

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50521182 (CHEMBL4456312 | US11260049, Ex. 123) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 expressed in BHK/AC9 cells assessed as inhibition of GSK634775-induced calcium immobilization pre-incubated for 10... | J Med Chem 61: 11209-11220 (2018) Article DOI: 10.1021/acs.jmedchem.8b01344 BindingDB Entry DOI: 10.7270/Q2KW5KD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50521192 (CHEMBL4547537 | US11260049, Ex. 121) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 expressed in BHK/AC9 cells assessed as inhibition of GSK634775-induced calcium immobilization pre-incubated for 10... | J Med Chem 61: 11209-11220 (2018) Article DOI: 10.1021/acs.jmedchem.8b01344 BindingDB Entry DOI: 10.7270/Q2KW5KD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50521183 (CHEMBL4461475 | US11260049, Ex. 125) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 expressed in BHK/AC9 cells assessed as inhibition of GSK634775-induced calcium immobilization pre-incubated for 10... | J Med Chem 61: 11209-11220 (2018) Article DOI: 10.1021/acs.jmedchem.8b01344 BindingDB Entry DOI: 10.7270/Q2KW5KD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Rattus norvegicus) | BDBM50521183 (CHEMBL4461475 | US11260049, Ex. 125) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at rat TRPV4 expressed in BHK/AC9 cells assessed as inhibition of GSK634775-induced calcium immobilization pre-incubated for 10 m... | J Med Chem 61: 11209-11220 (2018) Article DOI: 10.1021/acs.jmedchem.8b01344 BindingDB Entry DOI: 10.7270/Q2KW5KD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50521185 (CHEMBL4439448 | US11260049, Ex. 83) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 expressed in BHK/AC9 cells assessed as inhibition of GSK634775-induced calcium immobilization pre-incubated for 10... | J Med Chem 61: 11209-11220 (2018) Article DOI: 10.1021/acs.jmedchem.8b01344 BindingDB Entry DOI: 10.7270/Q2KW5KD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50521195 (CHEMBL4586959 | US11260049, Ex. 84) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 expressed in BHK/AC9 cells assessed as inhibition of GSK634775-induced calcium immobilization pre-incubated for 10... | J Med Chem 61: 11209-11220 (2018) Article DOI: 10.1021/acs.jmedchem.8b01344 BindingDB Entry DOI: 10.7270/Q2KW5KD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50521186 (CHEMBL4547101 | US11260049, Ex. 45) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 expressed in BHK/AC9 cells assessed as inhibition of GSK634775-induced calcium immobilization pre-incubated for 10... | J Med Chem 61: 11209-11220 (2018) Article DOI: 10.1021/acs.jmedchem.8b01344 BindingDB Entry DOI: 10.7270/Q2KW5KD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50505550 (CHEMBL4439190 | US11260049, Ex. 