 Found 75 hits with Last Name = 'tsujii' and Initial = 's'
Found 75 hits with Last Name = 'tsujii' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50222709
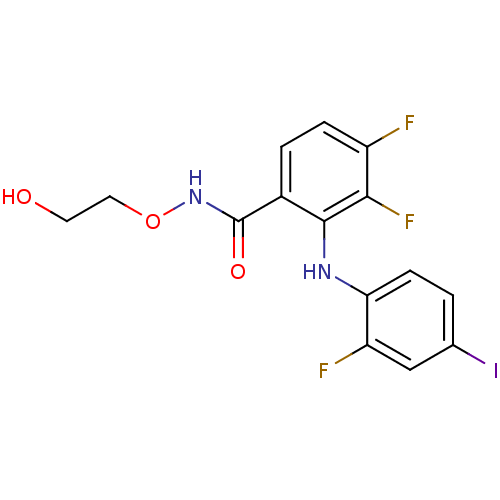
(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-N-(2-h...)Show InChI InChI=1S/C15H12F3IN2O3/c16-10-3-2-9(15(23)21-24-6-5-22)14(13(10)18)20-12-4-1-8(19)7-11(12)17/h1-4,7,20,22H,5-6H2,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM25764
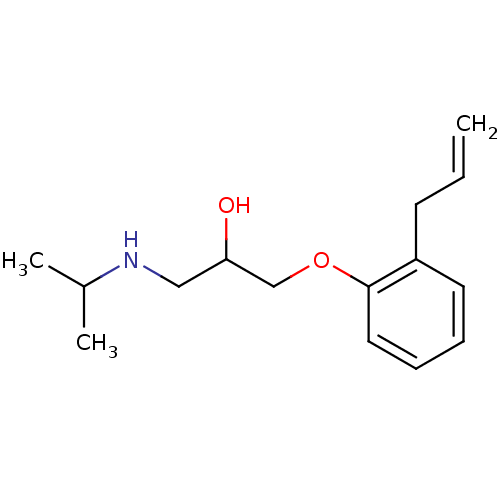
(ALPRENOLOL,(+) | ALPRENOLOL,(-) | Alfeprol | Alphe...)Show InChI InChI=1S/C15H23NO2/c1-4-7-13-8-5-6-9-15(13)18-11-14(17)10-16-12(2)3/h4-6,8-9,12,14,16-17H,1,7,10-11H2,2-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Compound was evaluated for its Beta adrenergic receptor blocking action |
Bioorg Med Chem Lett 11: 1833-7 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6TRW |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50218691

