

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50016966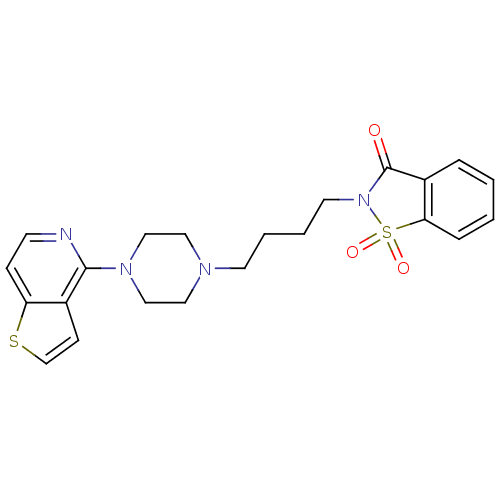 (1,1-Dioxo-2-[4-(4-thieno[3,2-c]pyridin-4-yl-pipera...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at serotonin 5-hydroxytryptamine 1A receptors of rat hippocampus by displacing [3H]8-OH-DPAT | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50016963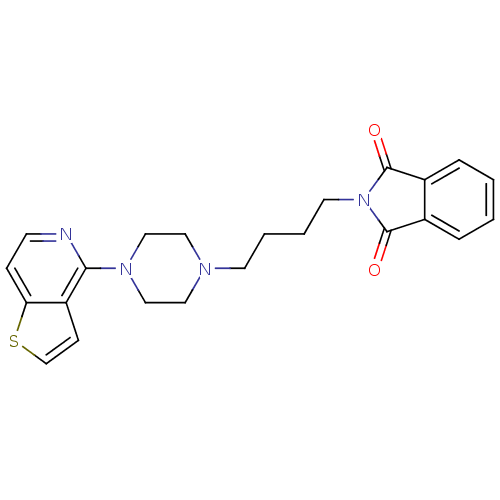 (2-[4-(4-Thieno[3,2-c]pyridin-4-yl-piperazin-1-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at alpha-1 adrenergic receptor by displacing [3H]WB-4101 | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50122418 (1-Benzo[1,3]dioxol-5-yl-4-[4-(2-fluoro-5-methoxy-b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of the 5-hydroxytryptamine 1A receptor in rat dorsal raphe | Bioorg Med Chem Lett 13: 285-8 (2002) BindingDB Entry DOI: 10.7270/Q2251HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50016955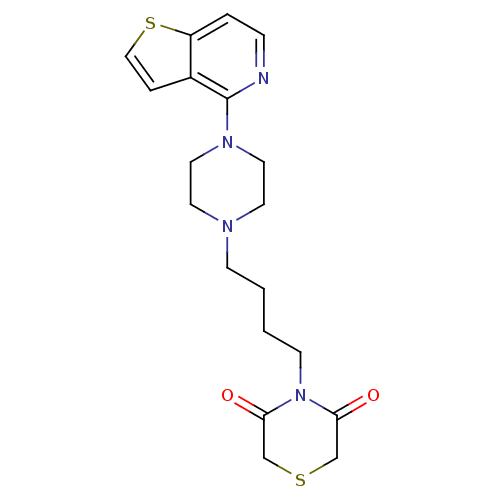 (4-[4-(4-Thieno[3,2-c]pyridin-4-yl-piperazin-1-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at alpha-1 adrenergic receptor by displacing [3H]WB-4101 | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50016966 (1,1-Dioxo-2-[4-(4-thieno[3,2-c]pyridin-4-yl-pipera...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at alpha-1 adrenergic receptor by displacing [3H]WB-4101 | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50122426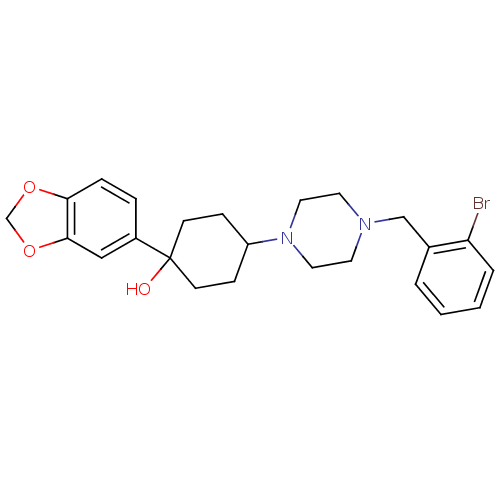 (1-Benzo[1,3]dioxol-5-yl-4-[4-(2-bromo-benzyl)-pipe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of the 5-hydroxytryptamine 1A receptor in rat dorsal raphe | Bioorg Med Chem Lett 13: 285-8 (2002) BindingDB Entry DOI: 10.7270/Q2251HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50122429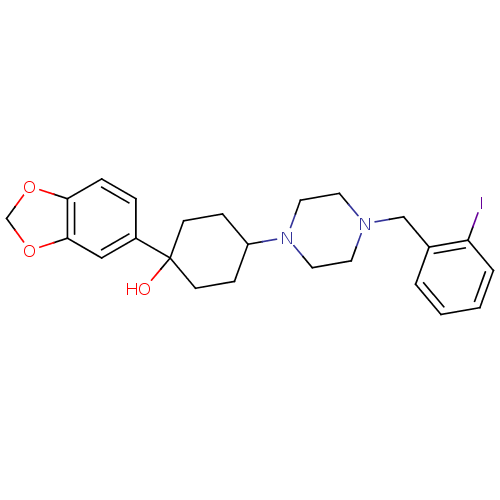 (1-Benzo[1,3]dioxol-5-yl-4-[4-(2-iodo-benzyl)-piper...