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 expressed in BHK/AC9 cells assessed as inhibition of GSK634775-induced calcium immobilization pre-incubated for 10... | J Med Chem 61: 11209-11220 (2018) Article DOI: 10.1021/acs.jmedchem.8b01344 BindingDB Entry DOI: 10.7270/Q2KW5KD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Rattus norvegicus) | BDBM50505550 (CHEMBL4439190 | US11260049, Ex. 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at rat TRPV4 expressed in BHK/AC9 cells assessed as inhibition of GSK634775-induced calcium immobilization pre-incubated for 10 m... | J Med Chem 61: 11209-11220 (2018) Article DOI: 10.1021/acs.jmedchem.8b01344 BindingDB Entry DOI: 10.7270/Q2KW5KD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50521180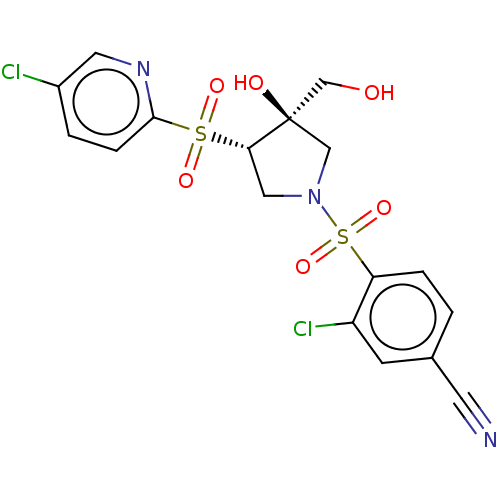 (CHEMBL4437115 | US11260049, Ex. 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 expressed in BHK/AC9 cells assessed as inhibition of GSK634775-induced calcium immobilization pre-incubated for 10... | J Med Chem 61: 11209-11220 (2018) Article DOI: 10.1021/acs.jmedchem.8b01344 BindingDB Entry DOI: 10.7270/Q2KW5KD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50521184 (CHEMBL4476783) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at TRPV4 (unknown origin) | J Med Chem 61: 11209-11220 (2018) Article DOI: 10.1021/acs.jmedchem.8b01344 BindingDB Entry DOI: 10.7270/Q2KW5KD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50505546 (CHEMBL4533534 | US11260049, Ex. 82) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 expressed in HEK cells assessed as inhibition of GSK634775-induced calcium immobilization pre-incubated for 10 min... | J Med Chem 61: 11209-11220 (2018) Article DOI: 10.1021/acs.jmedchem.8b01344 BindingDB Entry DOI: 10.7270/Q2KW5KD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50521187 (CHEMBL4441860) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 expressed in BHK/AC9 cells assessed as inhibition of GSK634775-induced calcium immobilization pre-incubated for 10... | J Med Chem 61: 11209-11220 (2018) Article DOI: 10.1021/acs.jmedchem.8b01344 BindingDB Entry DOI: 10.7270/Q2KW5KD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50521188 (CHEMBL4467225) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 expressed in BHK/AC9 cells assessed as inhibition of GSK634775-induced calcium immobilization pre-incubated for 10... | J Med Chem 61: 11209-11220 (2018) Article DOI: 10.1021/acs.jmedchem.8b01344 BindingDB