(CHEMBL51667)Show InChI InChI=1S/C19H27NO5/c1-6-23-18(22)17-12(2)16-14(8-7-9-15(16)25-17)24-11-13(21)10-20-19(3,4)5/h7-9,13,20-21H,6,10-11H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Compound was evaluated for its Beta adrenergic receptor blocking action |
Bioorg Med Chem Lett 11: 1833-7 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6TRW |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104963
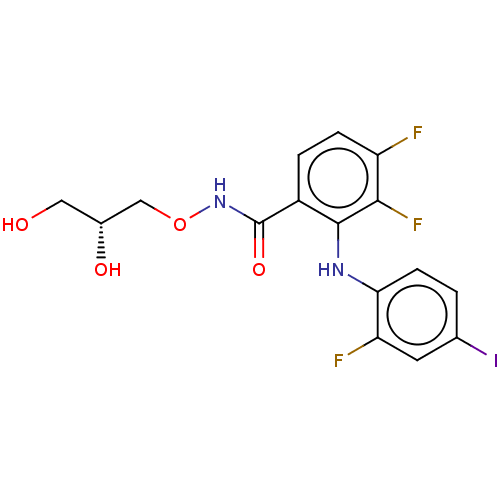
(CHEMBL507361 | US11147816, PD0325901 | US11701360,...)Show SMILES OC[C@@H](O)CONC(=O)c1ccc(F)c(F)c1Nc1ccc(I)cc1F |r| Show InChI InChI=1S/C16H14F3IN2O4/c17-11-3-2-10(16(25)22-26-7-9(24)6-23)15(14(11)19)21-13-4-1-8(20)5-12(13)18/h1-5,9,21,23-24H,6-7H2,(H,22,25)/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104959
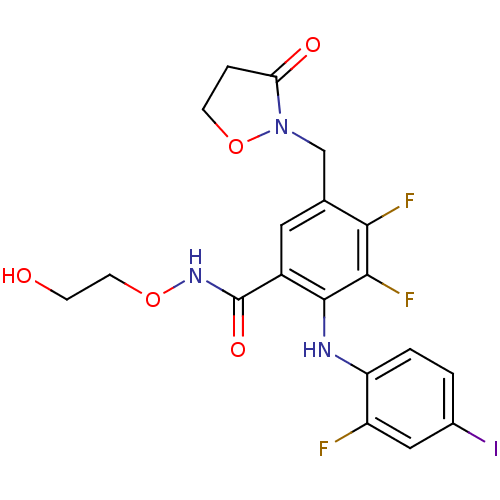
(US8575391, G-2)Show SMILES OCCONC(=O)c1cc(CN2OCCC2=O)c(F)c(F)c1Nc1ccc(I)cc1F Show InChI InChI=1S/C19H17F3IN3O5/c20-13-8-11(23)1-2-14(13)24-18-12(19(29)25-30-6-4-27)7-10(16(21)17(18)22)9-26-15(28)3-5-31-26/h1-2,7-8,24,27H,3-6,9H2,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104951
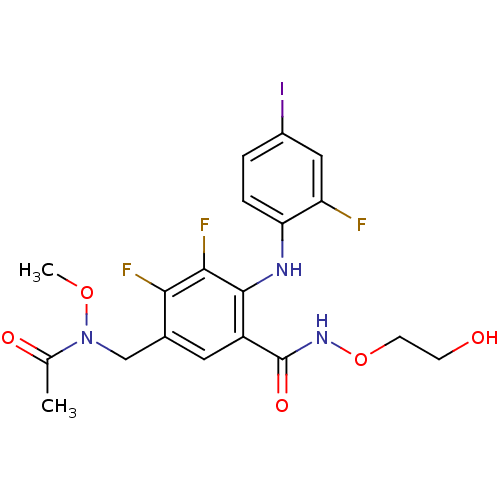
(US8575391, F-2)Show SMILES CON(Cc1cc(C(=O)NOCCO)c(Nc2ccc(I)cc2F)c(F)c1F)C(C)=O Show InChI InChI=1S/C19H19F3IN3O5/c1-10(28)26(30-2)9-11-7-13(19(29)25-31-6-5-27)18(17(22)16(11)21)24-15-4-3-12(23)8-14(15)20/h3-4,7-8,24,27H,5-6,9H2,1-2H3,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50338038
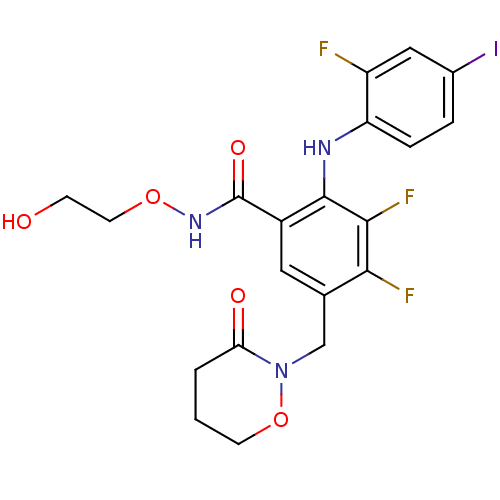
(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-N-(2-h...)Show SMILES OCCONC(=O)c1cc(CN2OCCCC2=O)c(F)c(F)c1Nc1ccc(I)cc1F Show InChI InChI=1S/C20H19F3IN3O5/c21-14-9-12(24)3-4-15(14)25-19-13(20(30)26-31-7-5-28)8-11(17(22)18(19)23)10-27-16(29)2-1-6-32-27/h3-4,8-9,25,28H,1-2,5-7,10H2,(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
US Patent
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104949
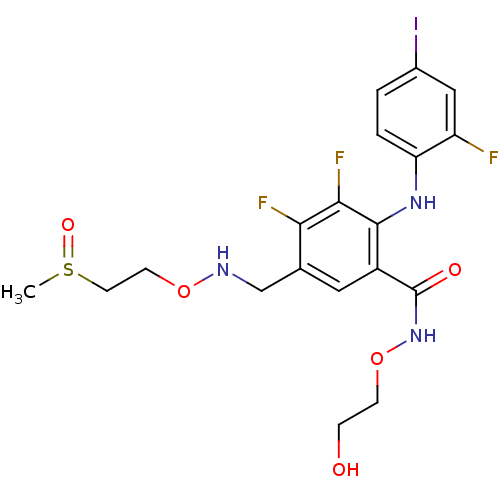
(US8575391, C-31)Show SMILES CS(=O)CCONCc1cc(C(=O)NOCCO)c(Nc2ccc(I)cc2F)c(F)c1F Show InChI InChI=1S/C19H21F3IN3O5S/c1-32(29)7-6-30-24-10-11-8-13(19(28)26-31-5-4-27)18(17(22)16(11)21)25-15-3-2-12(23)9-14(15)20/h2-3,8-9,24-25,27H,4-7,10H2,1H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104939

(US8575391, B-9)Show SMILES OCCONC(=O)c1cc(COCC(O)CO)c(F)c(F)c1Nc1ccc(I)cc1F Show InChI InChI=1S/C19H20F3IN2O6/c20-14-6-11(23)1-2-15(14)24-18-13(19(29)25-31-4-3-26)5-10(16(21)17(18)22)8-30-9-12(28)7-27/h1-2,5-6,12,24,26-28H,3-4,7-9H2,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104945

(US8575391, C-10)Show SMILES OCCCONCc1cc(C(=O)NOCCO)c(Nc2ccc(I)cc2F)c(F)c1F Show InChI InChI=1S/C19H21F3IN3O5/c20-14-9-12(23)2-3-15(14)25-18-13(19(29)26-31-7-5-28)8-11(16(21)17(18)22)10-24-30-6-1-4-27/h2-3,8-9,24-25,27-28H,1,4-7,10H2,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104941

(US8575391, C-1)Show SMILES OCCONCc1cc(C(=O)NOCCO)c(Nc2ccc(I)cc2F)c(F)c1F Show InChI InChI=1S/C18H19F3IN3O5/c19-13-8-11(22)1-2-14(13)24-17-12(18(28)25-30-6-4-27)7-10(15(20)16(17)21)9-23-29-5-3-26/h1-2,7-8,23-24,26-27H,3-6,9H2,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104936

(US8575391, B-1)Show SMILES OCCOCc1cc(C(=O)NOCCO)c(Nc2ccc(I)cc2F)c(F)c1F Show InChI InChI=1S/C18H18F3IN2O5/c19-13-8-11(22)1-2-14(13)23-17-12(18(27)24-29-6-4-26)7-10(9-28-5-3-25)15(20)16(17)21/h1-2,7-8,23,25-26H,3-6,9H2,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
US Patent
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104938

(US8575391, B-6)Show SMILES OCCCOCc1cc(C(=O)NOCCO)c(Nc2ccc(I)cc2F)c(F)c1F Show InChI InChI=1S/C19H20F3IN2O5/c20-14-9-12(23)2-3-15(14)24-18-13(19(28)25-30-7-5-27)8-11(16(21)17(18)22)10-29-6-1-4-26/h2-3,8-9,24,26-27H,1,4-7,10H2,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104946