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of the 5-hydroxytryptamine 1A receptor in rat dorsal raphe | Bioorg Med Chem Lett 13: 285-8 (2002) BindingDB Entry DOI: 10.7270/Q2251HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50016955 (4-[4-(4-Thieno[3,2-c]pyridin-4-yl-piperazin-1-yl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at serotonin 5-hydroxytryptamine 1A receptors of rat hippocampus by displacing [3H]8-OH-DPAT | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50016959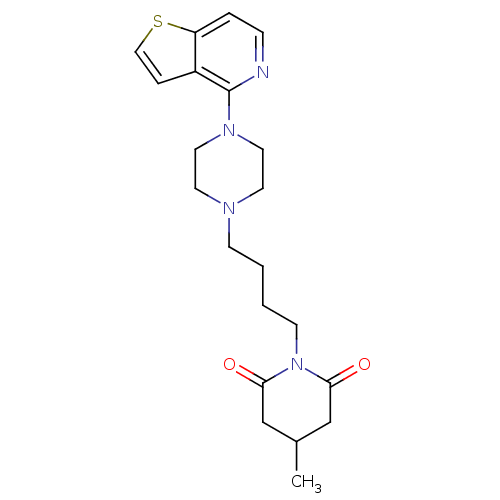 (4-Methyl-1-[4-(4-thieno[3,2-c]pyridin-4-yl-piperaz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at serotonin 5-hydroxytryptamine 1A receptors of rat hippocampus by displacing [3H]8-OH-DPAT | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50122428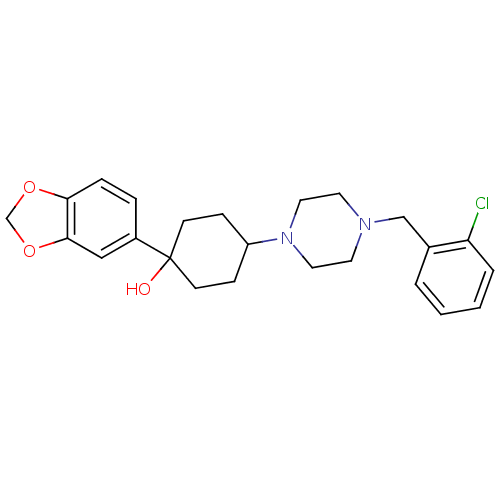 (1-Benzo[1,3]dioxol-5-yl-4-[4-(2-chloro-benzyl)-pip...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of the 5-hydroxytryptamine 1A receptor in rat dorsal raphe | Bioorg Med Chem Lett 13: 285-8 (2002) BindingDB Entry DOI: 10.7270/Q2251HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50122411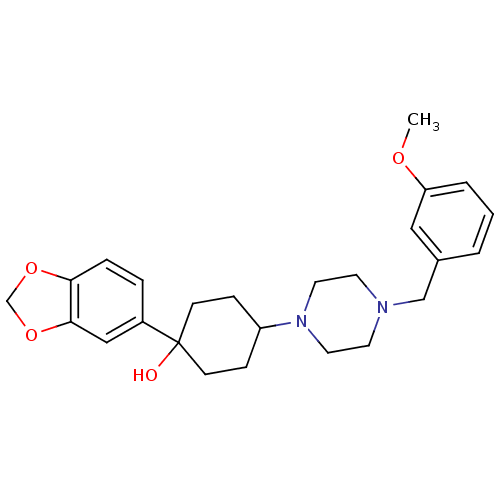 (1-Benzo[1,3]dioxol-5-yl-4-[4-(3-methoxy-benzyl)-pi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of the 5-hydroxytryptamine 1A receptor in rat dorsal raphe | Bioorg Med Chem Lett 13: 285-8 (2002) BindingDB Entry DOI: 10.7270/Q2251HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50016971 (3-{4-[4-(2-Methyl-furo[3,2-c]pyridin-4-yl)-piperaz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at 5-hydroxytryptamine 1A receptor of rat hippocampus by displacing [3H]8-OH-DPAT | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50016966 (1,1-Dioxo-2-[4-(4-thieno[3,2-c]pyridin-4-yl-pipera...