 Found 285 hits with Last Name = 'tupper' and Initial = 'd'
Found 285 hits with Last Name = 'tupper' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50132686
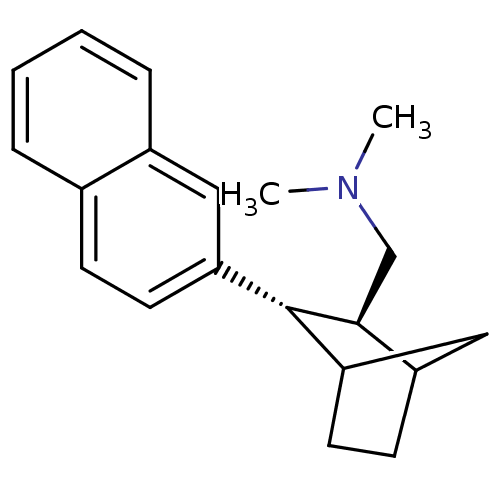
(CHEMBL326466 | Dimethyl-((2S,3S)-3-naphthalen-2-yl...)Show InChI InChI=1S/C20H25N/c1-21(2)13-19-16-8-10-18(12-16)20(19)17-9-7-14-5-3-4-6-15(14)11-17/h3-7,9,11,16,18-20H,8,10,12-13H2,1-2H3/t16?,18?,19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-citalopram from Serotonin transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50132687
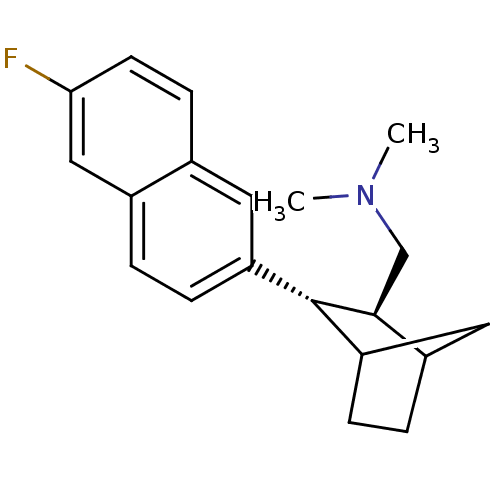
(CHEMBL324269 | [(2S,3S)-3-(6-Fluoro-naphthalen-2-y...)Show SMILES CN(C)C[C@H]1C2CCC(C2)[C@@H]1c1ccc2cc(F)ccc2c1 Show InChI InChI=1S/C20H24FN/c1-22(2)12-19-15-4-6-17(10-15)20(19)16-5-3-14-11-18(21)8-7-13(14)9-16/h3,5,7-9,11,15,17,19-20H,4,6,10,12H2,1-2H3/t15?,17?,19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-citalopram from Serotonin transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50132685

(CHEMBL109571 | Dimethyl-(3-naphthalen-2-yl-bicyclo...)Show InChI InChI=1S/C20H25N/c1-21(2)13-19-16-8-10-18(12-16)20(19)17-9-7-14-5-3-4-6-15(14)11-17/h3-7,9,11,16,18-20H,8,10,12-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-citalopram from Serotonin transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50132679
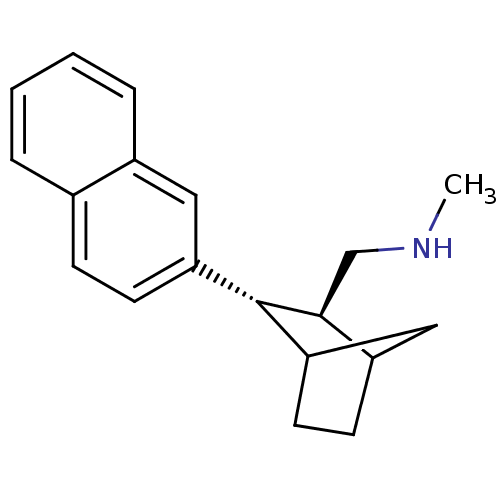
(CHEMBL111128 | Methyl-((2S,3S)-3-naphthalen-2-yl-b...)Show InChI InChI=1S/C19H23N/c1-20-12-18-15-7-9-17(11-15)19(18)16-8-6-13-4-2-3-5-14(13)10-16/h2-6,8,10,15,17-20H,7,9,11-12H2,1H3/t15?,17?,18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-citalopram from Serotonin transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50132678

(CHEMBL432022 | [(2S,3S)-3-(6-Fluoro-naphthalen-2-y...)Show InChI InChI=1S/C19H22FN/c1-21-11-18-14-3-5-16(9-14)19(18)15-4-2-13-10-17(20)7-6-12(13)8-15/h2,4,6-8,10,14,16,18-19,21H,3,5,9,11H2,1H3/t14?,16?,18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-citalopram from Serotonin transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50132684

(CHEMBL331799 | Methyl-(3-naphthalen-2-yl-bicyclo[2...)Show InChI InChI=1S/C19H23N/c1-20-12-18-15-7-9-17(11-15)19(18)16-8-6-13-4-2-3-5-14(13)10-16/h2-6,8,10,15,17-20H,7,9,11-12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-citalopram from Serotonin transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50132677

