 Found 498 hits with Last Name = 'ueno' and Initial = 'y'
Found 498 hits with Last Name = 'ueno' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50599576

(CHEMBL5200218)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@H]2NC(=O)CCc1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50274482
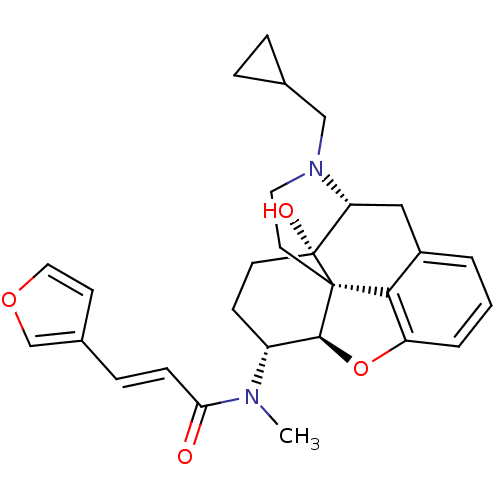
((2E)-N-[(5R,6R)-17-(Cyclopropylmethyl)-4,5-epoxy-1...)Show SMILES CN([C@@H]1CC[C@@]2(O)[C@H]3Cc4cccc5O[C@@H]1[C@]2(CCN3CC1CC1)c45)C(=O)\C=C\c1ccoc1 |r,TLB:4:5:9.24.8:17.19.18,THB:6:5:9.24.8:17.19.18| Show InChI InChI=1S/C28H32N2O4/c1-29(24(31)8-7-19-10-14-33-17-19)21-9-11-28(32)23-15-20-3-2-4-22-25(20)27(28,26(21)34-22)12-13-30(23)16-18-5-6-18/h2-4,7-8,10,14,17-18,21,23,26,32H,5-6,9,11-13,15-16H2,1H3/b8-7+/t21-,23-,26+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50599574
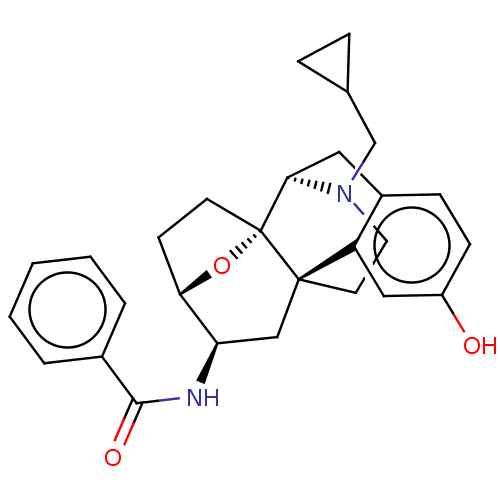
(CHEMBL5186730)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@H]2NC(=O)c1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50599574

(CHEMBL5186730)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@H]2NC(=O)c1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50599575

(CHEMBL5178511)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@H]2NC(=O)Cc1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50599576

(CHEMBL5200218)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@H]2NC(=O)CCc1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.197 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50599580

(CHEMBL5200050)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@H]2N(C)C(=O)c1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.256 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50599575

(CHEMBL5178511)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@H]2NC(=O)Cc1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.267 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50599581

(CHEMBL5185996)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@H]2N(C)C(=O)Cc1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.278 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50599582

(CHEMBL5200992)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@H]2N(C)C(=O)CCc1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.332 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50599575

(CHEMBL5178511)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@H]2NC(=O)Cc1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.522 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50599577
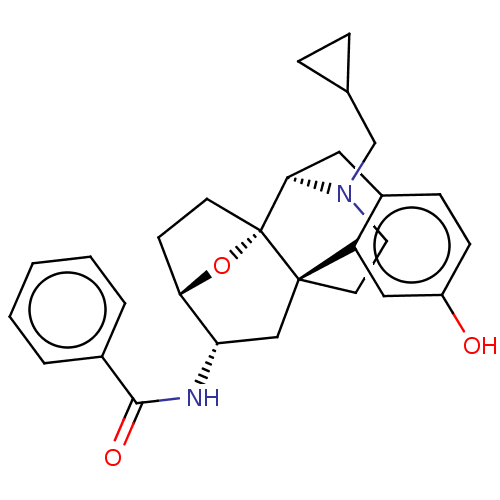
(CHEMBL5208385)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@@H]2NC(=O)c1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.564 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50274482