(US8575391, C-13)Show SMILES CNC(=O)CONCc1cc(C(=O)NOCCO)c(Nc2ccc(I)cc2F)c(F)c1F Show InChI InChI=1S/C19H20F3IN4O5/c1-24-15(29)9-32-25-8-10-6-12(19(30)27-31-5-4-28)18(17(22)16(10)21)26-14-3-2-11(23)7-13(14)20/h2-3,6-7,25-26,28H,4-5,8-9H2,1H3,(H,24,29)(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104955
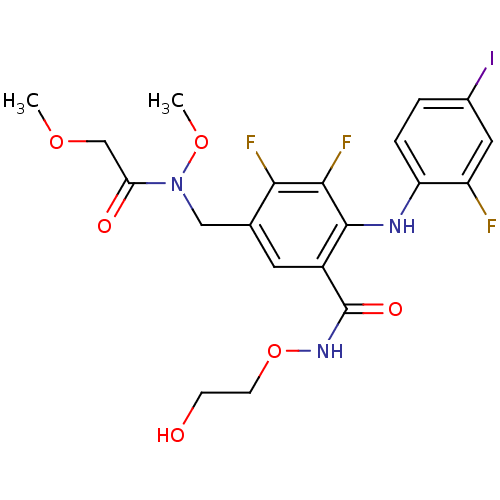
(US8575391, F-6)Show SMILES COCC(=O)N(Cc1cc(C(=O)NOCCO)c(Nc2ccc(I)cc2F)c(F)c1F)OC Show InChI InChI=1S/C20H21F3IN3O6/c1-31-10-16(29)27(32-2)9-11-7-13(20(30)26-33-6-5-28)19(18(23)17(11)22)25-15-4-3-12(24)8-14(15)21/h3-4,7-8,25,28H,5-6,9-10H2,1-2H3,(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104952

(US8575391, F-3)Show SMILES CCC(=O)N(Cc1cc(C(=O)NOCCO)c(Nc2ccc(I)cc2F)c(F)c1F)OC Show InChI InChI=1S/C20H21F3IN3O5/c1-3-16(29)27(31-2)10-11-8-13(20(30)26-32-7-6-28)19(18(23)17(11)22)25-15-5-4-12(24)9-14(15)21/h4-5,8-9,25,28H,3,6-7,10H2,1-2H3,(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104940

(US8575391, B-12)Show SMILES CS(=O)(=O)CCOCc1cc(C(=O)NOCCO)c(Nc2ccc(I)cc2F)c(F)c1F Show InChI InChI=1S/C19H20F3IN2O6S/c1-32(28,29)7-6-30-10-11-8-13(19(27)25-31-5-4-26)18(17(22)16(11)21)24-15-3-2-12(23)9-14(15)20/h2-3,8-9,24,26H,4-7,10H2,1H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104944
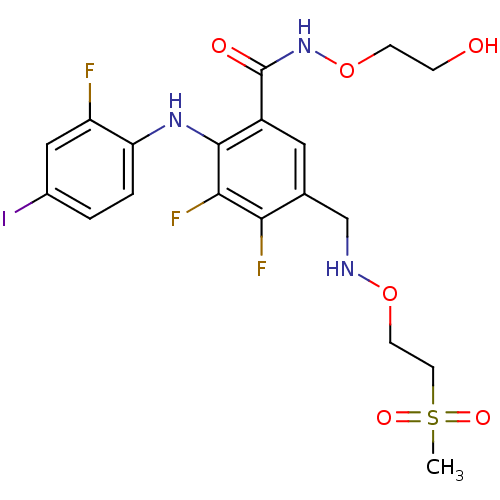
(US8575391, C-8)Show SMILES CS(=O)(=O)CCONCc1cc(C(=O)NOCCO)c(Nc2ccc(I)cc2F)c(F)c1F Show InChI InChI=1S/C19H21F3IN3O6S/c1-33(29,30)7-6-31-24-10-11-8-13(19(28)26-32-5-4-27)18(17(22)16(11)21)25-15-3-2-12(23)9-14(15)20/h2-3,8-9,24-25,27H,4-7,10H2,1H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104937
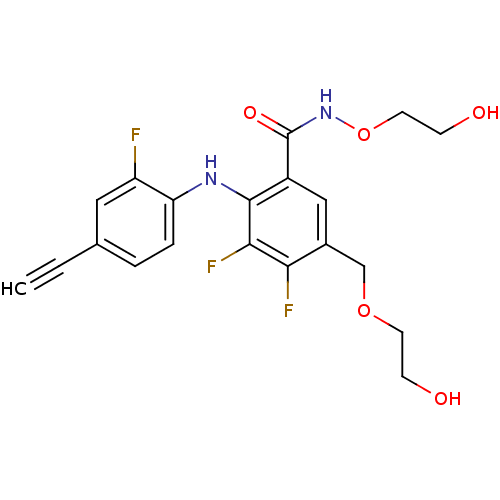
(US8575391, B-2)Show SMILES OCCOCc1cc(C(=O)NOCCO)c(Nc2ccc(cc2F)C#C)c(F)c1F Show InChI InChI=1S/C20H19F3N2O5/c1-2-12-3-4-16(15(21)9-12)24-19-14(20(28)25-30-8-6-27)10-13(11-29-7-5-26)17(22)18(19)23/h1,3-4,9-10,24,26-27H,5-8,11H2,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104953
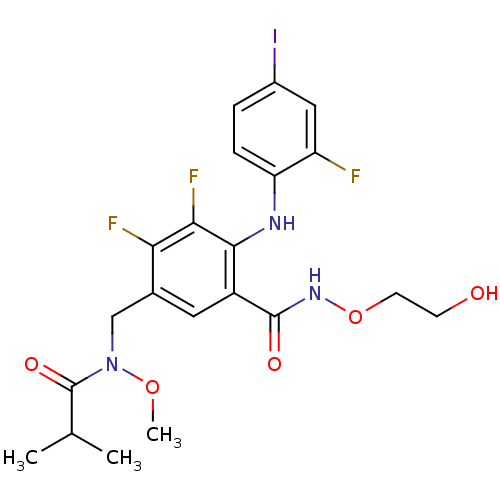
(US8575391, F-4)Show SMILES CON(Cc1cc(C(=O)NOCCO)c(Nc2ccc(I)cc2F)c(F)c1F)C(=O)C(C)C Show InChI InChI=1S/C21H23F3IN3O5/c1-11(2)21(31)28(32-3)10-12-8-14(20(30)27-33-7-6-29)19(18(24)17(12)23)26-16-5-4-13(25)9-15(16)22/h4-5,8-9,11,26,29H,6-7,10H2,1-3H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104950
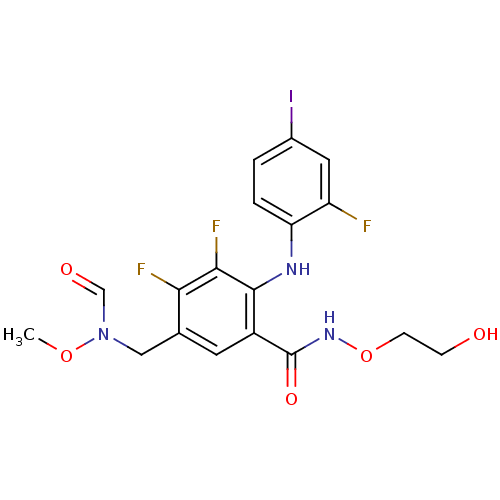
(US8575391, F-1)Show SMILES CON(Cc1cc(C(=O)NOCCO)c(Nc2ccc(I)cc2F)c(F)c1F)C=O Show InChI InChI=1S/C18H17F3IN3O5/c1-29-25(9-27)8-10-6-12(18(28)24-30-5-4-26)17(16(21)15(10)20)23-14-3-2-11(22)7-13(14)19/h2-3,6-7,9,23,26H,4-5,8H2,1H3,(H,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104960
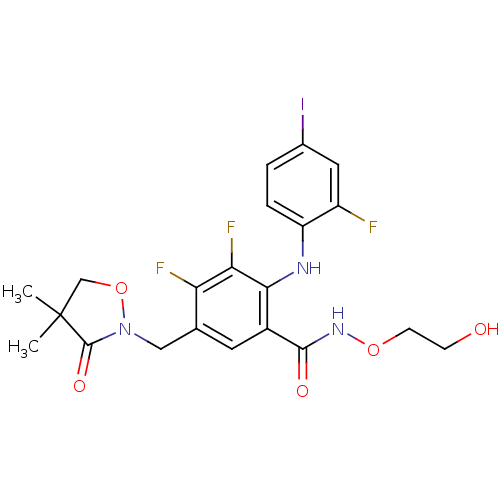
(US8575391, G-3)Show SMILES CC1(C)CON(Cc2cc(C(=O)NOCCO)c(Nc3ccc(I)cc3F)c(F)c2F)C1=O Show InChI InChI=1S/C21H21F3IN3O5/c1-21(2)10-33-28(20(21)31)9-11-7-13(19(30)27-32-6-5-29)18(17(24)16(11)23)26-15-4-3-12(25)8-14(15)22/h3-4,7-8,26,29H,5-6,9-10H2,1-2H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104962