(CHEMBL109517 | Dimethyl-((2R,3R)-3-naphthalen-2-yl...)Show InChI InChI=1S/C20H25N/c1-21(2)13-19-16-8-10-18(12-16)20(19)17-9-7-14-5-3-4-6-15(14)11-17/h3-7,9,11,16,18-20H,8,10,12-13H2,1-2H3/t16?,18?,19-,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-citalopram from Serotonin transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50289291
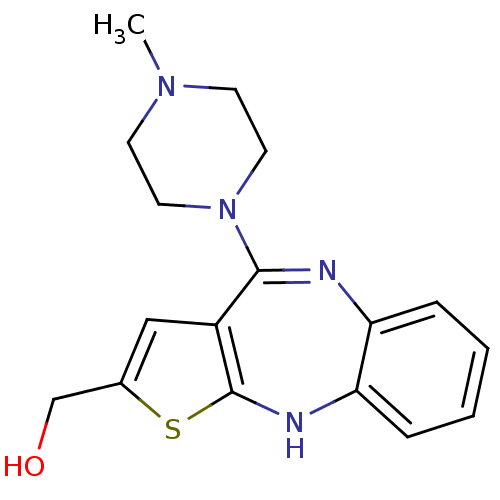
(2-Hydroxy-methylolanzapine | CHEMBL100454 | [10-(4...)Show InChI InChI=1S/C17H20N4OS/c1-20-6-8-21(9-7-20)16-13-10-12(11-22)23-17(13)19-15-5-3-2-4-14(15)18-16/h2-5,10,19,22H,6-9,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against histamine H1 neuronal receptor |
Bioorg Med Chem Lett 7: 25-30 (1997)
Article DOI: 10.1016/S0960-894X(96)00567-7
BindingDB Entry DOI: 10.7270/Q2KD1XXP |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50132683

(6-((2S,3S)-3-Dimethylaminomethyl-bicyclo[2.2.1]hep...)Show SMILES CN(C)C[C@H]1C2CCC(C2)[C@@H]1c1ccc2cc(O)ccc2c1 Show InChI InChI=1S/C20H25NO/c1-21(2)12-19-15-4-6-17(10-15)20(19)16-5-3-14-11-18(22)8-7-13(14)9-16/h3,5,7-9,11,15,17,19-20,22H,4,6,10,12H2,1-2H3/t15?,17?,19-,20-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-WIN-35,428 from Dopamine transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM35254

(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against histamine H1 neuronal receptor |
Bioorg Med Chem Lett 7: 25-30 (1997)
Article DOI: 10.1016/S0960-894X(96)00567-7
BindingDB Entry DOI: 10.7270/Q2KD1XXP |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50026952

((cis) [3-(3,4-Dichloro-phenyl)-bicyclo[2.2.2]oct-2...)Show SMILES CN(C)C[C@@H]1C2CCC(CC2)[C@@H]1c1ccc(Cl)c(Cl)c1 |wU:11.13,4.3,TLB:12:11:6.7:10.9,THB:3:4:6.7:10.9,(4.42,-5.07,;3.76,-3.67,;2.22,-3.53,;4.65,-2.41,;4,-1.01,;5.68,-.26,;5.79,1.35,;5.19,2.75,;5.16,1.21,;6.61,.69,;7.15,-.75,;3.76,.53,;2.43,1.3,;1.1,.53,;-.23,1.3,;-.23,2.84,;-1.58,3.63,;1.1,3.61,;1.1,5.15,;2.43,2.84,)| Show InChI InChI=1S/C17H23Cl2N/c1-20(2)10-14-11-3-5-12(6-4-11)17(14)13-7-8-15(18)16(19)9-13/h7-9,11-12,14,17H,3-6,10H2,1-2H3/t11?,12?,14-,17-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]-NE re-uptake into synaptosome |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50121539
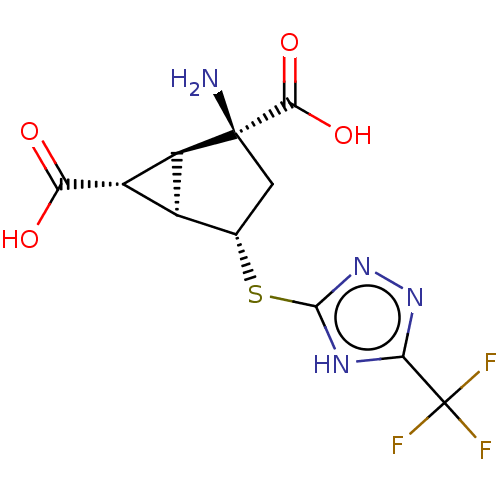
(CHEMBL3616856)Show SMILES [H][C@]12[C@H](C(O)=O)[C@@]1([H])[C@@](N)(C[C@@H]2Sc1nnc([nH]1)C(F)(F)F)C(O)=O |r| Show InChI InChI=1S/C11H11F3N4O4S/c12-11(13,14)7-16-9(18-17-7)23-2-1-10(15,8(21)22)5-3(2)4(5)6(19)20/h2-5H,1,15H2,(H,19,20)(H,21,22)(H,16,17,18)/t2-,3-,4-,5-,10-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]-459477 from human recombinant mGlu2 receptor expressed in AV12 cells after 90 mins by liquid scintillation counting |
J Med Chem 58: 7526-48 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01124
BindingDB Entry DOI: 10.7270/Q29S1ST0 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50240701
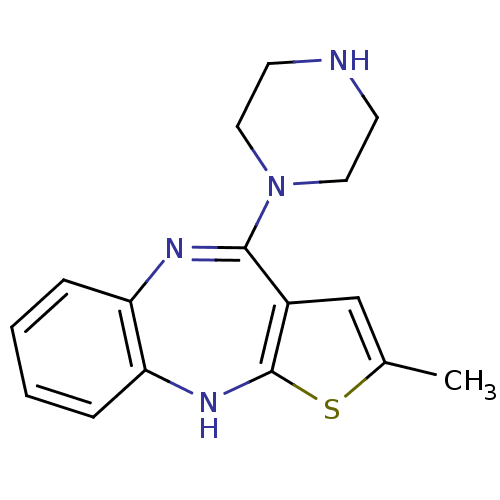
(2-Methyl-10-piperazin-1-yl-4H-3-thia-4,9-diaza-ben...)Show InChI InChI=1S/C16H18N4S/c1-11-10-12-15(20-8-6-17-7-9-20)18-13-4-2-3-5-14(13)19-16(12)21-11/h2-5,10,17,19H,6-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against neuronal Dopamine receptor D2 |
Bioorg Med Chem Lett 7: 25-30 (1997)
Article DOI: 10.1016/S0960-894X(96)00567-7
BindingDB Entry DOI: 10.7270/Q2KD1XXP |
More data for this
Ligand-Target Pair | |
Serotonin 2 (5-HT2) receptor
(Homo sapiens (Human)) | BDBM35254