((2E)-N-[(5R,6R)-17-(Cyclopropylmethyl)-4,5-epoxy-1...)Show SMILES CN([C@@H]1CC[C@@]2(O)[C@H]3Cc4cccc5O[C@@H]1[C@]2(CCN3CC1CC1)c45)C(=O)\C=C\c1ccoc1 |r,TLB:4:5:9.24.8:17.19.18,THB:6:5:9.24.8:17.19.18| Show InChI InChI=1S/C28H32N2O4/c1-29(24(31)8-7-19-10-14-33-17-19)21-9-11-28(32)23-15-20-3-2-4-22-25(20)27(28,26(21)34-22)12-13-30(23)16-18-5-6-18/h2-4,7-8,10,14,17-18,21,23,26,32H,5-6,9,11-13,15-16H2,1H3/b8-7+/t21-,23-,26+,27+,28-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50599579
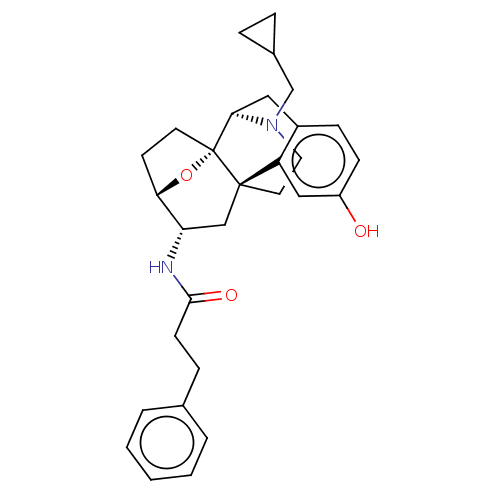
(CHEMBL5170301)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@@H]2NC(=O)CCc1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50599580

(CHEMBL5200050)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@H]2N(C)C(=O)c1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50599582

(CHEMBL5200992)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@H]2N(C)C(=O)CCc1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50599581

(CHEMBL5185996)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@H]2N(C)C(=O)Cc1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50599578
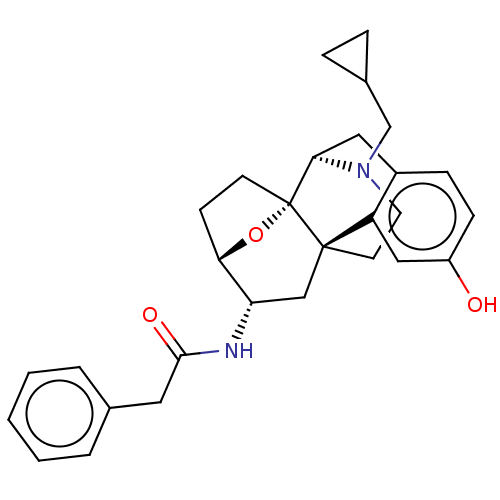
(CHEMBL5175717)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@@H]2NC(=O)Cc1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50599574

(CHEMBL5186730)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@H]2NC(=O)c1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50599579

(CHEMBL5170301)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@@H]2NC(=O)CCc1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50599578

(CHEMBL5175717)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@@H]2NC(=O)Cc1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50599578

(CHEMBL5175717)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@@H]2NC(=O)Cc1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50599579

(CHEMBL5170301)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@@H]2NC(=O)CCc1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50599576

(CHEMBL5200218)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@H]2NC(=O)CCc1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50599577