(US8575391, G-5)Show SMILES OCCONC(=O)c1cc(CN2OCCC2=O)c(F)c(F)c1Nc1ccc(cc1F)C#C Show InChI InChI=1S/C21H18F3N3O5/c1-2-12-3-4-16(15(22)9-12)25-20-14(21(30)26-31-8-6-28)10-13(18(23)19(20)24)11-27-17(29)5-7-32-27/h1,3-4,9-10,25,28H,5-8,11H2,(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104954

(US8575391, F-5)Show SMILES CON(Cc1cc(C(=O)NOCCO)c(Nc2ccc(I)cc2F)c(F)c1F)C(=O)CO Show InChI InChI=1S/C19H19F3IN3O6/c1-31-26(15(29)9-28)8-10-6-12(19(30)25-32-5-4-27)18(17(22)16(10)21)24-14-3-2-11(23)7-13(14)20/h2-3,6-7,24,27-28H,4-5,8-9H2,1H3,(H,25,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104956

(US8575391, F-7)Show SMILES CON(Cc1cc(C(=O)NOCCO)c(Nc2ccc(cc2F)C#C)c(F)c1F)C(C)=O Show InChI InChI=1S/C21H20F3N3O5/c1-4-13-5-6-17(16(22)9-13)25-20-15(21(30)26-32-8-7-28)10-14(18(23)19(20)24)11-27(31-3)12(2)29/h1,5-6,9-10,25,28H,7-8,11H2,2-3H3,(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104942
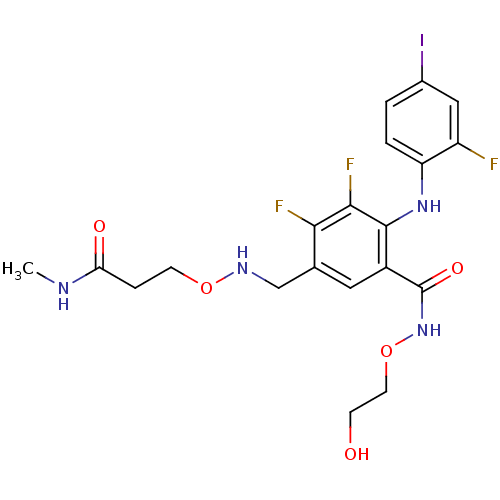
(US8575391, C-6)Show SMILES CNC(=O)CCONCc1cc(C(=O)NOCCO)c(Nc2ccc(I)cc2F)c(F)c1F Show InChI InChI=1S/C20H22F3IN4O5/c1-25-16(30)4-6-32-26-10-11-8-13(20(31)28-33-7-5-29)19(18(23)17(11)22)27-15-3-2-12(24)9-14(15)21/h2-3,8-9,26-27,29H,4-7,10H2,1H3,(H,25,30)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104961