(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against neuronal 5-hydroxytryptamine 2 receptor |
Bioorg Med Chem Lett 7: 25-30 (1997)
Article DOI: 10.1016/S0960-894X(96)00567-7
BindingDB Entry DOI: 10.7270/Q2KD1XXP |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50289291

(2-Hydroxy-methylolanzapine | CHEMBL100454 | [10-(4...)Show InChI InChI=1S/C17H20N4OS/c1-20-6-8-21(9-7-20)16-13-10-12(11-22)23-17(13)19-15-5-3-2-4-14(15)18-16/h2-5,10,19,22H,6-9,11H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Alpha-1 adrenergic receptor |
Bioorg Med Chem Lett 7: 25-30 (1997)
Article DOI: 10.1016/S0960-894X(96)00567-7
BindingDB Entry DOI: 10.7270/Q2KD1XXP |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50132680

(CHEMBL322348 | [(2S,3S)-3-(6-Methoxy-naphthalen-2-...)Show SMILES CNC[C@H]1C2CCC(C2)[C@@H]1c1ccc2cc(OC)ccc2c1 Show InChI InChI=1S/C20H25NO/c1-21-12-19-15-4-6-17(10-15)20(19)16-5-3-14-11-18(22-2)8-7-13(14)9-16/h3,5,7-9,11,15,17,19-21H,4,6,10,12H2,1-2H3/t15?,17?,19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-citalopram from Serotonin transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50121540
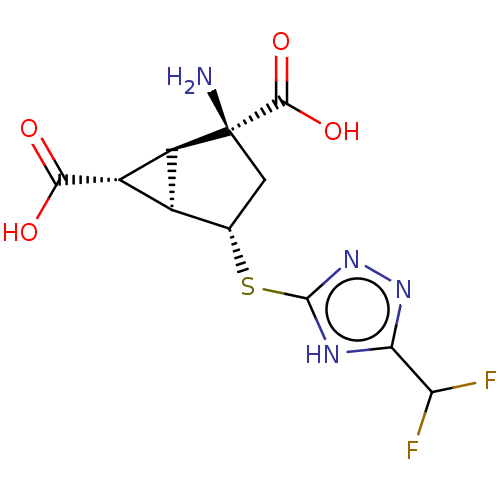
(CHEMBL3616857)Show SMILES [H][C@]12[C@H](C(O)=O)[C@@]1([H])[C@@](N)(C[C@@H]2Sc1nnc([nH]1)C(F)F)C(O)=O |r| Show InChI InChI=1S/C11H12F2N4O4S/c12-6(13)7-15-10(17-16-7)22-2-1-11(14,9(20)21)5-3(2)4(5)8(18)19/h2-6H,1,14H2,(H,18,19)(H,20,21)(H,15,16,17)/t2-,3-,4-,5-,11-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]-459477 from human recombinant mGlu2 receptor expressed in AV12 cells after 90 mins by liquid scintillation counting |
J Med Chem 58: 7526-48 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01124
BindingDB Entry DOI: 10.7270/Q29S1ST0 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM35254

(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Alpha-1 adrenergic receptor |
Bioorg Med Chem Lett 7: 25-30 (1997)
Article DOI: 10.1016/S0960-894X(96)00567-7
BindingDB Entry DOI: 10.7270/Q2KD1XXP |
More data for this
Ligand-Target Pair | |
Serotonin 2 (5-HT2) receptor
(Homo sapiens (Human)) | BDBM50289291

(2-Hydroxy-methylolanzapine | CHEMBL100454 | [10-(4...)Show InChI InChI=1S/C17H20N4OS/c1-20-6-8-21(9-7-20)16-13-10-12(11-22)23-17(13)19-15-5-3-2-4-14(15)18-16/h2-5,10,19,22H,6-9,11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against neuronal 5-hydroxytryptamine 2 receptor |
Bioorg Med Chem Lett 7: 25-30 (1997)
Article DOI: 10.1016/S0960-894X(96)00567-7
BindingDB Entry DOI: 10.7270/Q2KD1XXP |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50132685

(CHEMBL109571 | Dimethyl-(3-naphthalen-2-yl-bicyclo...)Show InChI InChI=1S/C20H25N/c1-21(2)13-19-16-8-10-18(12-16)20(19)17-9-7-14-5-3-4-6-15(14)11-17/h3-7,9,11,16,18-20H,8,10,12-13H2,1-2H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-WIN-35,428 from Dopamine transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM35254