(CHEMBL5208385)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@@H]2NC(=O)c1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50032379
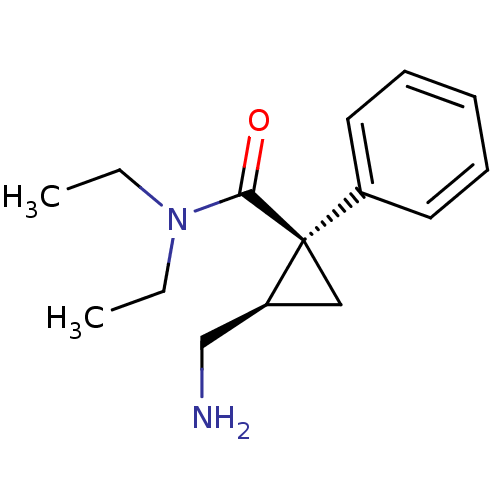
((1S,2R)-2-(aminomethyl)-N,N-diethyl-1-phenylcyclop...)Show InChI InChI=1S/C15H22N2O/c1-3-17(4-2)14(18)15(10-13(15)11-16)12-8-6-5-7-9-12/h5-9,13H,3-4,10-11,16H2,1-2H3/t13-,15+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-paroxetine binding on serotonin transporter of rat cerebral cortical synaptic membrane |
J Med Chem 39: 4844-52 (1997)
Article DOI: 10.1021/jm960495w
BindingDB Entry DOI: 10.7270/Q2M907R2 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50599577

(CHEMBL5208385)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@@H]2NC(=O)c1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50054804
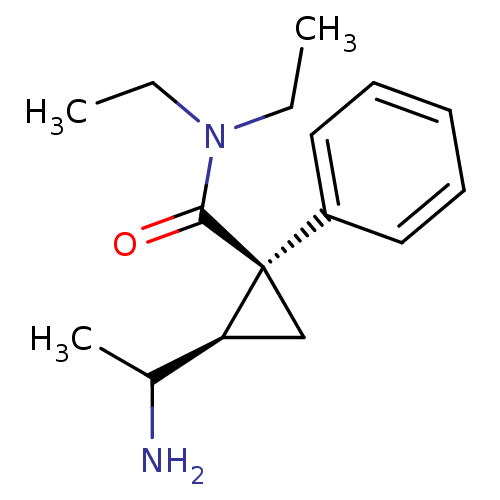
((1S,2R)-2-(1-Amino-ethyl)-1-phenyl-cyclopropanecar...)Show InChI InChI=1S/C16H24N2O/c1-4-18(5-2)15(19)16(11-14(16)12(3)17)13-9-7-6-8-10-13/h6-10,12,14H,4-5,11,17H2,1-3H3/t12?,14-,16+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-paroxetine binding on serotonin transporter of rat cerebral cortical synaptic membrane |
J Med Chem 39: 4844-52 (1997)
Article DOI: 10.1021/jm960495w
BindingDB Entry DOI: 10.7270/Q2M907R2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50054804

((1S,2R)-2-(1-Amino-ethyl)-1-phenyl-cyclopropanecar...)Show InChI InChI=1S/C16H24N2O/c1-4-18(5-2)15(19)16(11-14(16)12(3)17)13-9-7-6-8-10-13/h6-10,12,14H,4-5,11,17H2,1-3H3/t12?,14-,16+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-paroxetine binding on serotonin transporter of rat cerebral cortical synaptic membrane |
J Med Chem 39: 4844-52 (1997)
Article DOI: 10.1021/jm960495w
BindingDB Entry DOI: 10.7270/Q2M907R2 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50599581

(CHEMBL5185996)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@H]2N(C)C(=O)Cc1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50599582

(CHEMBL5200992)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@H]2N(C)C(=O)CCc1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50599580

(CHEMBL5200050)Show SMILES [H][C@@]12CC[C@@]3(O1)[C@@]1([H])Cc4ccc(O)cc4[C@@]3(CCN1CC1CC1)C[C@H]2N(C)C(=O)c1ccccc1 |r,TLB:20:19:4:8.9.15| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50054802