Entry DOI: 10.7270/Q2KW5KD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50521193 (CHEMBL4468326) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 expressed in HEK cells assessed as inhibition of GSK634775-induced calcium immobilization pre-incubated for 10 min... | J Med Chem 61: 11209-11220 (2018) Article DOI: 10.1021/acs.jmedchem.8b01344 BindingDB Entry DOI: 10.7270/Q2KW5KD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50521181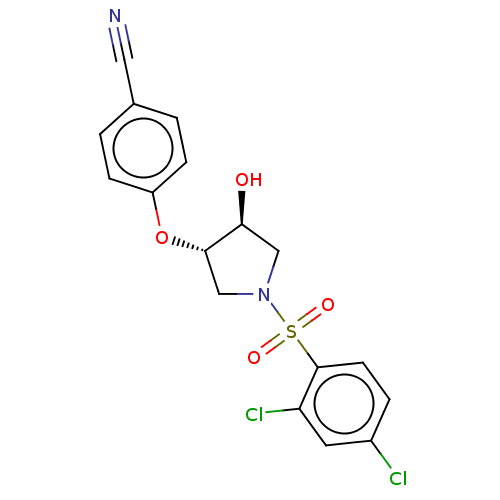 (CHEMBL4465119) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 expressed in HEK cells assessed as inhibition of GSK634775-induced calcium immobilization pre-incubated for 10 min... | J Med Chem 61: 11209-11220 (2018) Article DOI: 10.1021/acs.jmedchem.8b01344 BindingDB Entry DOI: 10.7270/Q2KW5KD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50521189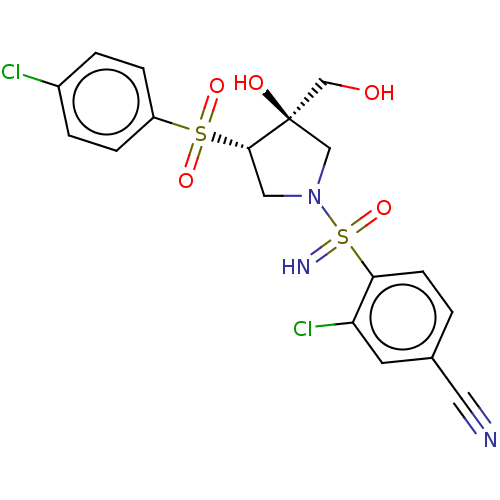 (CHEMBL4473984) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 expressed in BHK/AC9 cells assessed as inhibition of GSK634775-induced calcium immobilization pre-incubated for 10... | J Med Chem 61: 11209-11220 (2018) Article DOI: 10.1021/acs.jmedchem.8b01344 BindingDB Entry DOI: 10.7270/Q2KW5KD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50232797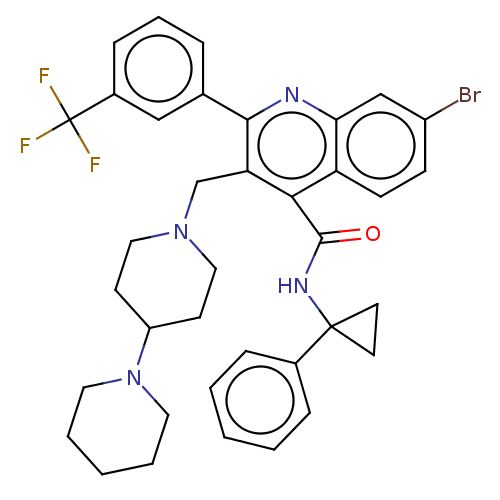 (CHEMBL4073922) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 expressed in HEK cells assessed as inhibition of GSK634775-induced Ca2+ influx by FLIPR assay | J Med Chem 61: 11209-11220 (2018) Article DOI: 10.1021/acs.jmedchem.8b01344 BindingDB Entry DOI: 10.7270/Q2KW5KD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50521191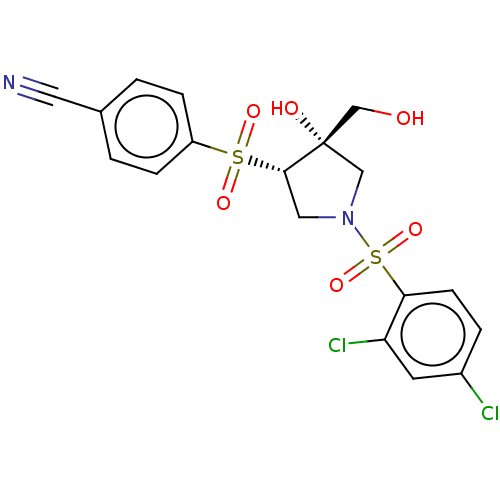 (CHEMBL4534649) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 expressed in HEK cells assessed as inhibition of GSK634775-induced calcium immobilization pre-incubated for 10 min... | J Med Chem 61: 11209-11220 (2018) Article DOI: 10.1021/acs.jmedchem.8b01344 BindingDB Entry DOI: 10.7270/Q2KW5KD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50521182 (CHEMBL4456312 | US11260049, Ex. 123) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible inhibition of CYP3A4 (unknown origin) in presence of NADPH by vivid red substrate-based assay | J Med Chem 61: 11209-11220 (2018) Article DOI: 10.1021/acs.jmedchem.8b01344 BindingDB Entry DOI: 10.7270/Q2KW5KD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50311424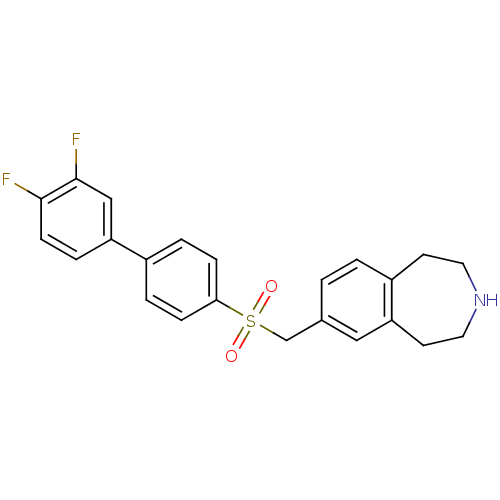 (7-((3',4'-difluorobiphenyl-4-ylsulfonyl)methyl)-2,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2D6 | Bioorg Med Chem Lett 19: 6452-8 (2009) Article DOI: 10.1016/j.bmcl.2009.09.027 BindingDB Entry DOI: 10.7270/Q2DV1K0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI (Staphylococcus aureus) | BDBM8767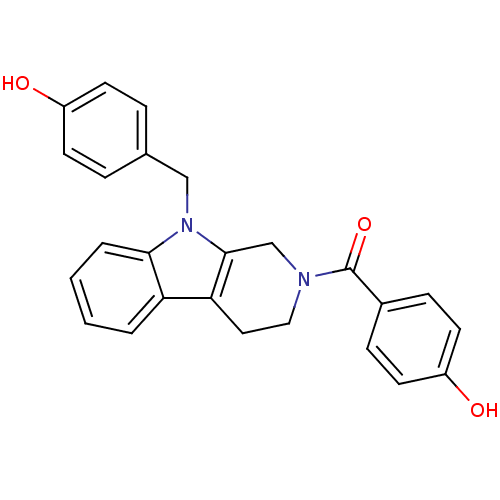 (1,2,3,4-Tetrahydropyrido[3,4-b]indole 3 | 4-({9-[(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | 6.5 | 30 |