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at 5-hydroxytryptamine 1 receptor of rat hippocampus by displacing [3H]5-HT | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50016969 (1-[4-(4-Thieno[3,2-c]pyridin-4-yl-piperazin-1-yl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at serotonin 5-hydroxytryptamine 1A receptors of rat hippocampus by displacing [3H]8-OH-DPAT | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50122437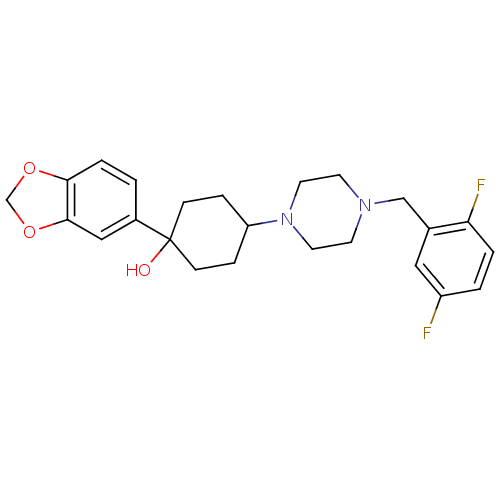 (1-Benzo[1,3]dioxol-5-yl-4-[4-(2,5-difluoro-benzyl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of the 5-hydroxytryptamine 1A receptor in rat dorsal raphe | Bioorg Med Chem Lett 13: 285-8 (2002) BindingDB Entry DOI: 10.7270/Q2251HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50016963 (2-[4-(4-Thieno[3,2-c]pyridin-4-yl-piperazin-1-yl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at serotonin 5-hydroxytryptamine 1A receptors of rat hippocampus by displacing [3H]8-OH-DPAT | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50016963 (2-[4-(4-Thieno[3,2-c]pyridin-4-yl-piperazin-1-yl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at serotonin 5-hydroxytryptamine 2 receptors of rat hippocampus by displacing [3H]spiperone | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50122433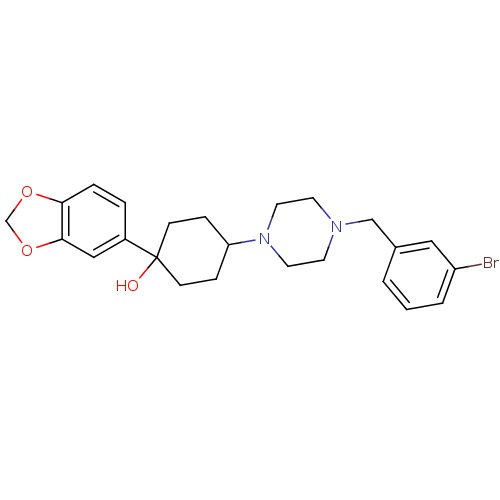 (1-Benzo[1,3]dioxol-5-yl-4-[4-(3-bromo-benzyl)-pipe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of the 5-hydroxytryptamine 1A receptor in rat dorsal raphe | Bioorg Med Chem Lett 13: 285-8 (2002) BindingDB Entry DOI: 10.7270/Q2251HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50016960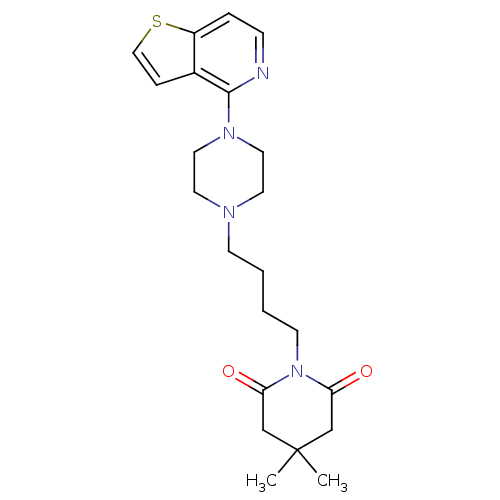 (4,4-Dimethyl-1-[4-(4-thieno[3,2-c]pyridin-4-yl-pip...