(US8575391, G-4)Show SMILES OCCONC(=O)c1cc(CN2OCCCC2=O)c(F)c(F)c1Nc1ccc(cc1F)C#C Show InChI InChI=1S/C22H20F3N3O5/c1-2-13-5-6-17(16(23)10-13)26-21-15(22(31)27-32-9-7-29)11-14(19(24)20(21)25)12-28-18(30)4-3-8-33-28/h1,5-6,10-11,26,29H,3-4,7-9,12H2,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104957

(US8575391, F-8)Show SMILES CCC(=O)N(Cc1cc(C(=O)NOCCO)c(Nc2ccc(cc2F)C#C)c(F)c1F)OC Show InChI InChI=1S/C22H22F3N3O5/c1-4-13-6-7-17(16(23)10-13)26-21-15(22(31)27-33-9-8-29)11-14(19(24)20(21)25)12-28(32-3)18(30)5-2/h1,6-7,10-11,26,29H,5,8-9,12H2,2-3H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104947

(US8575391, C-24)Show SMILES CONCc1cc(C(=O)NOCCO)c(Nc2ccc(I)cc2F)c(F)c1F Show InChI InChI=1S/C17H17F3IN3O4/c1-27-22-8-9-6-11(17(26)24-28-5-4-25)16(15(20)14(9)19)23-13-3-2-10(21)7-12(13)18/h2-3,6-7,22-23,25H,4-5,8H2,1H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104948

(US8575391, C-28)Show SMILES CC(C)(O)CONCc1cc(C(=O)NOCCO)c(Nc2ccc(I)cc2F)c(F)c1F Show InChI InChI=1S/C20H23F3IN3O5/c1-20(2,30)10-32-25-9-11-7-13(19(29)27-31-6-5-28)18(17(23)16(11)22)26-15-4-3-12(24)8-14(15)21/h3-4,7-8,25-26,28,30H,5-6,9-10H2,1-2H3,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104958

(US8575391, F-9)Show SMILES CON(Cc1cc(C(=O)NOCCO)c(Nc2ccc(cc2F)C#C)c(F)c1F)C(=O)C(C)C Show InChI InChI=1S/C23H24F3N3O5/c1-5-14-6-7-18(17(24)10-14)27-21-16(22(31)28-34-9-8-30)11-15(19(25)20(21)26)12-29(33-4)23(32)13(2)3/h1,6-7,10-11,13,27,30H,8-9,12H2,2-4H3,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM96235
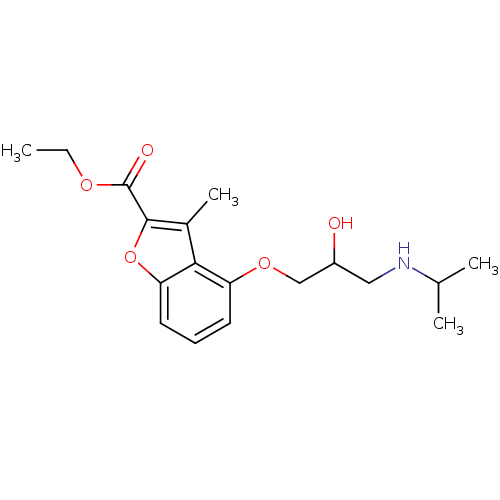
(4-[2-hydroxy-3-(isopropylamino)propoxy]-3-methyl-c...)Show InChI InChI=1S/C18H25NO5/c1-5-22-18(21)17-12(4)16-14(7-6-8-15(16)24-17)23-10-13(20)9-19-11(2)3/h6-8,11,13,19-20H,5,9-10H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Compound was evaluated for its Beta adrenergic receptor blocking action |
Bioorg Med Chem Lett 11: 1833-7 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6TRW |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1/2
(Homo sapiens (Human)) | BDBM50101969
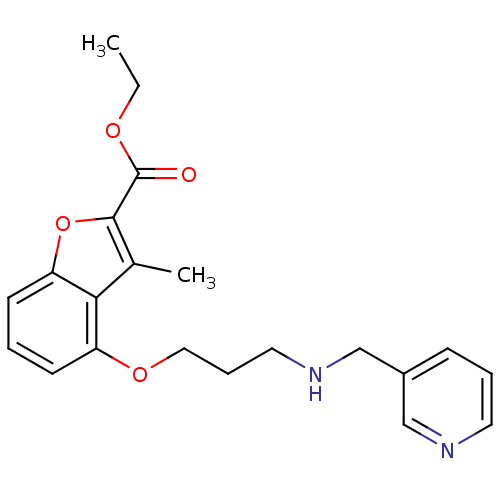
(3-Methyl-4-{3-[(pyridin-3-ylmethyl)-amino]-propoxy...)Show InChI InChI=1S/C21H24N2O4/c1-3-25-21(24)20-15(2)19-17(8-4-9-18(19)27-20)26-12-6-11-23-14-16-7-5-10-22-13-16/h4-5,7-10,13,23H,3,6,11-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Candida albicans N-myristoyltransferase (CaNmt) |
Bioorg Med Chem Lett 11: 1833-7 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6TRW |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50132260
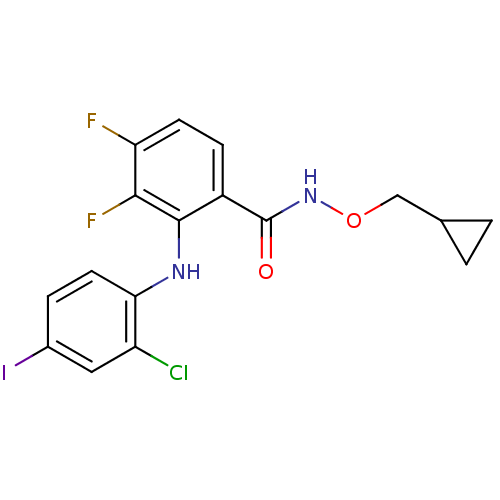
(2-(2-Chloro-4-iodo-phenylamino)-N-cyclopropylmetho...)Show InChI InChI=1S/C17H14ClF2IN2O2/c18-12-7-10(21)3-6-14(12)22-16-11(4-5-13(19)15(16)20)17(24)23-25-8-9-1-2-9/h3-7,9,22H,1-2,8H2,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104943