GlaxoSmithKline | Assay Description Assays were carried out in half-area, 96-well microtiter plates. Compounds were evaluated in assay mixtures containing components specific for each e... | Bioorg Med Chem Lett 11: 2241-4 (2001) Article DOI: 10.1016/s0960-894x(01)00405-x BindingDB Entry DOI: 10.7270/Q24X5610 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI (Staphylococcus aureus) | BDBM8786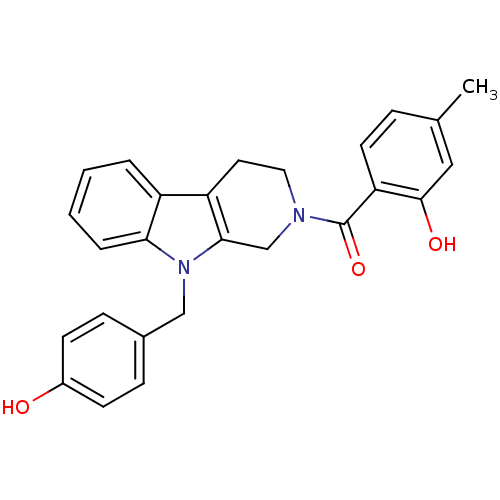 (1,2,3,4-tetrahydro pyrido[3,4-b]indole 30 | 2-({9-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | 30 |
GlaxoSmithKline | Assay Description Assays were carried out in half-area, 96-well microtiter plates. Compounds were evaluated in assay mixtures containing components specific for each e... | Bioorg Med Chem Lett 11: 2241-4 (2001) Article DOI: 10.1016/s0960-894x(01)00405-x BindingDB Entry DOI: 10.7270/Q24X5610 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50521194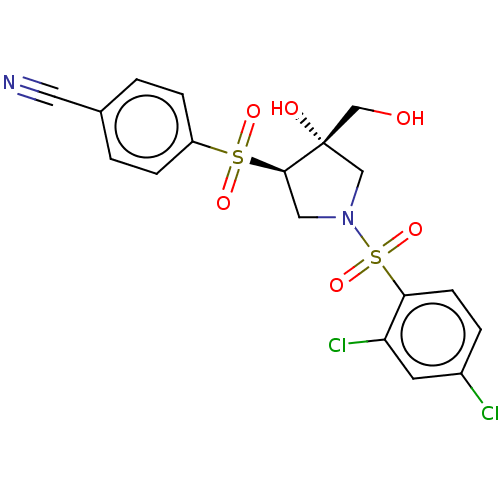 (CHEMBL4444971) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 expressed in HEK cells assessed as inhibition of GSK634775-induced calcium immobilization pre-incubated for 10 min... | J Med Chem 61: 11209-11220 (2018) Article DOI: 10.1021/acs.jmedchem.8b01344 BindingDB Entry DOI: 10.7270/Q2KW5KD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI (Staphylococcus aureus) | BDBM8789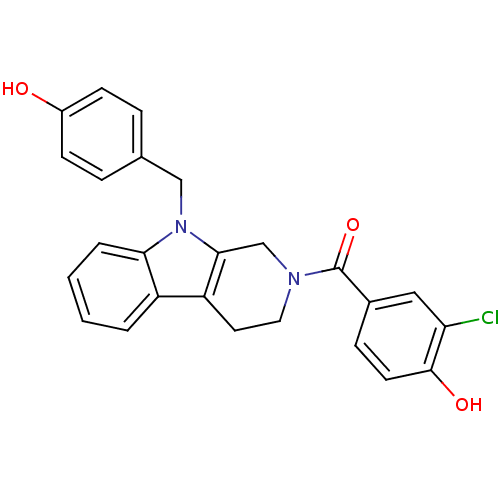 (1,2,3,4-tetrahydro pyrido[3,4-b]indole 33 | 2-chlo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | 30 |
GlaxoSmithKline | Assay Description Assays were carried out in half-area, 96-well microtiter plates. Compounds were evaluated in assay mixtures containing components specific for each e... | Bioorg Med Chem Lett 11: 2241-4 (2001) Article DOI: 10.1016/s0960-894x(01)00405-x BindingDB Entry DOI: 10.7270/Q24X5610 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI (Staphylococcus aureus) | BDBM8787 (1,2,3,4-tetrahydro pyrido[3,4-b]indole 31 | 5-chlo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | 30 |
GlaxoSmithKline | Assay Description Assays were carried out in half-area, 96-well microtiter plates. Compounds were evaluated in assay mixtures containing components specific for each e... | Bioorg Med Chem Lett 11: 2241-4 (2001) Article DOI: 10.1016/s0960-894x(01)00405-x BindingDB Entry DOI: 10.7270/Q24X5610 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI (Staphylococcus aureus) | BDBM8778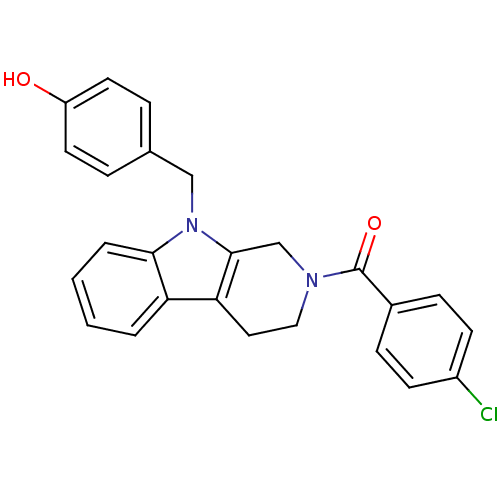 (1,2,3,4-tetrahydro pyrido[3,4-b]indole 22 | 4-({2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | 30 |