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at serotonin 5-hydroxytryptamine 2 receptors of rat hippocampus by displacing [3H]spiperone | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50016967 (3-{4-[4-(2-Methyl-thieno[3,2-c]pyridin-4-yl)-piper...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at 5-hydroxytryptamine 1 receptor of rat hippocampus by displacing [3H]5-HT | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50016957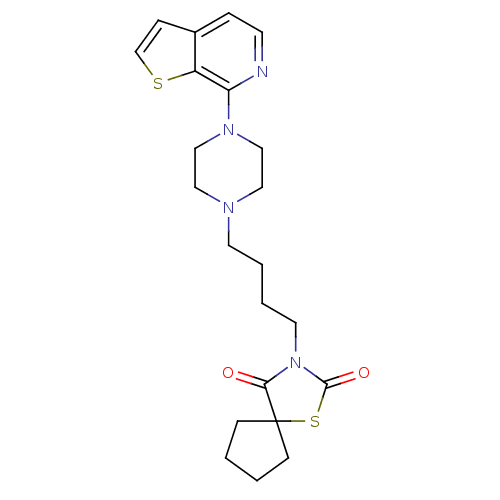 (3-[4-(4-Thieno[2,3-c]pyridin-7-yl-piperazin-1-yl)-...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description In vitro evaluation for arachidonic acid induced platelet aggregation of human platelet-rich plasma | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50016956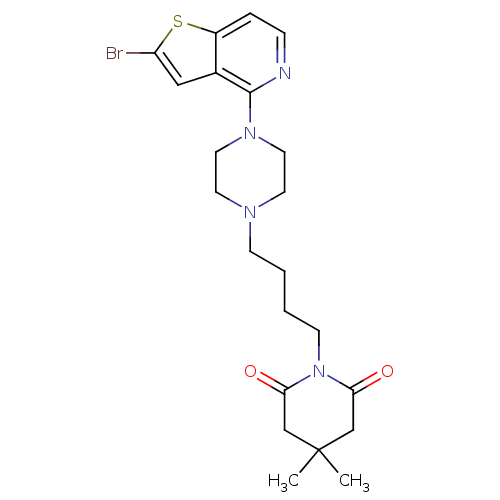 (1-{4-[4-(2-Bromo-thieno[3,2-c]pyridin-4-yl)-pipera...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at 5-hydroxytryptamine 1 receptor of rat hippocampus by displacing [3H]5-HT | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50016958 (1-(4-Fluoro-phenyl)-4-(4-thieno[3,2-c]pyridin-4-yl...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at 5-hydroxytryptamine 1 receptor of rat hippocampus by displacing [3H]5-HT | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50016964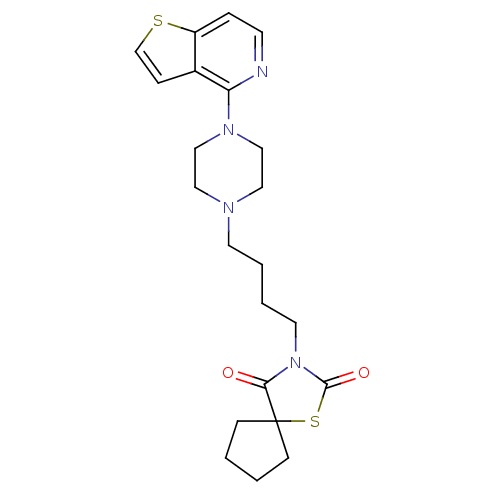 (3-[4-(4-Thieno[3,2-c]pyridin-4-yl-piperazin-1-yl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at 5-hydroxytryptamine 2 receptor of rat hippocampus by displacing [3H]spiperone | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50016959 (4-Methyl-1-[4-(4-thieno[3,2-c]pyridin-4-yl-piperaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at