(US8575391, C-7)Show SMILES CC(=O)NCCONCc1cc(C(=O)NOCCO)c(Nc2ccc(I)cc2F)c(F)c1F Show InChI InChI=1S/C20H22F3IN4O5/c1-11(30)25-4-6-32-26-10-12-8-14(20(31)28-33-7-5-29)19(18(23)17(12)22)27-16-3-2-13(24)9-15(16)21/h2-3,8-9,26-27,29H,4-7,10H2,1H3,(H,25,30)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Seiyaku Kabushiki Kaisha
US Patent
| Assay Description
Inhibition assay using MEK kinase. |
US Patent US8575391 (2013)
BindingDB Entry DOI: 10.7270/Q22B8WPF |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1/2
(Homo sapiens (Human)) | BDBM50034993
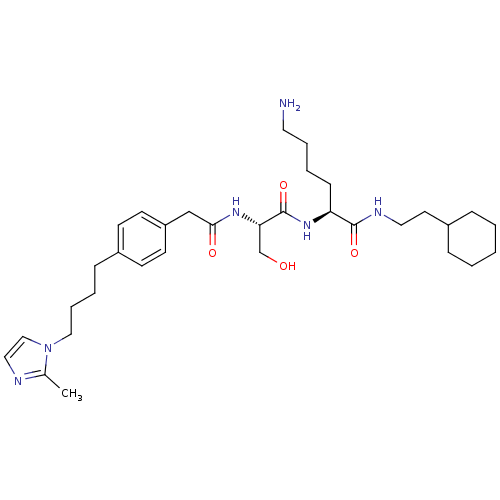
((S)-6-Amino-2-[(S)-3-hydroxy-2-(2-{4-[4-(2-methyl-...)Show SMILES Cc1nccn1CCCCc1ccc(CC(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)NCCC2CCCCC2)cc1 Show InChI InChI=1S/C33H52N6O4/c1-25-35-20-22-39(25)21-8-6-11-27-13-15-28(16-14-27)23-31(41)37-30(24-40)33(43)38-29(12-5-7-18-34)32(42)36-19-17-26-9-3-2-4-10-26/h13-16,20,22,26,29-30,40H,2-12,17-19,21,23-24,34H2,1H3,(H,36,42)(H,37,41)(H,38,43)/t29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Candida albicans N-myristoyltransferase (CaNmt) |
Bioorg Med Chem Lett 11: 1833-7 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6TRW |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1/2
(Homo sapiens (Human)) | BDBM96235

(4-[2-hydroxy-3-(isopropylamino)propoxy]-3-methyl-c...)Show InChI InChI=1S/C18H25NO5/c1-5-22-18(21)17-12(4)16-14(7-6-8-15(16)24-17)23-10-13(20)9-19-11(2)3/h6-8,11,13,19-20H,5,9-10H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Candida albicans N-myristoyltransferase (CaNmt) |
Bioorg Med Chem Lett 11: 1833-7 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6TRW |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1/2
(Homo sapiens (Human)) | BDBM50218691