(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against neuronal Dopamine receptor D2 |
Bioorg Med Chem Lett 7: 25-30 (1997)
Article DOI: 10.1016/S0960-894X(96)00567-7
BindingDB Entry DOI: 10.7270/Q2KD1XXP |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50240701

(2-Methyl-10-piperazin-1-yl-4H-3-thia-4,9-diaza-ben...)Show InChI InChI=1S/C16H18N4S/c1-11-10-12-15(20-8-6-17-7-9-20)18-13-4-2-3-5-14(13)19-16(12)21-11/h2-5,10,17,19H,6-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against histamine H1 neuronal receptor |
Bioorg Med Chem Lett 7: 25-30 (1997)
Article DOI: 10.1016/S0960-894X(96)00567-7
BindingDB Entry DOI: 10.7270/Q2KD1XXP |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50289291

(2-Hydroxy-methylolanzapine | CHEMBL100454 | [10-(4...)Show InChI InChI=1S/C17H20N4OS/c1-20-6-8-21(9-7-20)16-13-10-12(11-22)23-17(13)19-15-5-3-2-4-14(15)18-16/h2-5,10,19,22H,6-9,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against neuronal Dopamine receptor D2 |
Bioorg Med Chem Lett 7: 25-30 (1997)
Article DOI: 10.1016/S0960-894X(96)00567-7
BindingDB Entry DOI: 10.7270/Q2KD1XXP |
More data for this
Ligand-Target Pair | |
Serotonin 2 (5-HT2) receptor
(Homo sapiens (Human)) | BDBM50240701

(2-Methyl-10-piperazin-1-yl-4H-3-thia-4,9-diaza-ben...)Show InChI InChI=1S/C16H18N4S/c1-11-10-12-15(20-8-6-17-7-9-20)18-13-4-2-3-5-14(13)19-16(12)21-11/h2-5,10,17,19H,6-9H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against neuronal 5-hydroxytryptamine 2 receptor |
Bioorg Med Chem Lett 7: 25-30 (1997)
Article DOI: 10.1016/S0960-894X(96)00567-7
BindingDB Entry DOI: 10.7270/Q2KD1XXP |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50132686

(CHEMBL326466 | Dimethyl-((2S,3S)-3-naphthalen-2-yl...)Show InChI InChI=1S/C20H25N/c1-21(2)13-19-16-8-10-18(12-16)20(19)17-9-7-14-5-3-4-6-15(14)11-17/h3-7,9,11,16,18-20H,8,10,12-13H2,1-2H3/t16?,18?,19-,20-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-WIN-35,428 from Dopamine transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50132684

(CHEMBL331799 | Methyl-(3-naphthalen-2-yl-bicyclo[2...)Show InChI InChI=1S/C19H23N/c1-20-12-18-15-7-9-17(11-15)19(18)16-8-6-13-4-2-3-5-14(13)10-16/h2-6,8,10,15,17-20H,7,9,11-12H2,1H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
In vitro inhibition of DA re-uptake into synaptosome |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50132682

(CHEMBL113136 | [(2S,3S)-3-(3-Chloro-phenyl)-bicycl...)Show SMILES CN(C)C[C@H]1C2CCC(CC2)[C@@H]1c1cccc(Cl)c1 |wU:11.13,wD:4.3,TLB:12:11:6.7:10.9,THB:3:4:6.7:10.9,(3.18,-2.78,;1.64,-2.8,;.89,-4.14,;.86,-1.47,;1.62,-.12,;3.16,.53,;3.41,2.42,;2.97,3.54,;2.9,1.88,;4.25,1.28,;4.53,-.1,;1.43,1.25,;.09,2.02,;-1.25,1.25,;-2.58,2.02,;-2.58,3.56,;-1.25,4.33,;-1.25,5.87,;.09,3.56,)| Show InChI InChI=1S/C17H24ClN/c1-19(2)11-16-12-6-8-13(9-7-12)17(16)14-4-3-5-15(18)10-14/h3-5,10,12-13,16-17H,6-9,11H2,1-2H3/t12?,13?,16-,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
In vitro ability of compound to inhibit 5-HT re-uptake of radiolabelled [3H]-tritium trasmitter into synaptosome |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50132681

(CHEMBL323678 | [(2S,3S)-3-(6-Methoxy-naphthalen-2-...)Show SMILES COc1ccc2cc(ccc2c1)[C@H]1C2CCC(C2)[C@@H]1CN(C)C Show InChI InChI=1S/C21H27NO/c1-22(2)13-20-16-5-7-18(11-16)21(20)17-6-4-15-12-19(23-3)9-8-14(15)10-17/h4,6,8-10,12,16,18,20-21H,5,7,11,13H2,1-3H3/t16?,18?,20-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-citalopram from Serotonin transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50121599
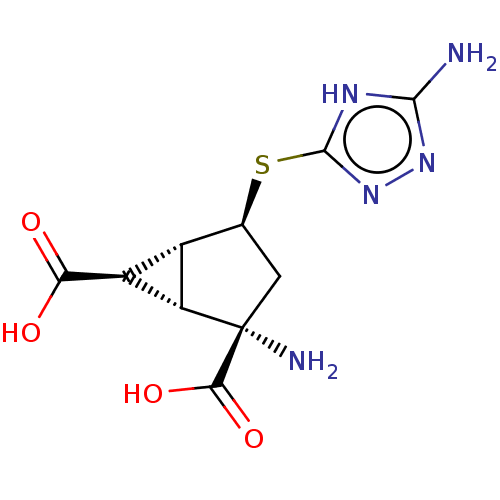
(CHEMBL3616858)Show SMILES [H][C@]12[C@H](C(O)=O)[C@@]1([H])[C@@](N)(C[C@@H]2Sc1nnc(N)[nH]1)C(O)=O |r| Show InChI InChI=1S/C10H13N5O4S/c11-8-13-9(15-14-8)20-2-1-10(12,7(18)19)5-3(2)4(5)6(16)17/h2-5H,1,12H2,(H,16,17)(H,18,19)(H3,11,13,14,15)/t2-,3-,4-,5-,10-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]-459477 from human recombinant mGlu2 receptor expressed in AV12 cells after 90 mins by liquid scintillation counting |
J Med Chem 58: 7526-48 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01124
BindingDB Entry DOI: 10.7270/Q29S1ST0 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50132677