((1S,2R)-2-(1-Amino-allyl)-1-phenyl-cyclopropanecar...)Show InChI InChI=1S/C17H24N2O/c1-4-15(18)14-12-17(14,13-10-8-7-9-11-13)16(20)19(5-2)6-3/h4,7-11,14-15H,1,5-6,12,18H2,2-3H3/t14-,15?,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-paroxetine binding on serotonin transporter of rat cerebral cortical synaptic membrane |
J Med Chem 39: 4844-52 (1997)
Article DOI: 10.1021/jm960495w
BindingDB Entry DOI: 10.7270/Q2M907R2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50054800
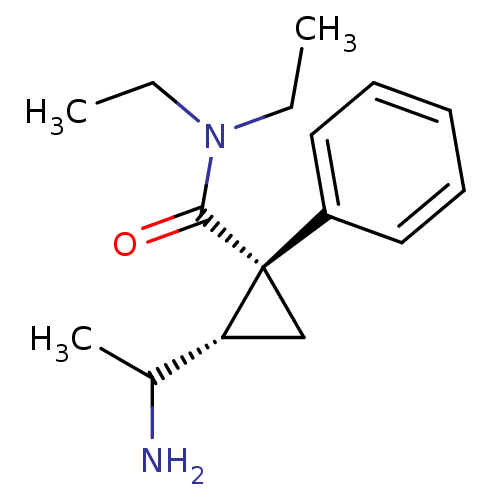
((1R,2S)-2-(1-Amino-ethyl)-1-phenyl-cyclopropanecar...)Show InChI InChI=1S/C16H24N2O/c1-4-18(5-2)15(19)16(11-14(16)12(3)17)13-9-7-6-8-10-13/h6-10,12,14H,4-5,11,17H2,1-3H3/t12?,14-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-paroxetine binding on serotonin transporter of rat cerebral cortical synaptic membrane |
J Med Chem 39: 4844-52 (1997)
Article DOI: 10.1021/jm960495w
BindingDB Entry DOI: 10.7270/Q2M907R2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50054800

((1R,2S)-2-(1-Amino-ethyl)-1-phenyl-cyclopropanecar...)Show InChI InChI=1S/C16H24N2O/c1-4-18(5-2)15(19)16(11-14(16)12(3)17)13-9-7-6-8-10-13/h6-10,12,14H,4-5,11,17H2,1-3H3/t12?,14-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-paroxetine binding on serotonin transporter of rat cerebral cortical synaptic membrane |
J Med Chem 39: 4844-52 (1997)
Article DOI: 10.1021/jm960495w
BindingDB Entry DOI: 10.7270/Q2M907R2 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50274482

((2E)-N-[(5R,6R)-17-(Cyclopropylmethyl)-4,5-epoxy-1...)Show SMILES CN([C@@H]1CC[C@@]2(O)[C@H]3Cc4cccc5O[C@@H]1[C@]2(CCN3CC1CC1)c45)C(=O)\C=C\c1ccoc1 |r,TLB:4:5:9.24.8:17.19.18,THB:6:5:9.24.8:17.19.18| Show InChI InChI=1S/C28H32N2O4/c1-29(24(31)8-7-19-10-14-33-17-19)21-9-11-28(32)23-15-20-3-2-4-22-25(20)27(28,26(21)34-22)12-13-30(23)16-18-5-6-18/h2-4,7-8,10,14,17-18,21,23,26,32H,5-6,9,11-13,15-16H2,1H3/b8-7+/t21-,23-,26+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 408 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116552
BindingDB Entry DOI: 10.7270/Q2BZ6B34 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50054798
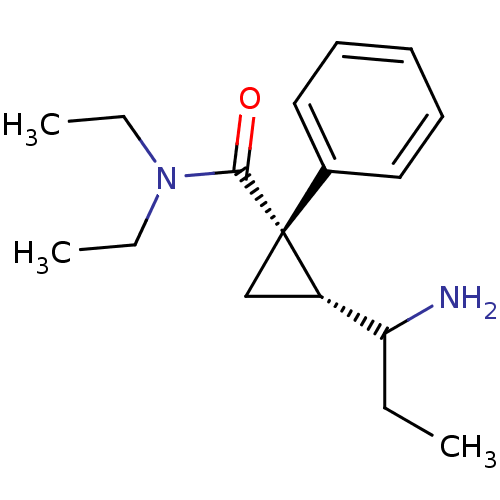
((1S,2R)-2-(1-Amino-propyl)-1-phenyl-cyclopropaneca...)Show InChI InChI=1S/C17H26N2O/c1-4-15(18)14-12-17(14,13-10-8-7-9-11-13)16(20)19(5-2)6-3/h7-11,14-15H,4-6,12,18H2,1-3H3/t14-,15?,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-paroxetine binding on serotonin transporter of rat cerebral cortical synaptic membrane |
J Med Chem 39: 4844-52 (1997)
Article DOI: 10.1021/jm960495w
BindingDB Entry DOI: 10.7270/Q2M907R2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50054798