GlaxoSmithKline | Assay Description Assays were carried out in half-area, 96-well microtiter plates. Compounds were evaluated in assay mixtures containing components specific for each e... | Bioorg Med Chem Lett 11: 2241-4 (2001) Article DOI: 10.1016/s0960-894x(01)00405-x BindingDB Entry DOI: 10.7270/Q24X5610 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI (Staphylococcus aureus) | BDBM8788 (1,2,3,4-tetrahydro pyrido[3,4-b]indole 32 | 4-({9-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | 30 |
GlaxoSmithKline | Assay Description Assays were carried out in half-area, 96-well microtiter plates. Compounds were evaluated in assay mixtures containing components specific for each e... | Bioorg Med Chem Lett 11: 2241-4 (2001) Article DOI: 10.1016/s0960-894x(01)00405-x BindingDB Entry DOI: 10.7270/Q24X5610 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50311414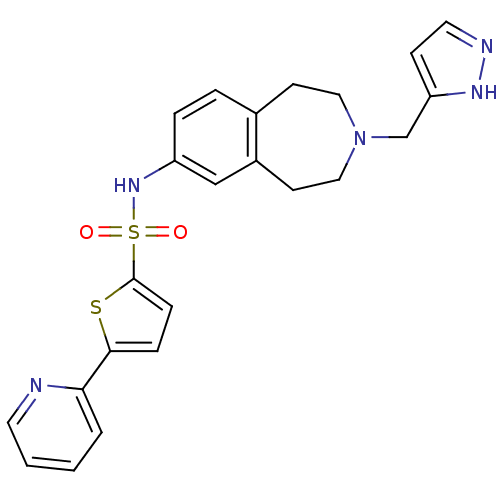 (CHEMBL1078492 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2D6 | Bioorg Med Chem Lett 19: 6452-8 (2009) Article DOI: 10.1016/j.bmcl.2009.09.027 BindingDB Entry DOI: 10.7270/Q2DV1K0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50311425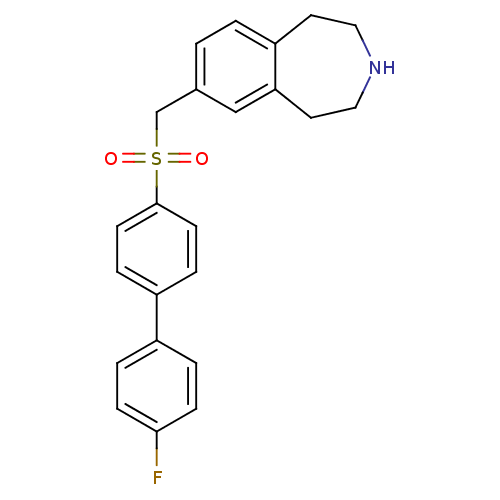 (7-((4'-fluorobiphenyl-4-ylsulfonyl)methyl)-2,3,4,5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2D6 | Bioorg Med Chem Lett 19: 6452-8 (2009) Article DOI: 10.1016/j.bmcl.2009.09.027 BindingDB Entry DOI: 10.7270/Q2DV1K0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50311413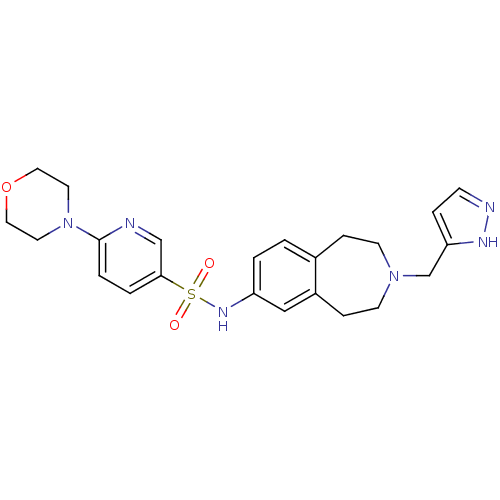 (CHEMBL1078595 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2C9 | Bioorg Med Chem Lett 19: 6452-8 (2009) Article DOI: 10.1016/j.bmcl.2009.09.027 BindingDB Entry DOI: 10.7270/Q2DV1K0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI (Staphylococcus aureus) | BDBM8776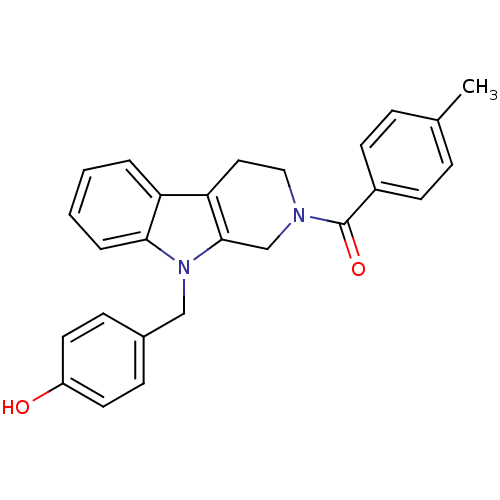 (1,2,3,4-tetrahydro pyrido[3,4-b]indole 20 | 4-({2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | 30 |
GlaxoSmithKline | Assay Description Assays were carried out in half-area, 96-well microtiter plates. Compounds were evaluated in assay mixtures containing components specific for each e... | Bioorg Med Chem Lett 11: 2241-4 (2001) Article DOI: 10.1016/s0960-894x(01)00405-x BindingDB Entry DOI: 10.7270/Q24X5610 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI (Staphylococcus aureus) | BDBM8744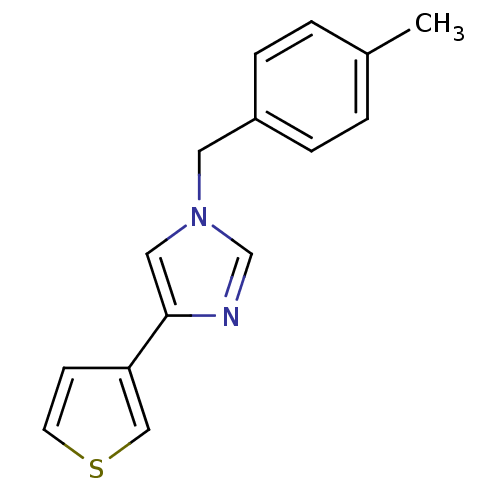 (1-[(4-methylphenyl)methyl]-4-(thiophen-3-yl)-1H-im...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | 6.5 | 30 |