(CHEMBL109517 | Dimethyl-((2R,3R)-3-naphthalen-2-yl...)Show InChI InChI=1S/C20H25N/c1-21(2)13-19-16-8-10-18(12-16)20(19)17-9-7-14-5-3-4-6-15(14)11-17/h3-7,9,11,16,18-20H,8,10,12-13H2,1-2H3/t16?,18?,19-,20-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-WIN-35,428 from Dopamine transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50132679

(CHEMBL111128 | Methyl-((2S,3S)-3-naphthalen-2-yl-b...)Show InChI InChI=1S/C19H23N/c1-20-12-18-15-7-9-17(11-15)19(18)16-8-6-13-4-2-3-5-14(13)10-16/h2-6,8,10,15,17-20H,7,9,11-12H2,1H3/t15?,17?,18-,19-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-WIN-35,428 from Dopamine transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 3
(Homo sapiens (Human)) | BDBM50121539

(CHEMBL3616856)Show SMILES [H][C@]12[C@H](C(O)=O)[C@@]1([H])[C@@](N)(C[C@@H]2Sc1nnc([nH]1)C(F)(F)F)C(O)=O |r| Show InChI InChI=1S/C11H11F3N4O4S/c12-11(13,14)7-16-9(18-17-7)23-2-1-10(15,8(21)22)5-3(2)4(5)6(19)20/h2-5H,1,15H2,(H,19,20)(H,21,22)(H,16,17,18)/t2-,3-,4-,5-,10-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]-459477 from human recombinant mGlu3 receptor expressed in AV12 cells after 90 mins by liquid scintillation counting |
J Med Chem 58: 7526-48 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01124
BindingDB Entry DOI: 10.7270/Q29S1ST0 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50026952

((cis) [3-(3,4-Dichloro-phenyl)-bicyclo[2.2.2]oct-2...)Show SMILES CN(C)C[C@@H]1C2CCC(CC2)[C@@H]1c1ccc(Cl)c(Cl)c1 |wU:11.13,4.3,TLB:12:11:6.7:10.9,THB:3:4:6.7:10.9,(4.42,-5.07,;3.76,-3.67,;2.22,-3.53,;4.65,-2.41,;4,-1.01,;5.68,-.26,;5.79,1.35,;5.19,2.75,;5.16,1.21,;6.61,.69,;7.15,-.75,;3.76,.53,;2.43,1.3,;1.1,.53,;-.23,1.3,;-.23,2.84,;-1.58,3.63,;1.1,3.61,;1.1,5.15,;2.43,2.84,)| Show InChI InChI=1S/C17H23Cl2N/c1-20(2)10-14-11-3-5-12(6-4-11)17(14)13-7-8-15(18)16(19)9-13/h7-9,11-12,14,17H,3-6,10H2,1-2H3/t11?,12?,14-,17-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-WIN-35,428 from Dopamine transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50132683

(6-((2S,3S)-3-Dimethylaminomethyl-bicyclo[2.2.1]hep...)Show SMILES CN(C)C[C@H]1C2CCC(C2)[C@@H]1c1ccc2cc(O)ccc2c1 Show InChI InChI=1S/C20H25NO/c1-21(2)12-19-15-4-6-17(10-15)20(19)16-5-3-14-11-18(22)8-7-13(14)9-16/h3,5,7-9,11,15,17,19-20,22H,4,6,10,12H2,1-2H3/t15?,17?,19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-citalopram from Serotonin transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50132687

(CHEMBL324269 | [(2S,3S)-3-(6-Fluoro-naphthalen-2-y...)Show SMILES CN(C)C[C@H]1C2CCC(C2)[C@@H]1c1ccc2cc(F)ccc2c1 Show InChI InChI=1S/C20H24FN/c1-22(2)12-19-15-4-6-17(10-15)20(19)16-5-3-14-11-18(21)8-7-13(14)9-16/h3,5,7-9,11,15,17,19-20H,4,6,10,12H2,1-2H3/t15?,17?,19-,20-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-WIN-35,428 from Dopamine transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50132683

(6-((2S,3S)-3-Dimethylaminomethyl-bicyclo[2.2.1]hep...)Show SMILES CN(C)C[C@H]1C2CCC(C2)[C@@H]1c1ccc2cc(O)ccc2c1 Show InChI InChI=1S/C20H25NO/c1-21(2)12-19-15-4-6-17(10-15)20(19)16-5-3-14-11-18(22)8-7-13(14)9-16/h3,5,7-9,11,15,17,19-20,22H,4,6,10,12H2,1-2H3/t15?,17?,19-,20-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Nisoxetine from norepinephrin transpoter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50132682