((1S,2R)-2-(1-Amino-propyl)-1-phenyl-cyclopropaneca...)Show InChI InChI=1S/C17H26N2O/c1-4-15(18)14-12-17(14,13-10-8-7-9-11-13)16(20)19(5-2)6-3/h7-11,14-15H,4-6,12,18H2,1-3H3/t14-,15?,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-paroxetine binding on serotonin transporter of rat cerebral cortical synaptic membrane |
J Med Chem 39: 4844-52 (1997)
Article DOI: 10.1021/jm960495w
BindingDB Entry DOI: 10.7270/Q2M907R2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50054805
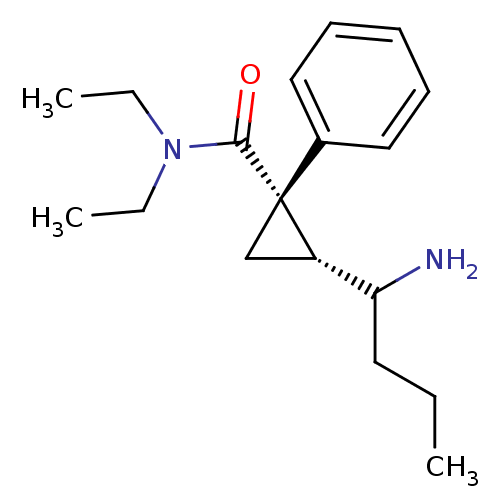
((1S,2R)-2-(1-Amino-butyl)-1-phenyl-cyclopropanecar...)Show SMILES CCCC(N)[C@@H]1C[C@@]1(C(=O)N(CC)CC)c1ccccc1 Show InChI InChI=1S/C18H28N2O/c1-4-10-16(19)15-13-18(15,14-11-8-7-9-12-14)17(21)20(5-2)6-3/h7-9,11-12,15-16H,4-6,10,13,19H2,1-3H3/t15-,16?,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-paroxetine binding on serotonin transporter of rat cerebral cortical synaptic membrane |
J Med Chem 39: 4844-52 (1997)
Article DOI: 10.1021/jm960495w
BindingDB Entry DOI: 10.7270/Q2M907R2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50054799
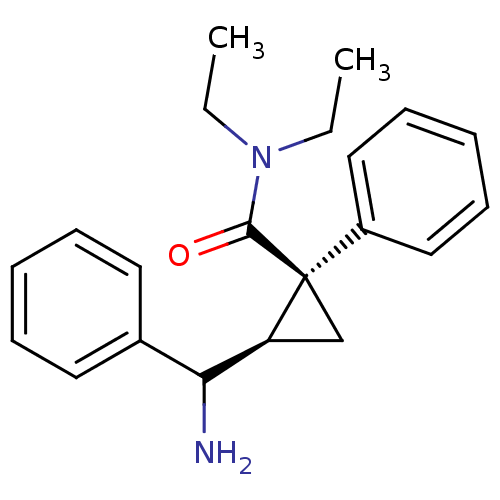
((1S,2R)-2-(Amino-phenyl-methyl)-1-phenyl-cycloprop...)Show SMILES CCN(CC)C(=O)[C@]1(C[C@H]1C(N)c1ccccc1)c1ccccc1 Show InChI InChI=1S/C21H26N2O/c1-3-23(4-2)20(24)21(17-13-9-6-10-14-17)15-18(21)19(22)16-11-7-5-8-12-16/h5-14,18-19H,3-4,15,22H2,1-2H3/t18-,19?,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-paroxetine binding on serotonin transporter of rat cerebral cortical synaptic membrane |
J Med Chem 39: 4844-52 (1997)
Article DOI: 10.1021/jm960495w
BindingDB Entry DOI: 10.7270/Q2M907R2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50054803
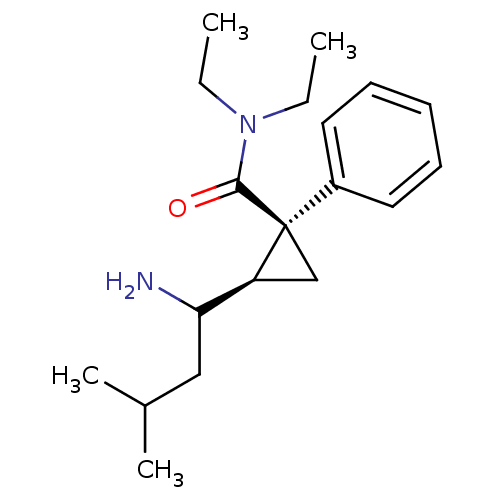
((1S,2R)-2-(1-Amino-3-methyl-butyl)-1-phenyl-cyclop...)Show SMILES CCN(CC)C(=O)[C@]1(C[C@H]1C(N)CC(C)C)c1ccccc1 Show InChI InChI=1S/C19H30N2O/c1-5-21(6-2)18(22)19(15-10-8-7-9-11-15)13-16(19)17(20)12-14(3)4/h7-11,14,16-17H,5-6,12-13,20H2,1-4H3/t16-,17?,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-paroxetine binding on serotonin transporter of rat cerebral cortical synaptic membrane |
J Med Chem 39: 4844-52 (1997)
Article DOI: 10.1021/jm960495w
BindingDB Entry DOI: 10.7270/Q2M907R2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50054801
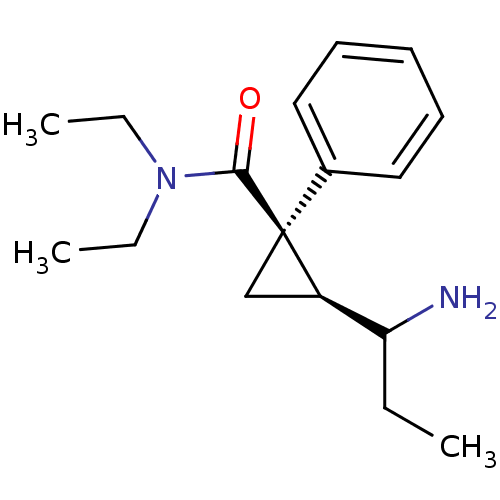
((1R,2S)-2-(1-Amino-propyl)-1-phenyl-cyclopropaneca...)Show InChI InChI=1S/C17H26N2O/c1-4-15(18)14-12-17(14,13-10-8-7-9-11-13)16(20)19(5-2)6-3/h7-11,14-15H,4-6,12,18H2,1-3H3/t14-,15?,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-paroxetine binding on serotonin transporter of rat cerebral cortical synaptic membrane |
J Med Chem 39: 4844-52 (1997)
Article DOI: 10.1021/jm960495w
BindingDB Entry DOI: 10.7270/Q2M907R2 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50054801