GlaxoSmithKline | Assay Description Assays were carried out in half-area, 96-well microtiter plates. Compounds were evaluated in assay mixtures containing components specific for each e... | Bioorg Med Chem Lett 11: 2061-5 (2001) Article DOI: 10.1016/s0960-894x(01)00404-8 BindingDB Entry DOI: 10.7270/Q28P5XQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI (Staphylococcus aureus) | BDBM8769 (1,2,3,4-Tetrahydropyrido[3,4-b]indole 13 | 4-({9-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | 6.5 | 30 |
GlaxoSmithKline | Assay Description Assays were carried out in half-area, 96-well microtiter plates. Compounds were evaluated in assay mixtures containing components specific for each e... | Bioorg Med Chem Lett 11: 2241-4 (2001) Article DOI: 10.1016/s0960-894x(01)00405-x BindingDB Entry DOI: 10.7270/Q24X5610 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50311419 (CHEMBL1078464 | N-(4-fluorophenyl)-5-((2,3,4,5-tet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2D6 | Bioorg Med Chem Lett 19: 6452-8 (2009) Article DOI: 10.1016/j.bmcl.2009.09.027 BindingDB Entry DOI: 10.7270/Q2DV1K0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50521192 (CHEMBL4547537 | US11260049, Ex. 121) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible inhibition of CYP3A4 (unknown origin) in presence of NADPH by vivid red substrate-based assay | J Med Chem 61: 11209-11220 (2018) Article DOI: 10.1021/acs.jmedchem.8b01344 BindingDB Entry DOI: 10.7270/Q2KW5KD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI (Staphylococcus aureus) | BDBM8770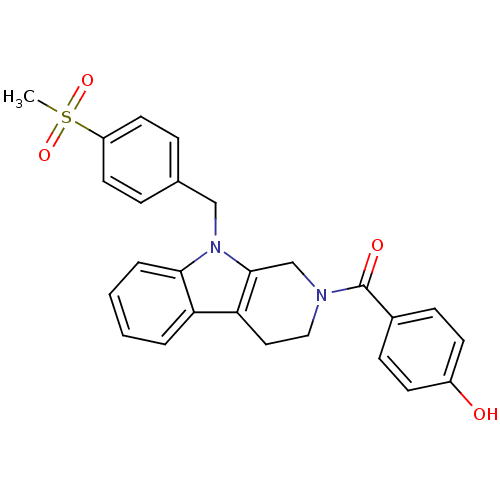 (1,2,3,4-Tetrahydropyrido[3,4-b]indole 14 | 4-({9-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | 6.5 | 30 |
GlaxoSmithKline | Assay Description Assays were carried out in half-area, 96-well microtiter plates. Compounds were evaluated in assay mixtures containing components specific for each e... | Bioorg Med Chem Lett 11: 2241-4 (2001) Article DOI: 10.1016/s0960-894x(01)00405-x BindingDB Entry DOI: 10.7270/Q24X5610 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI (Staphylococcus aureus) | BDBM8739 (4-(4-methoxyphenyl)-1-[(4-methylphenyl)methyl]-1H-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | 6.5 | 30 |
GlaxoSmithKline | Assay Description Assays were carried out in half-area, 96-well microtiter plates. Compounds were evaluated in assay mixtures containing components specific for each e... | Bioorg Med Chem Lett 11: 2061-5 (2001) Article DOI: 10.1016/s0960-894x(01)00404-8 BindingDB Entry DOI: 10.7270/Q28P5XQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI (Staphylococcus aureus) | BDBM8785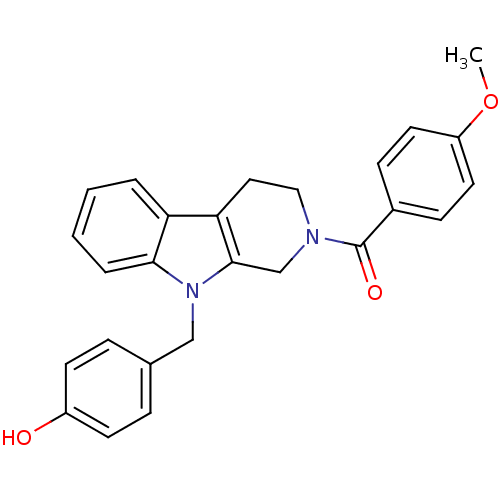 (1,2,3,4-tetrahydro pyrido[3,4-b]indole 29 | 4-({2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | 30 |