(CHEMBL113136 | [(2S,3S)-3-(3-Chloro-phenyl)-bicycl...)Show SMILES CN(C)C[C@H]1C2CCC(CC2)[C@@H]1c1cccc(Cl)c1 |wU:11.13,wD:4.3,TLB:12:11:6.7:10.9,THB:3:4:6.7:10.9,(3.18,-2.78,;1.64,-2.8,;.89,-4.14,;.86,-1.47,;1.62,-.12,;3.16,.53,;3.41,2.42,;2.97,3.54,;2.9,1.88,;4.25,1.28,;4.53,-.1,;1.43,1.25,;.09,2.02,;-1.25,1.25,;-2.58,2.02,;-2.58,3.56,;-1.25,4.33,;-1.25,5.87,;.09,3.56,)| Show InChI InChI=1S/C17H24ClN/c1-19(2)11-16-12-6-8-13(9-7-12)17(16)14-4-3-5-15(18)10-14/h3-5,10,12-13,16-17H,6-9,11H2,1-2H3/t12?,13?,16-,17+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Nisoxetine from norepinephrin transpoter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50132688
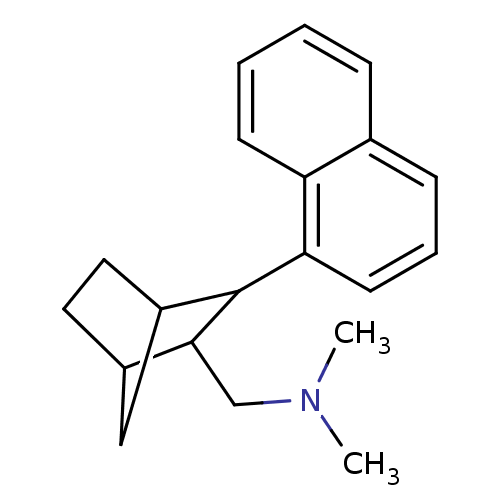
(CHEMBL323447 | Dimethyl-(3-naphthalen-1-yl-bicyclo...)Show InChI InChI=1S/C20H25N/c1-21(2)13-19-15-10-11-16(12-15)20(19)18-9-5-7-14-6-3-4-8-17(14)18/h3-9,15-16,19-20H,10-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-citalopram from Serotonin transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50026952

((cis) [3-(3,4-Dichloro-phenyl)-bicyclo[2.2.2]oct-2...)Show SMILES CN(C)C[C@@H]1C2CCC(CC2)[C@@H]1c1ccc(Cl)c(Cl)c1 |wU:11.13,4.3,TLB:12:11:6.7:10.9,THB:3:4:6.7:10.9,(4.42,-5.07,;3.76,-3.67,;2.22,-3.53,;4.65,-2.41,;4,-1.01,;5.68,-.26,;5.79,1.35,;5.19,2.75,;5.16,1.21,;6.61,.69,;7.15,-.75,;3.76,.53,;2.43,1.3,;1.1,.53,;-.23,1.3,;-.23,2.84,;-1.58,3.63,;1.1,3.61,;1.1,5.15,;2.43,2.84,)| Show InChI InChI=1S/C17H23Cl2N/c1-20(2)10-14-11-3-5-12(6-4-11)17(14)13-7-8-15(18)16(19)9-13/h7-9,11-12,14,17H,3-6,10H2,1-2H3/t11?,12?,14-,17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-WIN-35,428 from Dopamine transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 3
(Homo sapiens (Human)) | BDBM50121647
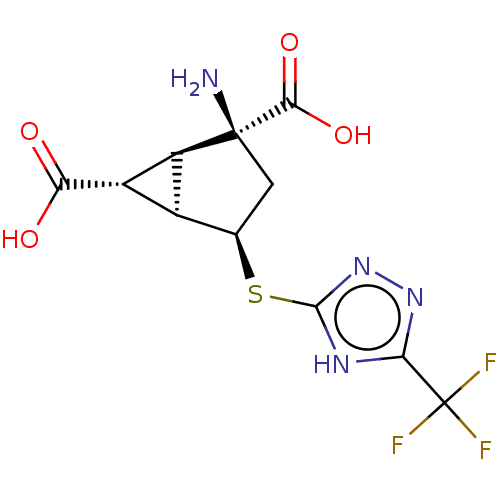
(CHEMBL3616849)Show SMILES [H][C@]12[C@H](C(O)=O)[C@@]1([H])[C@@](N)(C[C@H]2Sc1nnc([nH]1)C(F)(F)F)C(O)=O |r| Show InChI InChI=1S/C11H11F3N4O4S/c12-11(13,14)7-16-9(18-17-7)23-2-1-10(15,8(21)22)5-3(2)4(5)6(19)20/h2-5H,1,15H2,(H,19,20)(H,21,22)(H,16,17,18)/t2-,3+,4+,5+,10+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]-459477 from human recombinant mGlu3 receptor expressed in AV12 cells after 90 mins by liquid scintillation counting |
J Med Chem 58: 7526-48 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01124
BindingDB Entry DOI: 10.7270/Q29S1ST0 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 3
(Homo sapiens (Human)) | BDBM50121652
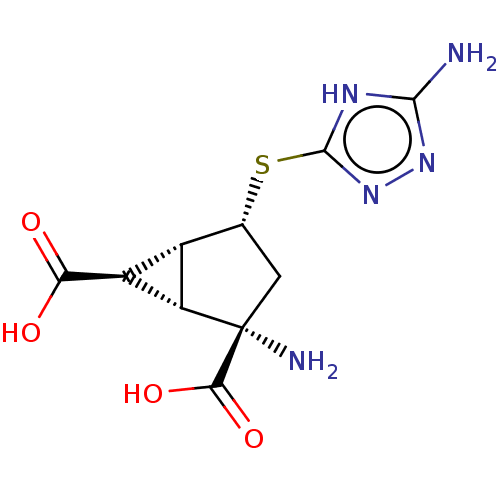
(CHEMBL3616851)Show SMILES [H][C@]12[C@H](C(O)=O)[C@@]1([H])[C@@](N)(C[C@H]2Sc1nnc(N)[nH]1)C(O)=O |r| Show InChI InChI=1S/C10H13N5O4S/c11-8-13-9(15-14-8)20-2-1-10(12,7(18)19)5-3(2)4(5)6(16)17/h2-5H,1,12H2,(H,16,17)(H,18,19)(H3,11,13,14,15)/t2-,3+,4+,5+,10+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]-459477 from human recombinant mGlu3 receptor expressed in AV12 cells after 90 mins by liquid scintillation counting |
J Med Chem 58: 7526-48 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01124
BindingDB Entry DOI: 10.7270/Q29S1ST0 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 3
(Homo sapiens (Human)) | BDBM50121540