((1R,2S)-2-(1-Amino-propyl)-1-phenyl-cyclopropaneca...)Show InChI InChI=1S/C17H26N2O/c1-4-15(18)14-12-17(14,13-10-8-7-9-11-13)16(20)19(5-2)6-3/h7-11,14-15H,4-6,12,18H2,1-3H3/t14-,15?,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-paroxetine binding on serotonin transporter of rat cerebral cortical synaptic membrane |
J Med Chem 39: 4844-52 (1997)
Article DOI: 10.1021/jm960495w
BindingDB Entry DOI: 10.7270/Q2M907R2 |
More data for this
Ligand-Target Pair | |
Stearoyl-CoA desaturase
(Homo sapiens (Human)) | BDBM50306131
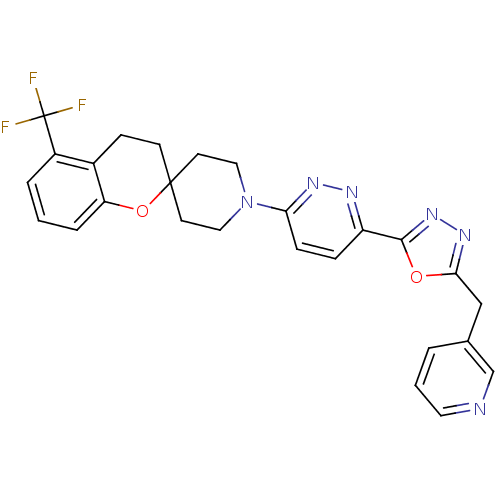
(1'-(6-(5-(pyridin-3-ylmethyl)-1,3,4-oxadiazol-2-yl...)Show SMILES FC(F)(F)c1cccc2OC3(CCN(CC3)c3ccc(nn3)-c3nnc(Cc4cccnc4)o3)CCc12 Show InChI InChI=1S/C26H23F3N6O2/c27-26(28,29)19-4-1-5-21-18(19)8-9-25(37-21)10-13-35(14-11-25)22-7-6-20(31-32-22)24-34-33-23(36-24)15-17-3-2-12-30-16-17/h1-7,12,16H,8-11,13-15H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of SCD1 in HEK293A cell microsomes assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 mins |
Eur J Med Chem 45: 4788-96 (2010)
Article DOI: 10.1016/j.ejmech.2010.07.044
BindingDB Entry DOI: 10.7270/Q2CZ37CN |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Mus musculus) | BDBM50306131