GlaxoSmithKline | Assay Description Assays were carried out in half-area, 96-well microtiter plates. Compounds were evaluated in assay mixtures containing components specific for each e... | Bioorg Med Chem Lett 11: 2241-4 (2001) Article DOI: 10.1016/s0960-894x(01)00405-x BindingDB Entry DOI: 10.7270/Q24X5610 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50311414 (CHEMBL1078492 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2C9 | Bioorg Med Chem Lett 19: 6452-8 (2009) Article DOI: 10.1016/j.bmcl.2009.09.027 BindingDB Entry DOI: 10.7270/Q2DV1K0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI (Staphylococcus aureus) | BDBM8766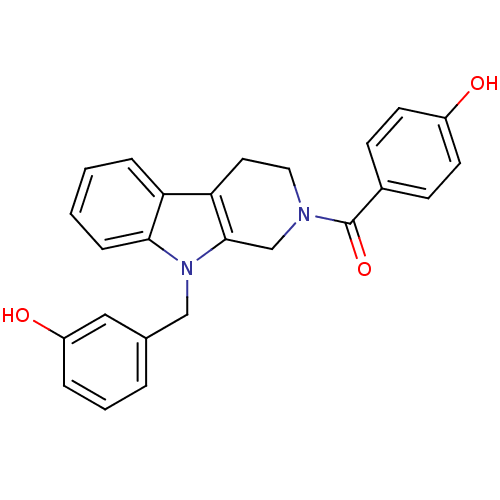 (1,2,3,4-Tetrahydropyrido[3,4-b]indole 11 | 4-({9-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | 6.5 | 30 |
GlaxoSmithKline | Assay Description Assays were carried out in half-area, 96-well microtiter plates. Compounds were evaluated in assay mixtures containing components specific for each e... | Bioorg Med Chem Lett 11: 2241-4 (2001) Article DOI: 10.1016/s0960-894x(01)00405-x BindingDB Entry DOI: 10.7270/Q24X5610 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] FabI (Escherichia coli) | BDBM8726 (5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | 30 |
GlaxoSmithKline | Assay Description Assays were carried out in half-area, 96-well microtiter plates. Compounds were evaluated in assay mixtures containing components specific for each e... | Bioorg Med Chem Lett 11: 2061-5 (2001) Article DOI: 10.1016/s0960-894x(01)00404-8 BindingDB Entry DOI: 10.7270/Q28P5XQG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] FabI (Escherichia coli) | BDBM8726 (5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | 30 |
GlaxoSmithKline | Assay Description Assays were carried out in half-area, 96-well microtiter plates. Compounds were evaluated in assay mixtures containing components specific for each e... | Bioorg Med Chem Lett 11: 2241-4 (2001) Article DOI: 10.1016/s0960-894x(01)00405-x BindingDB Entry DOI: 10.7270/Q24X5610 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI (Staphylococcus aureus) | BDBM8775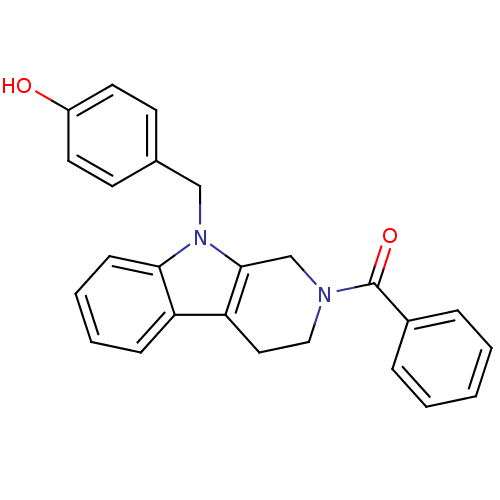 (1,2,3,4-tetrahydro pyrido[3,4-b]indole 19 | 4-({2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | 30 |