(CHEMBL3616857)Show SMILES [H][C@]12[C@H](C(O)=O)[C@@]1([H])[C@@](N)(C[C@@H]2Sc1nnc([nH]1)C(F)F)C(O)=O |r| Show InChI InChI=1S/C11H12F2N4O4S/c12-6(13)7-15-10(17-16-7)22-2-1-11(14,9(20)21)5-3(2)4(5)8(18)19/h2-6H,1,14H2,(H,18,19)(H,20,21)(H,15,16,17)/t2-,3-,4-,5-,11-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]-459477 from human recombinant mGlu3 receptor expressed in AV12 cells after 90 mins by liquid scintillation counting |
J Med Chem 58: 7526-48 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01124
BindingDB Entry DOI: 10.7270/Q29S1ST0 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50121655
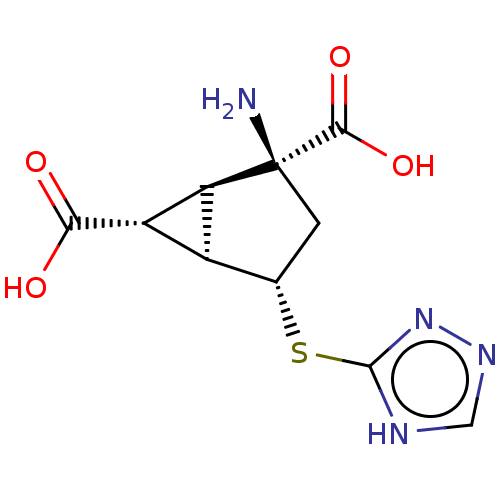
(CHEMBL3616854)Show SMILES [H][C@]12[C@H](C(O)=O)[C@@]1([H])[C@@](N)(C[C@@H]2Sc1nnc[nH]1)C(O)=O |r| Show InChI InChI=1S/C10H12N4O4S/c11-10(8(17)18)1-3(19-9-12-2-13-14-9)4-5(6(4)10)7(15)16/h2-6H,1,11H2,(H,15,16)(H,17,18)(H,12,13,14)/t3-,4-,5-,6-,10-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]-459477 from human recombinant mGlu2 receptor expressed in AV12 cells after 90 mins by liquid scintillation counting |
J Med Chem 58: 7526-48 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01124
BindingDB Entry DOI: 10.7270/Q29S1ST0 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50132680

(CHEMBL322348 | [(2S,3S)-3-(6-Methoxy-naphthalen-2-...)Show SMILES CNC[C@H]1C2CCC(C2)[C@@H]1c1ccc2cc(OC)ccc2c1 Show InChI InChI=1S/C20H25NO/c1-21-12-19-15-4-6-17(10-15)20(19)16-5-3-14-11-18(22-2)8-7-13(14)9-16/h3,5,7-9,11,15,17,19-21H,4,6,10,12H2,1-2H3/t15?,17?,19-,20-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-WIN-35,428 from Dopamine transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 3
(Homo sapiens (Human)) | BDBM50121646
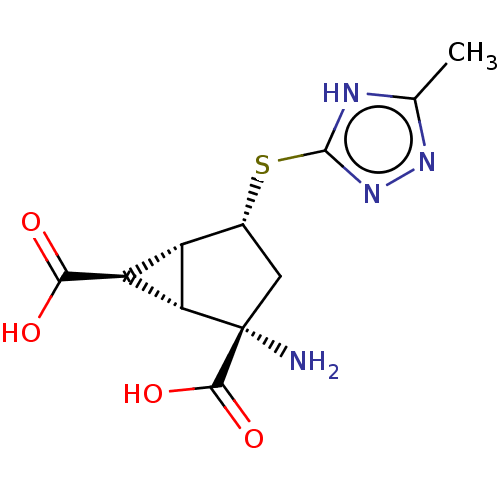
(CHEMBL3616848)Show SMILES [H][C@]12[C@H](C(O)=O)[C@@]1([H])[C@@](N)(C[C@H]2Sc1nnc(C)[nH]1)C(O)=O |r| Show InChI InChI=1S/C11H14N4O4S/c1-3-13-10(15-14-3)20-4-2-11(12,9(18)19)7-5(4)6(7)8(16)17/h4-7H,2,12H2,1H3,(H,16,17)(H,18,19)(H,13,14,15)/t4-,5+,6+,7+,11+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]-459477 from human recombinant mGlu3 receptor expressed in AV12 cells after 90 mins by liquid scintillation counting |
J Med Chem 58: 7526-48 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01124
BindingDB Entry DOI: 10.7270/Q29S1ST0 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 3
(Homo sapiens (Human)) | BDBM50121651