serotonin 5-hydroxytryptamine 2 receptors of rat hippocampus by displacing [3H]spiperone | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50016965 (4,4-Dimethyl-1-{4-[4-(2-methyl-furo[3,2-c]pyridin-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at serotonin 5-hydroxytryptamine 1A receptors of rat hippocampus by displacing [3H]8-OH-DPAT | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50016963 (2-[4-(4-Thieno[3,2-c]pyridin-4-yl-piperazin-1-yl)-...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description In vitro inhibition of arachidonic acid induced platelet aggregation in human platelet-rich plasma | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50016966 (1,1-Dioxo-2-[4-(4-thieno[3,2-c]pyridin-4-yl-pipera...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description In vitro inhibition of arachidonic acid induced platelet aggregation in human platelet-rich plasma | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50016960 (4,4-Dimethyl-1-[4-(4-thieno[3,2-c]pyridin-4-yl-pip...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at 5-hydroxytryptamine 1 receptor of rat hippocampus by displacing [3H]5-HT | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50016958 (1-(4-Fluoro-phenyl)-4-(4-thieno[3,2-c]pyridin-4-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at serotonin 5-hydroxytryptamine 2 receptors of rat hippocampus by displacing [3H]spiperone | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50122431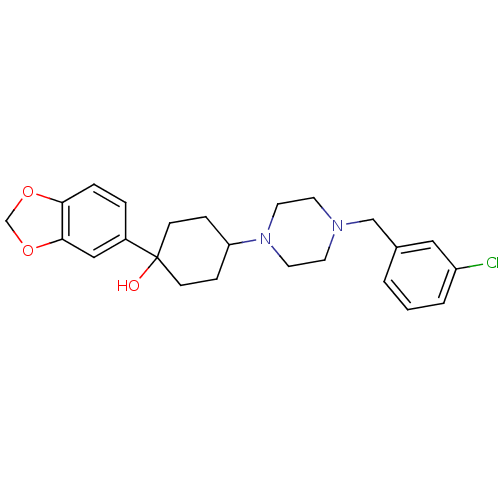 (1-Benzo[1,3]dioxol-5-yl-4-[4-(3-chloro-benzyl)-pip...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of the 5-hydroxytryptamine 1A receptor in rat dorsal raphe | Bioorg Med Chem Lett 13: 285-8 (2002) BindingDB Entry DOI: 10.7270/Q2251HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50016968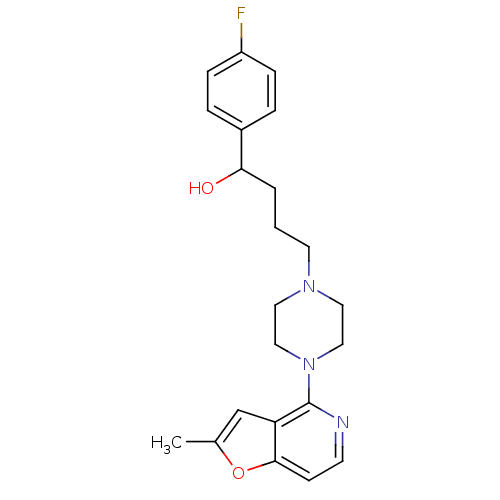 (1-(4-Fluoro-phenyl)-4-[4-(2-methyl-furo[3,2-c]pyri...