(CHEMBL51667)Show InChI InChI=1S/C19H27NO5/c1-6-23-18(22)17-12(2)16-14(8-7-9-15(16)25-17)24-11-13(21)10-20-19(3,4)5/h7-9,13,20-21H,6,10-11H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Candida albicans N-myristoyltransferase (CaNmt) |
Bioorg Med Chem Lett 11: 1833-7 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6TRW |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1/2
(Homo sapiens (Human)) | BDBM50218690
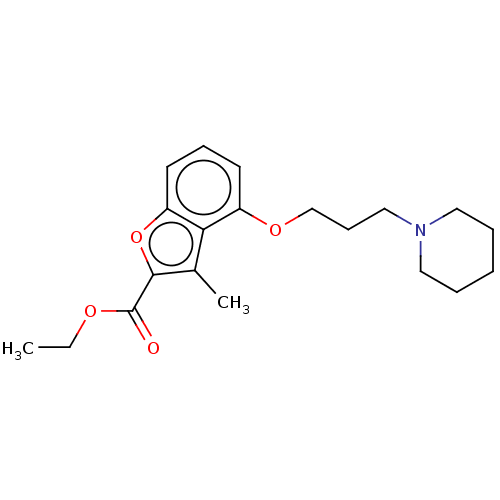
(CHEMBL53026)Show InChI InChI=1S/C20H27NO4/c1-3-23-20(22)19-15(2)18-16(9-7-10-17(18)25-19)24-14-8-13-21-11-5-4-6-12-21/h7,9-10H,3-6,8,11-14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Candida albicans N-myristoyltransferase (CaNmt) |
Bioorg Med Chem Lett 11: 1833-7 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6TRW |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1/2
(Homo sapiens (Human)) | BDBM50101968
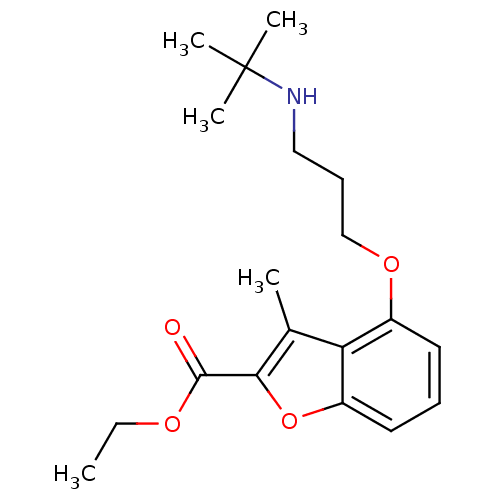
(4-(3-tert-Butylamino-propoxy)-3-methyl-benzofuran-...)Show InChI InChI=1S/C19H27NO4/c1-6-22-18(21)17-13(2)16-14(9-7-10-15(16)24-17)23-12-8-11-20-19(3,4)5/h7,9-10,20H,6,8,11-12H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Candida albicans N-myristoyltransferase (CaNmt) |
Bioorg Med Chem Lett 11: 1833-7 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6TRW |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1/2
(Homo sapiens (Human)) | BDBM50218696
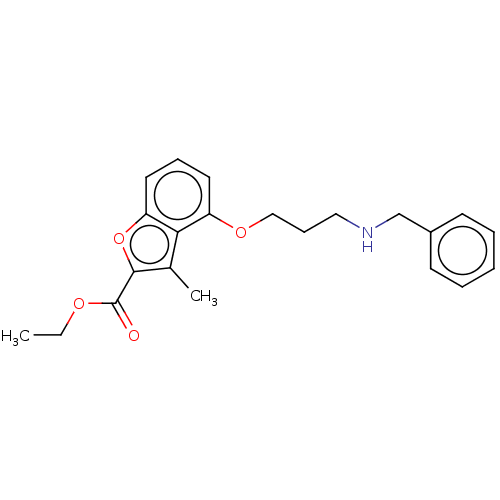
(CHEMBL299034)Show InChI InChI=1S/C22H25NO4/c1-3-25-22(24)21-16(2)20-18(11-7-12-19(20)27-21)26-14-8-13-23-15-17-9-5-4-6-10-17/h4-7,9-12,23H,3,8,13-15H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Candida albicans N-myristoyltransferase (CaNmt) |
Bioorg Med Chem Lett 11: 1833-7 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6TRW |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1/2
(Homo sapiens (Human)) | BDBM50218743
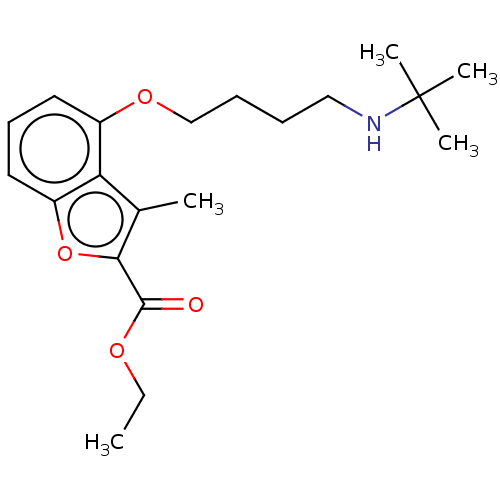
(CHEMBL53246)Show InChI InChI=1S/C20H29NO4/c1-6-23-19(22)18-14(2)17-15(10-9-11-16(17)25-18)24-13-8-7-12-21-20(3,4)5/h9-11,21H,6-8,12-13H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Candida albicans N-myristoyltransferase (CaNmt) |
Bioorg Med Chem Lett 11: 1833-7 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6TRW |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1/2
(Homo sapiens (Human)) | BDBM50218688
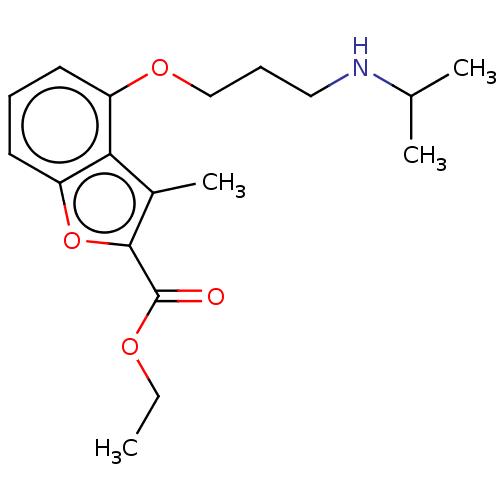
(CHEMBL416300)Show InChI InChI=1S/C18H25NO4/c1-5-21-18(20)17-13(4)16-14(8-6-9-15(16)23-17)22-11-7-10-19-12(2)3/h6,8-9,12,19H,5,7,10-11H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Candida albicans N-myristoyltransferase (CaNmt) |
Bioorg Med Chem Lett 11: 1833-7 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6TRW |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1/2
(Homo sapiens (Human)) | BDBM50218744
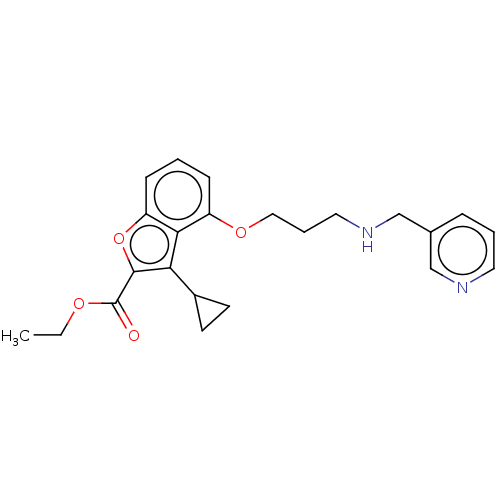
(CHEMBL53257)Show InChI InChI=1S/C23H26N2O4/c1-2-27-23(26)22-20(17-9-10-17)21-18(7-3-8-19(21)29-22)28-13-5-12-25-15-16-6-4-11-24-14-16/h3-4,6-8,11,14,17,25H,2,5,9-10,12-13,15H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Candida albicans N-myristoyltransferase (CaNmt) |
Bioorg Med Chem Lett 11: 1833-7 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6TRW |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1/2
(Homo sapiens (Human)) | BDBM50218692
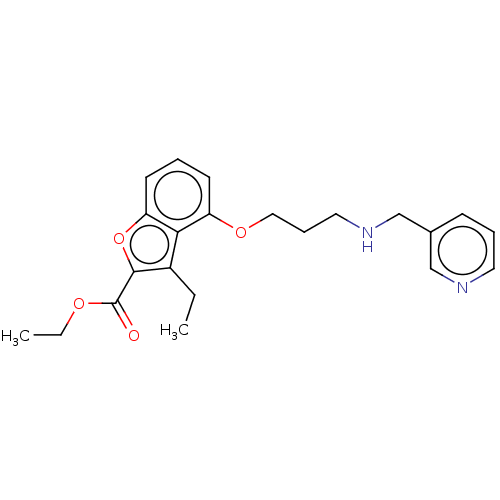
(CHEMBL52631)Show InChI InChI=1S/C22H26N2O4/c1-3-17-20-18(9-5-10-19(20)28-21(17)22(25)26-4-2)27-13-7-12-24-15-16-8-6-11-23-14-16/h5-6,8-11,14,24H,3-4,7,12-13,15H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Candida albicans N-myristoyltransferase (CaNmt) |
Bioorg Med Chem Lett 11: 1833-7 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6TRW |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1/2
(Homo sapiens (Human)) | BDBM50218694
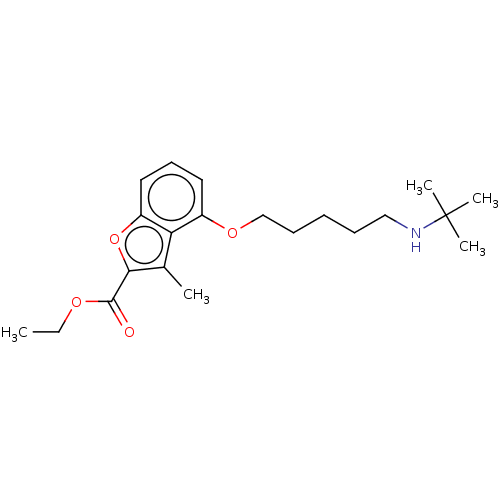
(CHEMBL52854)Show InChI InChI=1S/C21H31NO4/c1-6-24-20(23)19-15(2)18-16(11-10-12-17(18)26-19)25-14-9-7-8-13-22-21(3,4)5/h10-12,22H,6-9,13-14H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Candida albicans N-myristoyltransferase (CaNmt) |
Bioorg Med Chem Lett 11: 1833-7 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6TRW |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50101968