(1'-(6-(5-(pyridin-3-ylmethyl)-1,3,4-oxadiazol-2-yl...)Show SMILES FC(F)(F)c1cccc2OC3(CCN(CC3)c3ccc(nn3)-c3nnc(Cc4cccnc4)o3)CCc12 Show InChI InChI=1S/C26H23F3N6O2/c27-26(28,29)19-4-1-5-21-18(19)8-9-25(37-21)10-13-35(14-11-25)22-7-6-20(31-32-22)24-34-33-23(36-24)15-17-3-2-12-30-16-17/h1-7,12,16H,8-11,13-15H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of SCD1 in mouse microsomal liver S9 microsomal fraction assessed as conversion of [14C]stearate to [14C]oleate after 60 mins |
Bioorg Med Chem Lett 20: 746-54 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.043
BindingDB Entry DOI: 10.7270/Q28C9WBJ |
More data for this
Ligand-Target Pair | |
Stearoyl-CoA desaturase
(Homo sapiens (Human)) | BDBM50306131

(1'-(6-(5-(pyridin-3-ylmethyl)-1,3,4-oxadiazol-2-yl...)Show SMILES FC(F)(F)c1cccc2OC3(CCN(CC3)c3ccc(nn3)-c3nnc(Cc4cccnc4)o3)CCc12 Show InChI InChI=1S/C26H23F3N6O2/c27-26(28,29)19-4-1-5-21-18(19)8-9-25(37-21)10-13-35(14-11-25)22-7-6-20(31-32-22)24-34-33-23(36-24)15-17-3-2-12-30-16-17/h1-7,12,16H,8-11,13-15H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human SCD1 |
Eur J Med Chem 45: 4788-96 (2010)
Article DOI: 10.1016/j.ejmech.2010.07.044
BindingDB Entry DOI: 10.7270/Q2CZ37CN |
More data for this
Ligand-Target Pair | |
Stearoyl-CoA desaturase
(Homo sapiens (Human)) | BDBM50296309
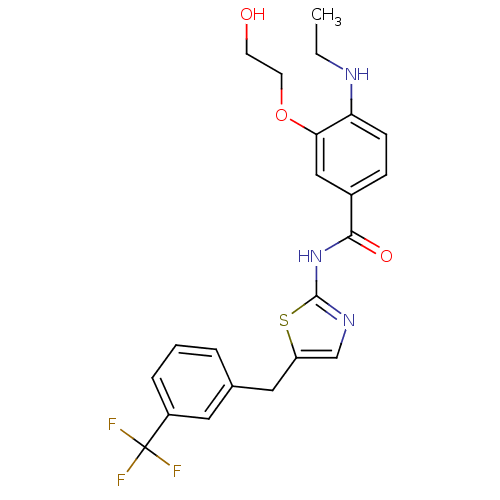
(4-(ethylamino)-3-(2-hydroxyethoxy)-N-(5-(3-(triflu...)Show SMILES CCNc1ccc(cc1OCCO)C(=O)Nc1ncc(Cc2cccc(c2)C(F)(F)F)s1 Show InChI InChI=1S/C22H22F3N3O3S/c1-2-26-18-7-6-15(12-19(18)31-9-8-29)20(30)28-21-27-13-17(32-21)11-14-4-3-5-16(10-14)22(23,24)25/h3-7,10,12-13,26,29H,2,8-9,11H2,1H3,(H,27,28,30) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of stearoyl-CoA desaturase 1 in human microsome assessed as conversion of [14C]stearate to [14C]oleate |
Bioorg Med Chem Lett 19: 4159-66 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.123
BindingDB Entry DOI: 10.7270/Q2PZ58VW |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Mus musculus) | BDBM50330378