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at serotonin 5-hydroxytryptamine 1A receptors of rat hippocampus by displacing [3H]8-OH-DPAT | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50122423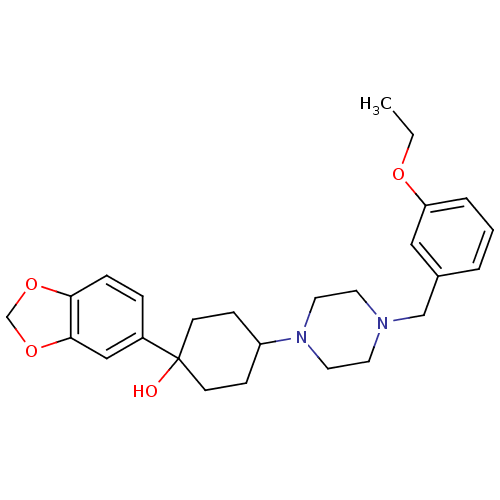 (1-Benzo[1,3]dioxol-5-yl-4-[4-(3-ethoxy-benzyl)-pip...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of the 5-hydroxytryptamine 1A receptor in rat dorsal raphe | Bioorg Med Chem Lett 13: 285-8 (2002) BindingDB Entry DOI: 10.7270/Q2251HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50016955 (4-[4-(4-Thieno[3,2-c]pyridin-4-yl-piperazin-1-yl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at serotonin 5-hydroxytryptamine 2 receptors of rat hippocampus by displacing [3H]spiperone | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50016964 (3-[4-(4-Thieno[3,2-c]pyridin-4-yl-piperazin-1-yl)-...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at 5-hydroxytryptamine 1 receptor of rat hippocampus by displacing [3H]5-HT | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50016975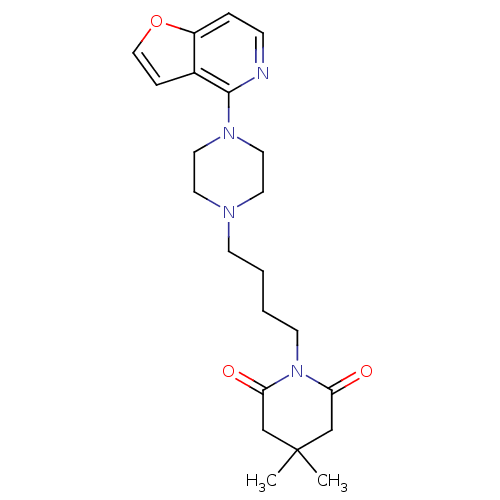 (1-[4-(4-Furo[3,2-c]pyridin-4-yl-piperazin-1-yl)-bu...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at 5-hydroxytryptamine 1A receptor of rat hippocampus by displacing [3H]8-OH-DPAT | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50001888 ((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at 5-hydroxytryptamine 2 receptor of rat hippocampus by displacing [3H]spiperone | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50016959 (4-Methyl-1-[4-(4-thieno[3,2-c]pyridin-4-yl-piperaz...