHEMBL1938879) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 438 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat hepatocytes expressing organic anion transporting polypeptides assessed as reduction in [1-14C] stearoyl CoA desa... | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362590 (CHEMBL1938878) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 506 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat hepatocytes expressing organic anion transporting polypeptides assessed as reduction in [1-14C] stearoyl CoA desa... | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50175303 (CHEMBL3810042) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50175304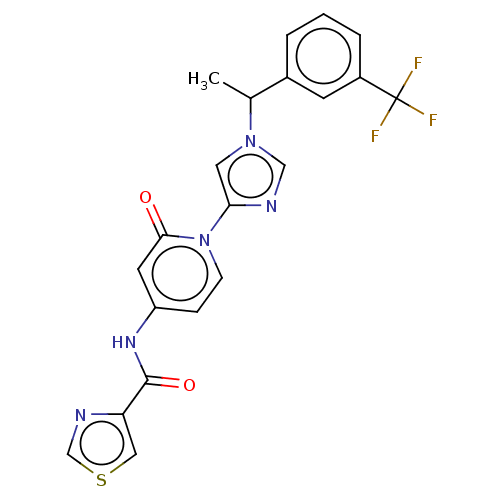 (CHEMBL3808842) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C8 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50175296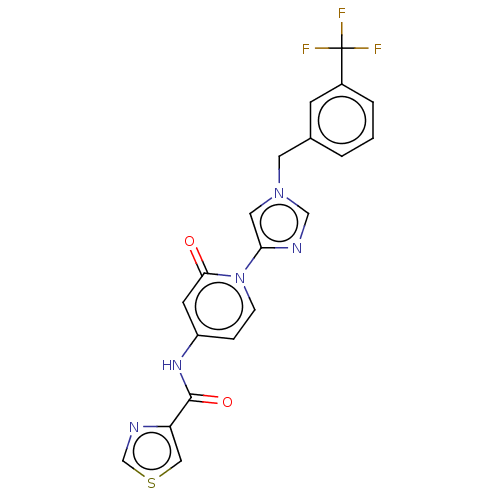 (CHEMBL3809896) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50175296 (CHEMBL3809896) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50175304 (CHEMBL3808842) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50362586 (CHEMBL1938874) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in organic anion transporting polypeptides-deficient human HepG2 cells assessed as reduction in [1-14C] stearoyl CoA des... | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50175299 (CHEMBL3808805) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50362587 (CHEMBL1938875) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in organic anion transporting polypeptides-deficient human HepG2 cells assessed as reduction in [1-14C] stearoyl CoA des... | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50175298 (CHEMBL3809890) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C8 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50093526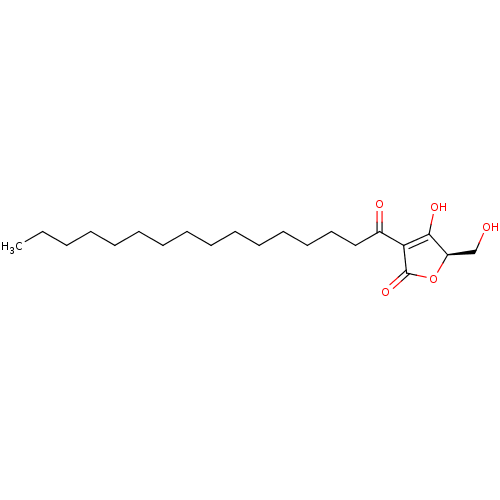 (CHEMBL426373 | RK-682) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Natural Product and Medicinal Chemistry Lab, Faculty of Chemistry, VNUHCM-University of Science, 227 Nguyen Van Cu, Ho Chi Minh City, Viet Nam. Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B pre-incubated for 10 mins before pnitrophenyl phosphate substrate addition and measured every 30 secs for 10 mi... | Bioorg Med Chem Lett 27: 3301-3304 (2017) Article DOI: 10.1016/j.bmcl.2017.06.021 BindingDB Entry DOI: 10.7270/Q20Z75QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50175299 (CHEMBL3808805) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C8 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50175298 (CHEMBL3809890) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50268304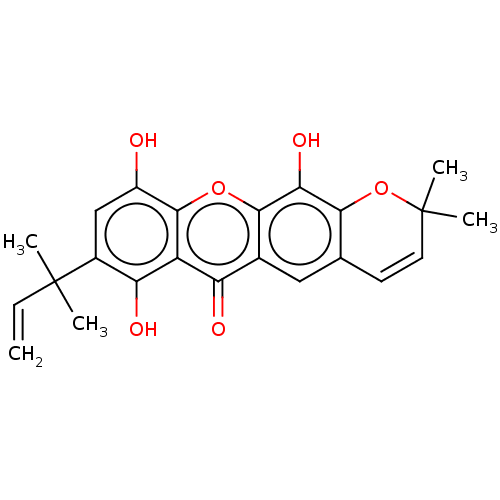 (CHEMBL4086905) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Natural Product and Medicinal Chemistry Lab, Faculty of Chemistry, VNUHCM-University of Science, 227 Nguyen Van Cu, Ho Chi Minh City, Viet Nam. Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B pre-incubated for 10 mins before pnitrophenyl phosphate substrate addition and measured every 30 secs for 10 mi... | Bioorg Med Chem Lett 27: 3301-3304 (2017) Article DOI: 10.1016/j.bmcl.2017.06.021 BindingDB Entry DOI: 10.7270/Q20Z75QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50362588 (CHEMBL1938876) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in organic anion transporting polypeptides-deficient human HepG2 cells assessed as reduction in [1-14C] stearoyl CoA des... | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50362583 (CHEMBL1938871) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in organic anion transporting polypeptides-deficient human HepG2 cells assessed as reduction in [1-14C] stearoyl CoA des... | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50362585 (CHEMBL1938873) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in organic anion transporting polypeptides-deficient human HepG2 cells assessed as reduction in [1-14C] stearoyl CoA des... | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50175300 (CHEMBL3810414) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50175301 (CHEMBL3809367) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50362584 (CHEMBL1938872) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in organic anion transporting polypeptides-deficient human HepG2 cells assessed as reduction in [1-14C] stearoyl CoA des... | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50175299 (CHEMBL3808805) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50175301 (CHEMBL3809367) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C8 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50175300 (CHEMBL3810414) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C8 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50268289 (CHEMBL4089362) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Natural Product and Medicinal Chemistry Lab, Faculty of Chemistry, VNUHCM-University of Science, 227 Nguyen Van Cu, Ho Chi Minh City, Viet Nam. Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B pre-incubated for 10 mins before pnitrophenyl phosphate substrate addition and measured every 30 secs for 10 mi... | Bioorg Med Chem Lett 27: 3301-3304 (2017) Article DOI: 10.1016/j.bmcl.2017.06.021 BindingDB Entry DOI: 10.7270/Q20Z75QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 78 total ) | Next | Last >> |