GlaxoSmithKline | Assay Description Assays were carried out in half-area, 96-well microtiter plates. Compounds were evaluated in assay mixtures containing components specific for each e... | Bioorg Med Chem Lett 11: 2241-4 (2001) Article DOI: 10.1016/s0960-894x(01)00405-x BindingDB Entry DOI: 10.7270/Q24X5610 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50311415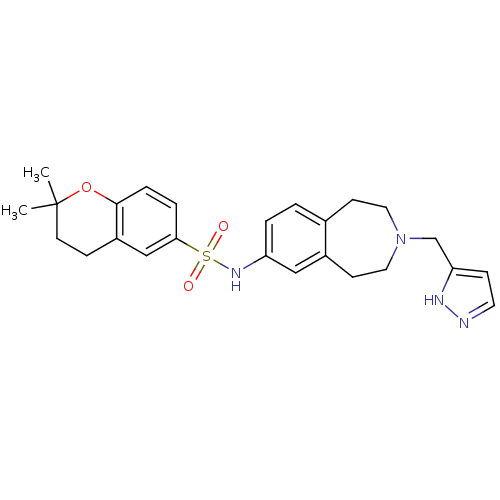 (CHEMBL1078490 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2D6 | Bioorg Med Chem Lett 19: 6452-8 (2009) Article DOI: 10.1016/j.bmcl.2009.09.027 BindingDB Entry DOI: 10.7270/Q2DV1K0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI (Staphylococcus aureus) | BDBM8777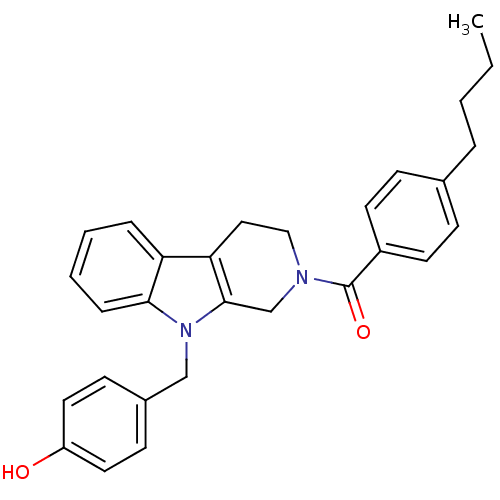 (1,2,3,4-tetrahydro pyrido[3,4-b]indole 21 | 4-({2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | 30 |
GlaxoSmithKline | Assay Description Assays were carried out in half-area, 96-well microtiter plates. Compounds were evaluated in assay mixtures containing components specific for each e... | Bioorg Med Chem Lett 11: 2241-4 (2001) Article DOI: 10.1016/s0960-894x(01)00405-x BindingDB Entry DOI: 10.7270/Q24X5610 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50311423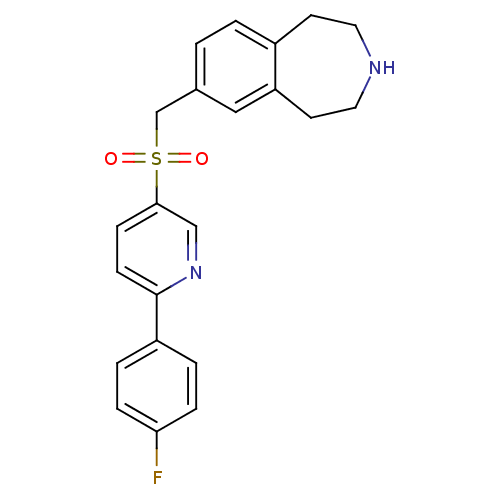 (7-((6-(4-fluorophenyl)pyridin-3-ylsulfonyl)methyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2D6 | Bioorg Med Chem Lett 19: 6452-8 (2009) Article DOI: 10.1016/j.bmcl.2009.09.027 BindingDB Entry DOI: 10.7270/Q2DV1K0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50311412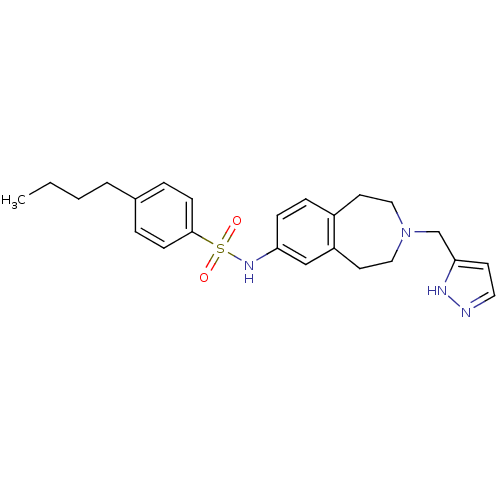 (CHEMBL1080046 | N-(3-((1H-pyrazol-3-yl)methyl)-2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP2C9 | Bioorg Med Chem Lett 19: 6452-8 (2009) Article DOI: 10.1016/j.bmcl.2009.09.027 BindingDB Entry DOI: 10.7270/Q2DV1K0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 4 (Homo sapiens (Human)) | BDBM50521196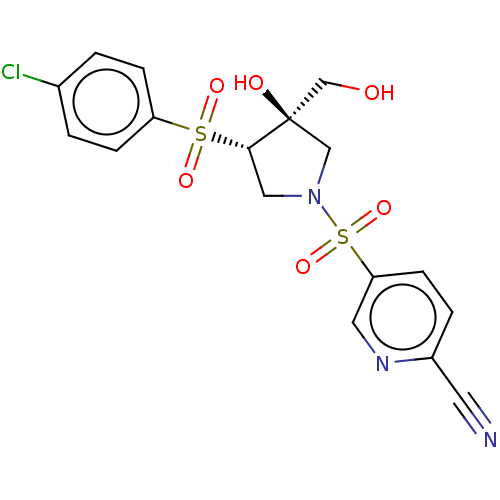 (CHEMBL4453093) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human TRPV4 expressed in BHK/AC9 cells assessed as inhibition of GSK634775-induced calcium immobilization pre-incubated for 10... | J Med Chem 61: 11209-11220 (2018) Article DOI: 10.1021/acs.jmedchem.8b01344 BindingDB Entry DOI: 10.7270/Q2KW5KD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50241425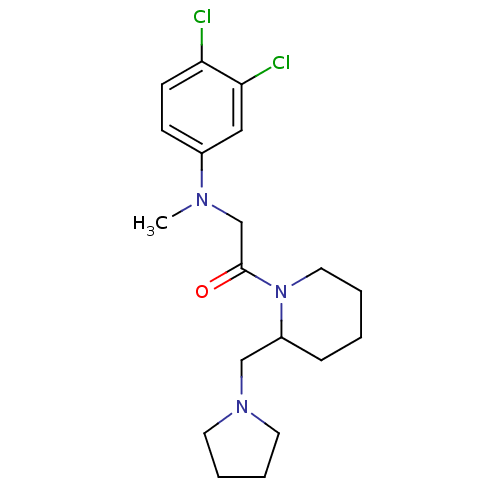 (2-((3,4-dichlorophenyl)(methyl)amino)-1-(2-(pyrrol...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 631 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant urotensin 2 receptor-mediated calcium mobilization expressed in HEK293 cells by FLIPR assay | Bioorg Med Chem Lett 18: 2860-4 (2008) Article DOI: 10.1016/j.bmcl.2008.03.078 BindingDB Entry DOI: 10.7270/Q29S1RXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 277 total ) | Next | Last >> |