(4-(3-tert-Butylamino-propoxy)-3-methyl-benzofuran-...)Show InChI InChI=1S/C19H27NO4/c1-6-22-18(21)17-13(2)16-14(9-7-10-15(16)24-17)23-12-8-11-20-19(3,4)5/h7,9-10,20H,6,8,11-12H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Compound was evaluated for its Beta adrenergic receptor blocking action |
Bioorg Med Chem Lett 11: 1833-7 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6TRW |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50218688

(CHEMBL416300)Show InChI InChI=1S/C18H25NO4/c1-5-21-18(20)17-13(4)16-14(8-6-9-15(16)23-17)22-11-7-10-19-12(2)3/h6,8-9,12,19H,5,7,10-11H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
In vitro ability of the compound to displace radiolabelled FTI from farnesyltransferase in cultured Ha-ras transformed RAT1 cells |
Bioorg Med Chem Lett 11: 1833-7 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6TRW |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1/2
(Homo sapiens (Human)) | BDBM50218699
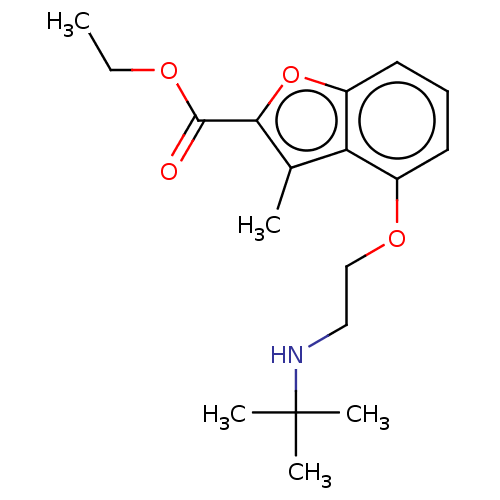
(CHEMBL55282)Show InChI InChI=1S/C18H25NO4/c1-6-21-17(20)16-12(2)15-13(8-7-9-14(15)23-16)22-11-10-19-18(3,4)5/h7-9,19H,6,10-11H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Candida albicans N-myristoyltransferase (CaNmt) |
Bioorg Med Chem Lett 11: 1833-7 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6TRW |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1/2
(Homo sapiens (Human)) | BDBM50218697
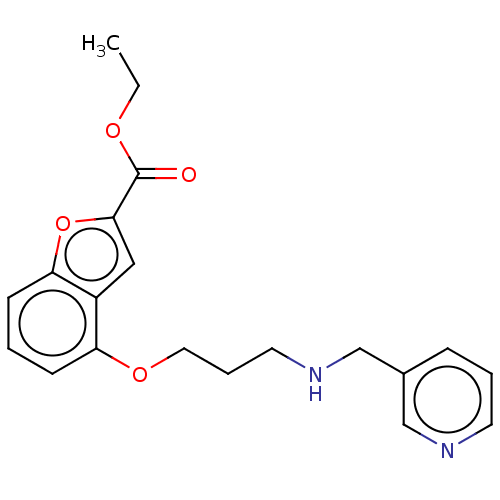
(CHEMBL51868)Show InChI InChI=1S/C20H22N2O4/c1-2-24-20(23)19-12-16-17(7-3-8-18(16)26-19)25-11-5-10-22-14-15-6-4-9-21-13-15/h3-4,6-9,12-13,22H,2,5,10-11,14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Candida albicans N-myristoyltransferase (CaNmt) |
Bioorg Med Chem Lett 11: 1833-7 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6TRW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data