

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50554287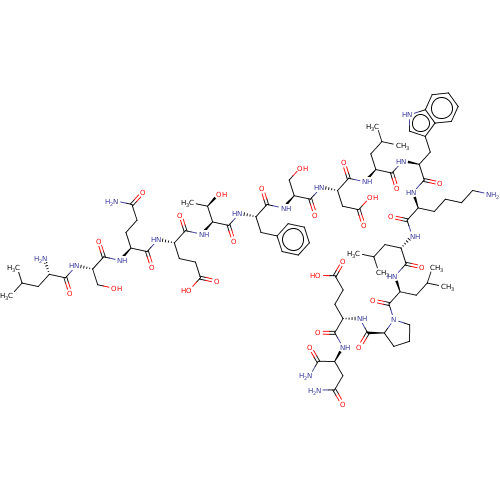 (CHEMBL4745237) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 237 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive binding affinity to human MDM2 assessed as displacement of p1-LC4f peptide by fluorescence polarization competitive binding assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127605 BindingDB Entry DOI: 10.7270/Q2C82DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50554287 (CHEMBL4745237) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 461 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive binding affinity to human MDM2 assessed as displacement of p3-BPf peptide by fluorescence polarization competitive binding assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127605 BindingDB Entry DOI: 10.7270/Q2C82DZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50391561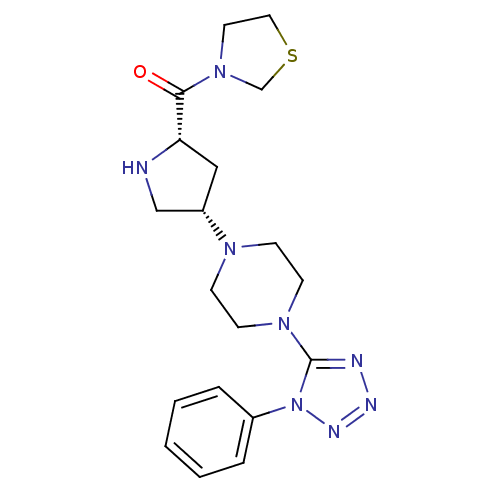 (CHEMBL2147704) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in rat plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50391559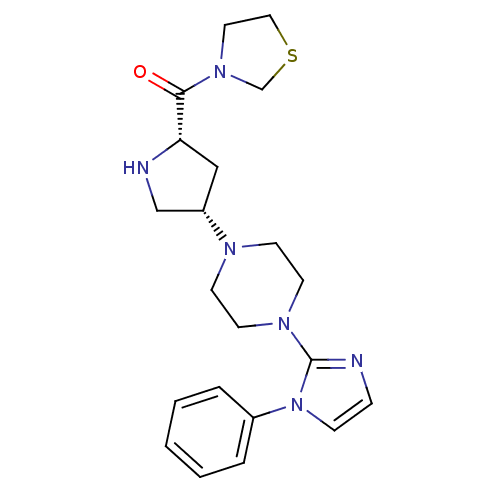 (CHEMBL2147703) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in rat plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391561 (CHEMBL2147704) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391559 (CHEMBL2147703) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50391565 (CHEMBL2147777) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in rat plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391570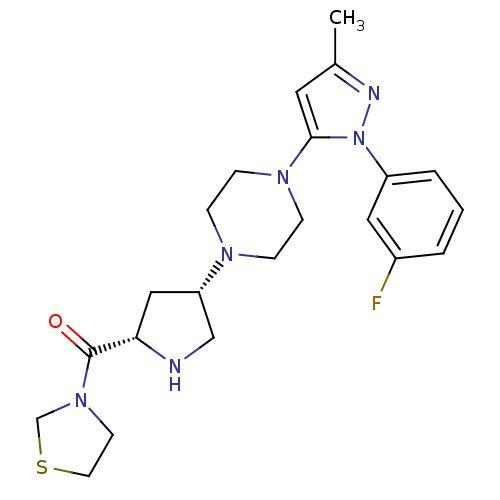 (CHEMBL2147712) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50391567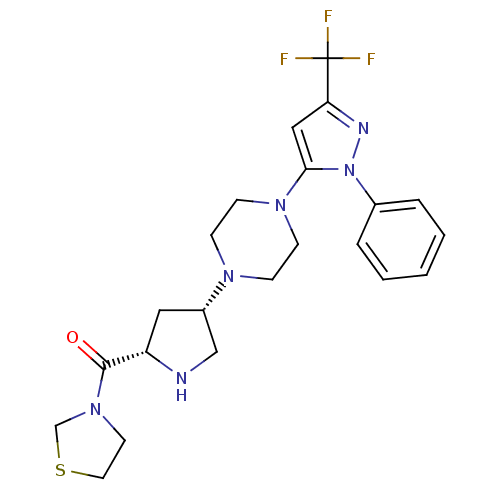 (CHEMBL2147709) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in rat plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391567 (CHEMBL2147709) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391565 (CHEMBL2147777) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50388112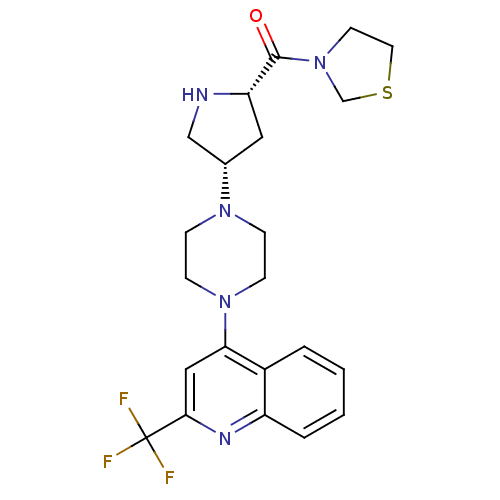 (CHEMBL2058971) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50391570 (CHEMBL2147712) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in rat plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50391571 (CHEMBL2147713) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in rat plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391576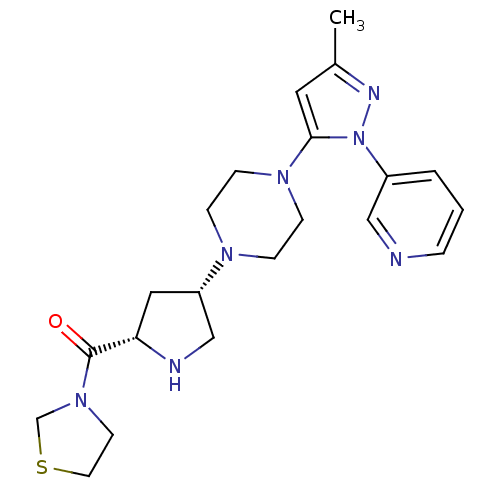 (CHEMBL2147773) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391568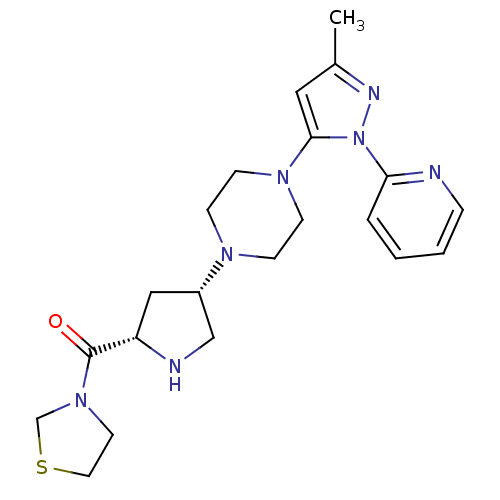 (CHEMBL2147771) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50391564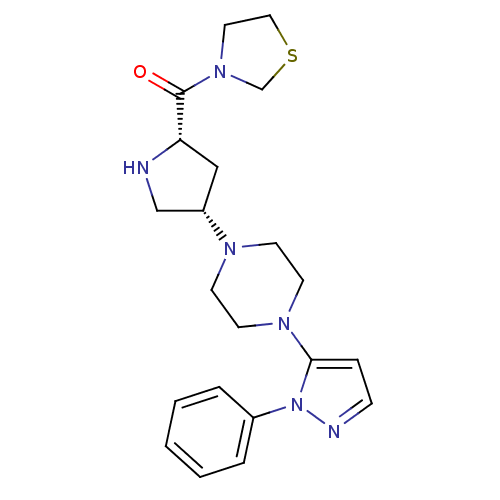 (CHEMBL2147707) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in rat plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50391568 (CHEMBL2147771) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in rat plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50391576 (CHEMBL2147773) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in rat plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391571 (CHEMBL2147713) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391564 (CHEMBL2147707) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50388117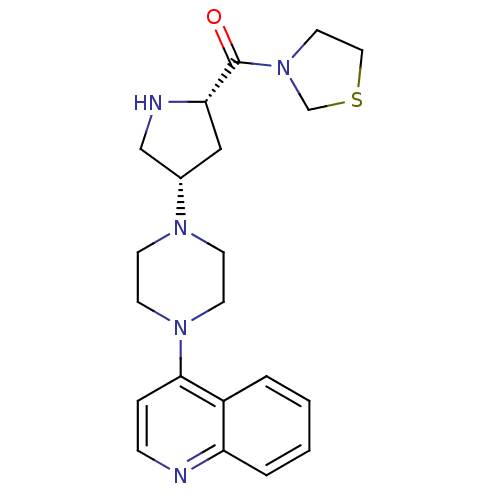 (CHEMBL2058969) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50388117 (CHEMBL2058969) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in rat plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50206038 (((2S,4S)-4-(4-(4-nitrophenyl)piperazin-1-yl)pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50206058 (6-(4-((3S,5S)-5-(thiazolidine-3-carbonyl)pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50388116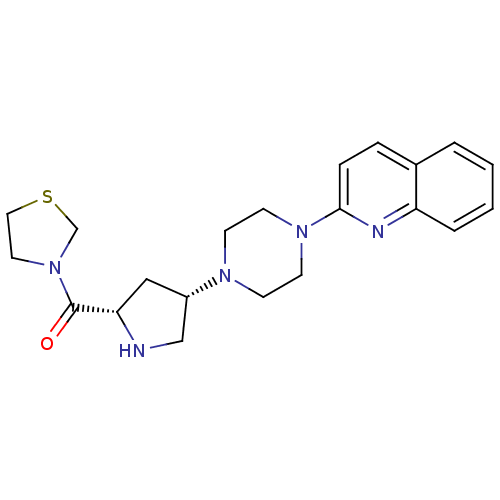 (CHEMBL2058968) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50391573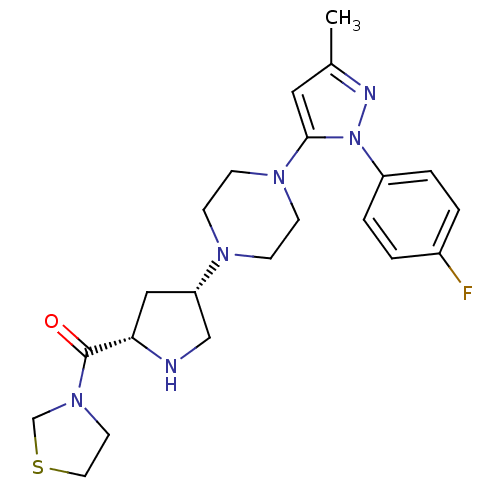 (CHEMBL2147711) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in rat plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50391574 (CHEMBL2147710) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in rat plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50391569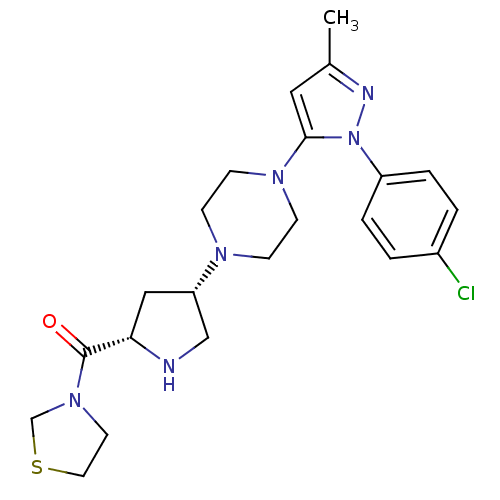 (CHEMBL2147714) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in rat plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50391560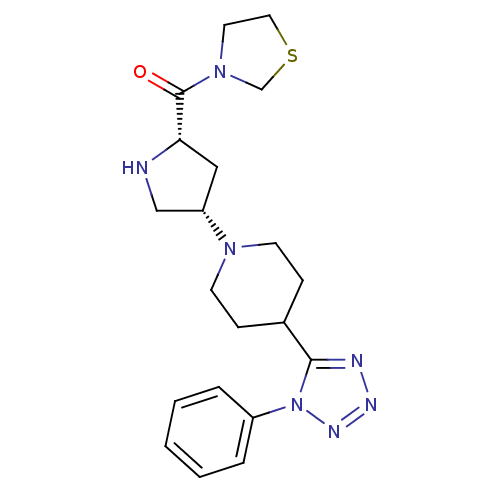 (CHEMBL2147705) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in rat plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391573 (CHEMBL2147711) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50388116 (CHEMBL2058968) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in rat plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50391563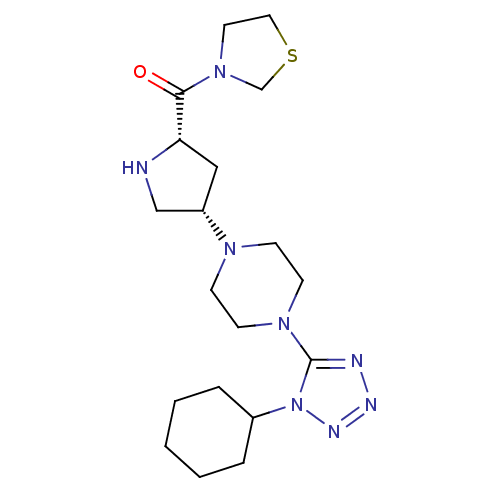 (CHEMBL2147706) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in rat plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391569 (CHEMBL2147714) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391560 (CHEMBL2147705) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50482161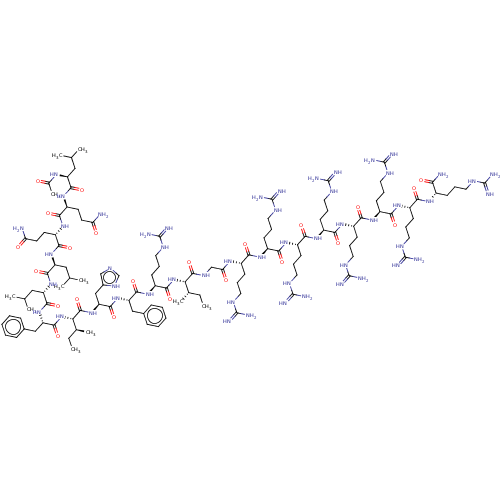 (CHEMBL1082256) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity after 1 hr by densitometric analysis | J Med Chem 53: 5356-60 (2010) Article DOI: 10.1021/jm1003528 BindingDB Entry DOI: 10.7270/Q2BG2RT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391563 (CHEMBL2147706) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391558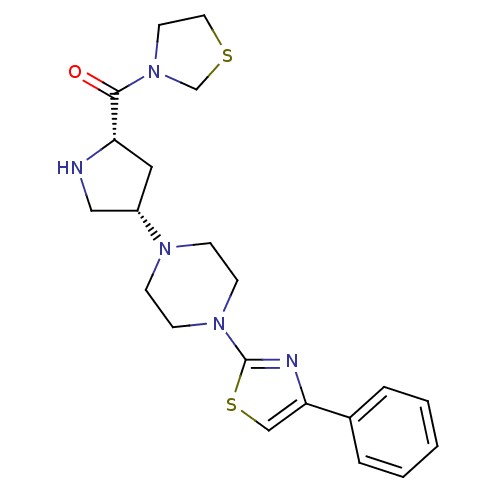 (CHEMBL2147702) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50391566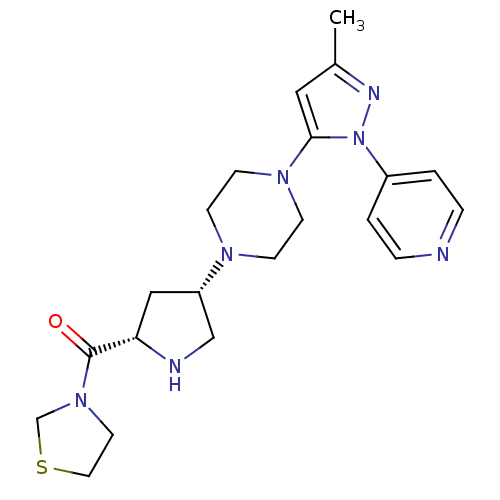 (CHEMBL2147772) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in rat plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50482160 (CHEMBL1082257) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity after 1 hr by densitometric analysis | J Med Chem 53: 5356-60 (2010) Article DOI: 10.1021/jm1003528 BindingDB Entry DOI: 10.7270/Q2BG2RT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391566 (CHEMBL2147772) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50391558 (CHEMBL2147702) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in rat plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391574 (CHEMBL2147710) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50391562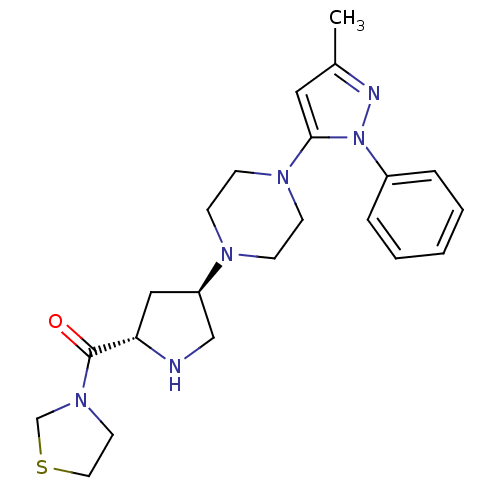 (CHEMBL2147775) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in rat plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50482160 (CHEMBL1082257) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase 3'-end processing activity after 1 hr by densitometric analysis | J Med Chem 53: 5356-60 (2010) Article DOI: 10.1021/jm1003528 BindingDB Entry DOI: 10.7270/Q2BG2RT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391562 (CHEMBL2147775) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50482161 (CHEMBL1082256) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase 3'-end processing activity after 1 hr by densitometric analysis | J Med Chem 53: 5356-60 (2010) Article DOI: 10.1021/jm1003528 BindingDB Entry DOI: 10.7270/Q2BG2RT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50482699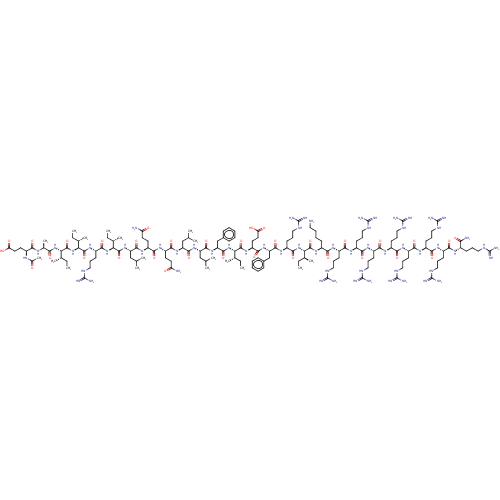 (CHEMBL1241174) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity expressed in Escherichia coli after 60 mins | Bioorg Med Chem 18: 6771-5 (2010) Article DOI: 10.1016/j.bmc.2010.07.050 BindingDB Entry DOI: 10.7270/Q23N2661 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM50206038 (((2S,4S)-4-(4-(4-nitrophenyl)piperazin-1-yl)pyrrol...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human DPP8 using GLY-Pro-MCA as substrate after 30 mins by cell-based fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391577 (CHEMBL2147715) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 139 total ) | Next | Last >> |