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at 5-hydroxytryptamine 1 receptor of rat hippocampus by displacing [3H]5-HT | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50122410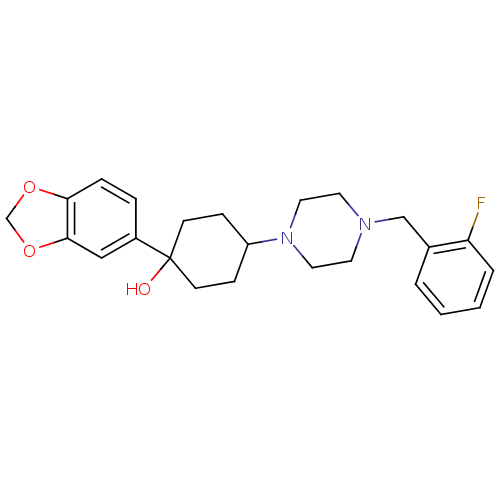 (1-Benzo[1,3]dioxol-5-yl-4-[4-(2-fluoro-benzyl)-pip...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of the 5-hydroxytryptamine 1A receptor in rat dorsal raphe | Bioorg Med Chem Lett 13: 285-8 (2002) BindingDB Entry DOI: 10.7270/Q2251HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50016974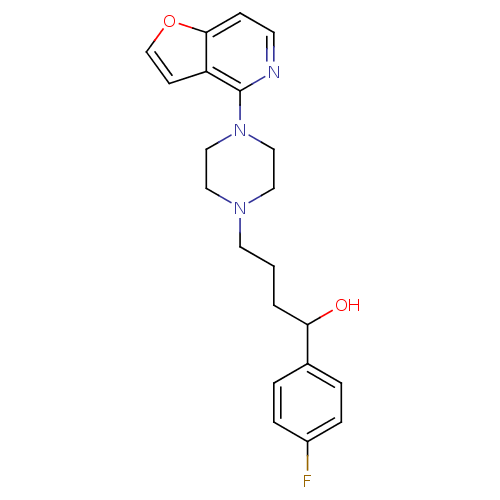 (1-(4-Fluoro-phenyl)-4-(4-furo[3,2-c]pyridin-4-yl-p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at serotonin 5-hydroxytryptamine 1A receptors of rat hippocampus by displacing [3H]8-OH-DPAT | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50122435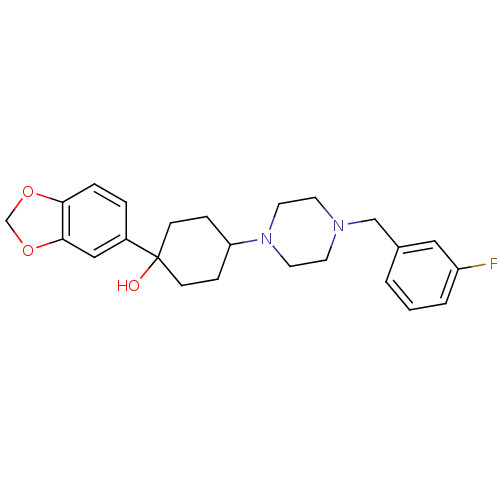 (1-Benzo[1,3]dioxol-5-yl-4-[4-(3-fluoro-benzyl)-pip...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of the 5-hydroxytryptamine 1A receptor in rat dorsal raphe | Bioorg Med Chem Lett 13: 285-8 (2002) BindingDB Entry DOI: 10.7270/Q2251HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50122436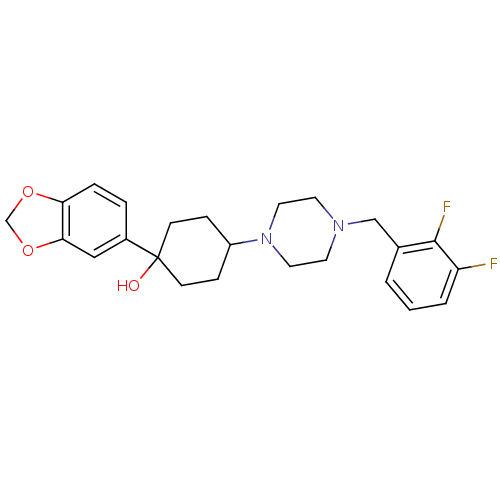 (1-Benzo[1,3]dioxol-5-yl-4-[4-(2,3-difluoro-benzyl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of the 5-hydroxytryptamine 1A receptor in rat dorsal raphe | Bioorg Med Chem Lett 13: 285-8 (2002) BindingDB Entry DOI: 10.7270/Q2251HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50016960 (4,4-Dimethyl-1-[4-(4-thieno[3,2-c]pyridin-4-yl-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at alpha-1 adrenergic receptor by displacing [3H]WB-4101 | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50016969 (1-[4-(4-Thieno[3,2-c]pyridin-4-yl-piperazin-1-yl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at