(CHEMBL3616850)Show SMILES [H][C@]12[C@H](C(O)=O)[C@@]1([H])[C@@](N)(C[C@H]2Sc1nnc([nH]1)C(F)F)C(O)=O |r| Show InChI InChI=1S/C11H12F2N4O4S/c12-6(13)7-15-10(17-16-7)22-2-1-11(14,9(20)21)5-3(2)4(5)8(18)19/h2-6H,1,14H2,(H,18,19)(H,20,21)(H,15,16,17)/t2-,3+,4+,5+,11+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]-459477 from human recombinant mGlu3 receptor expressed in AV12 cells after 90 mins by liquid scintillation counting |
J Med Chem 58: 7526-48 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01124
BindingDB Entry DOI: 10.7270/Q29S1ST0 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50132682

(CHEMBL113136 | [(2S,3S)-3-(3-Chloro-phenyl)-bicycl...)Show SMILES CN(C)C[C@H]1C2CCC(CC2)[C@@H]1c1cccc(Cl)c1 |wU:11.13,wD:4.3,TLB:12:11:6.7:10.9,THB:3:4:6.7:10.9,(3.18,-2.78,;1.64,-2.8,;.89,-4.14,;.86,-1.47,;1.62,-.12,;3.16,.53,;3.41,2.42,;2.97,3.54,;2.9,1.88,;4.25,1.28,;4.53,-.1,;1.43,1.25,;.09,2.02,;-1.25,1.25,;-2.58,2.02,;-2.58,3.56,;-1.25,4.33,;-1.25,5.87,;.09,3.56,)| Show InChI InChI=1S/C17H24ClN/c1-19(2)11-16-12-6-8-13(9-7-12)17(16)14-4-3-5-15(18)10-14/h3-5,10,12-13,16-17H,6-9,11H2,1-2H3/t12?,13?,16-,17+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
In vitro inhibition of DA re-uptake into synaptosome |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50132681

(CHEMBL323678 | [(2S,3S)-3-(6-Methoxy-naphthalen-2-...)Show SMILES COc1ccc2cc(ccc2c1)[C@H]1C2CCC(C2)[C@@H]1CN(C)C Show InChI InChI=1S/C21H27NO/c1-22(2)13-20-16-5-7-18(11-16)21(20)17-6-4-15-12-19(23-3)9-8-14(15)10-17/h4,6,8-10,12,16,18,20-21H,5,7,11,13H2,1-3H3/t16?,18?,20-,21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [3H]-WIN-35,428 from Dopamine transporter |
Bioorg Med Chem Lett 13: 3277-80 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DXF |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 3
(Homo sapiens (Human)) | BDBM50121642
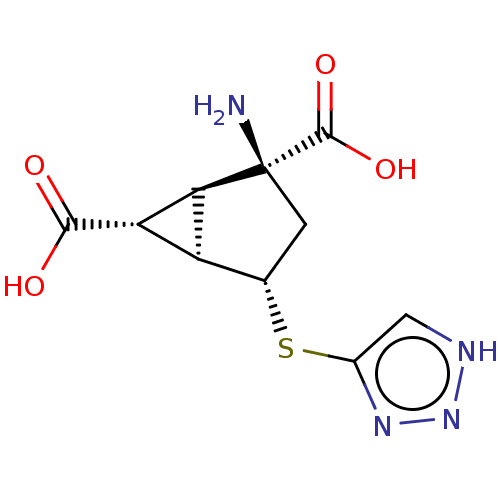
(CHEMBL3616860)Show SMILES [H][C@]12[C@H](C(O)=O)[C@@]1([H])[C@@](N)(C[C@@H]2Sc1c[nH]nn1)C(O)=O |r| Show InChI InChI=1S/C10H12N4O4S/c11-10(9(17)18)1-3(19-4-2-12-14-13-4)5-6(7(5)10)8(15)16/h2-3,5-7H,1,11H2,(H,15,16)(H,17,18)(H,12,13,14)/t3-,5-,6-,7-,10-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]-459477 from human recombinant mGlu3 receptor expressed in AV12 cells after 90 mins by liquid scintillation counting |
J Med Chem 58: 7526-48 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01124
BindingDB Entry DOI: 10.7270/Q29S1ST0 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50289291

(2-Hydroxy-methylolanzapine | CHEMBL100454 | [10-(4...)Show InChI InChI=1S/C17H20N4OS/c1-20-6-8-21(9-7-20)16-13-10-12(11-22)23-17(13)19-15-5-3-2-4-14(15)18-16/h2-5,10,19,22H,6-9,11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against dopamine neuronal Dopamine receptor D1 |
Bioorg Med Chem Lett 7: 25-30 (1997)
Article DOI: 10.1016/S0960-894X(96)00567-7
BindingDB Entry DOI: 10.7270/Q2KD1XXP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data