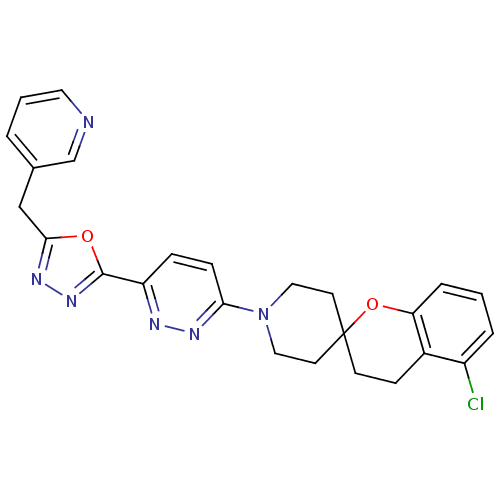
(5-Chloro-1'-{6-[5-(pyridin-3-ylmethyl)-1,3,4-oxadi...)Show SMILES Clc1cccc2OC3(CCN(CC3)c3ccc(nn3)-c3nnc(Cc4cccnc4)o3)CCc12 Show InChI InChI=1S/C25H23ClN6O2/c26-19-4-1-5-21-18(19)8-9-25(34-21)10-13-32(14-11-25)22-7-6-20(28-29-22)24-31-30-23(33-24)15-17-3-2-12-27-16-17/h1-7,12,16H,8-11,13-15H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of SCD1 in mouse microsome assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 mins |
Eur J Med Chem 45: 4788-96 (2010)
Article DOI: 10.1016/j.ejmech.2010.07.044
BindingDB Entry DOI: 10.7270/Q2CZ37CN |
More data for this
Ligand-Target Pair | |
Acyl-CoA desaturase 1
(Mus musculus) | BDBM50306131

(1'-(6-(5-(pyridin-3-ylmethyl)-1,3,4-oxadiazol-2-yl...)Show SMILES FC(F)(F)c1cccc2OC3(CCN(CC3)c3ccc(nn3)-c3nnc(Cc4cccnc4)o3)CCc12 Show InChI InChI=1S/C26H23F3N6O2/c27-26(28,29)19-4-1-5-21-18(19)8-9-25(37-21)10-13-35(14-11-25)22-7-6-20(31-32-22)24-34-33-23(36-24)15-17-3-2-12-30-16-17/h1-7,12,16H,8-11,13-15H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of SCD1 in mouse microsome assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 mins |
Eur J Med Chem 45: 4788-96 (2010)
Article DOI: 10.1016/j.ejmech.2010.07.044
BindingDB Entry DOI: 10.7270/Q2CZ37CN |
More data for this
Ligand-Target Pair | |
Stearoyl-CoA desaturase
(Homo sapiens (Human)) | BDBM50306131

(1'-(6-(5-(pyridin-3-ylmethyl)-1,3,4-oxadiazol-2-yl...)Show SMILES FC(F)(F)c1cccc2OC3(CCN(CC3)c3ccc(nn3)-c3nnc(Cc4cccnc4)o3)CCc12 Show InChI InChI=1S/C26H23F3N6O2/c27-26(28,29)19-4-1-5-21-18(19)8-9-25(37-21)10-13-35(14-11-25)22-7-6-20(31-32-22)24-34-33-23(36-24)15-17-3-2-12-30-16-17/h1-7,12,16H,8-11,13-15H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of SCD1 in human 293A cells assessed as conversion of [14C]stearate to [14C]oleate after 60 mins in presence of S9 microsomal fraction |
Bioorg Med Chem Lett 20: 746-54 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.043
BindingDB Entry DOI: 10.7270/Q28C9WBJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data