serotonin 5-hydroxytryptamine 2 receptors of rat hippocampus by displacing [3H]spiperone | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50122427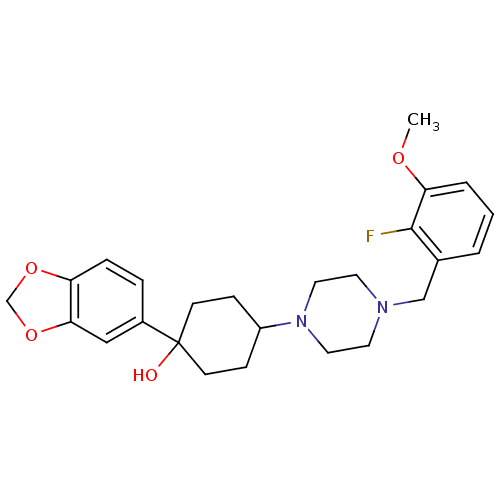 (1-Benzo[1,3]dioxol-5-yl-4-[4-(2-fluoro-3-methoxy-b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of the 5-hydroxytryptamine 1A receptor in rat dorsal raphe | Bioorg Med Chem Lett 13: 285-8 (2002) BindingDB Entry DOI: 10.7270/Q2251HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50016957 (3-[4-(4-Thieno[2,3-c]pyridin-7-yl-piperazin-1-yl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at 5-hydroxytryptamine 2 receptor of rat hippocampus by displacing [3H]spiperone | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50122415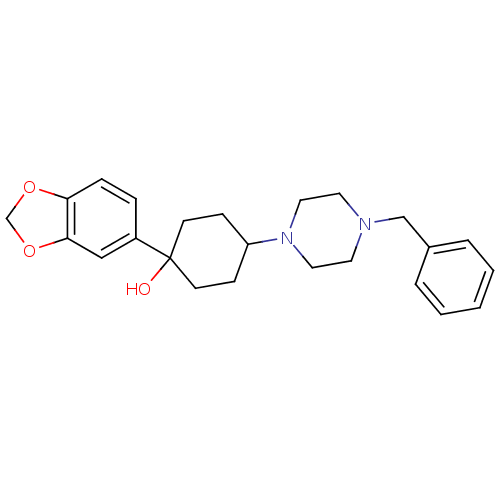 (1-Benzo[1,3]dioxol-5-yl-4-(4-benzyl-piperazin-1-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of the 5-hydroxytryptamine 1A receptor in rat dorsal raphe | Bioorg Med Chem Lett 13: 285-8 (2002) BindingDB Entry DOI: 10.7270/Q2251HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50001884 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at 5-hydroxytryptamine 2 receptor of rat hippocampus by displacing [3H]spiperone | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50122432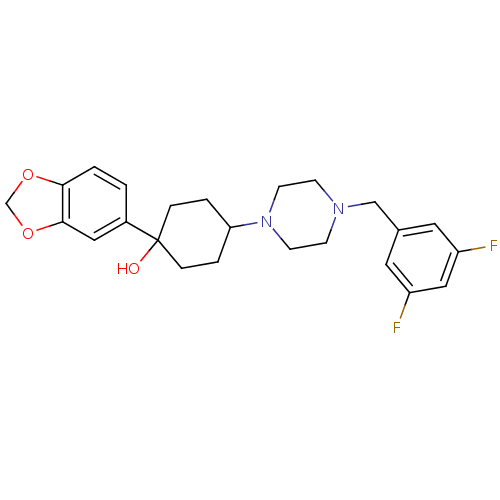 (1-Benzo[1,3]dioxol-5-yl-4-[4-(3,5-difluoro-benzyl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of the 5-hydroxytryptamine 1A receptor in rat dorsal raphe | Bioorg Med Chem Lett 13: 285-8 (2002) BindingDB Entry DOI: 10.7270/Q2251HJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50016958 (1-(4-Fluoro-phenyl)-4-(4-thieno[3,2-c]pyridin-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description Ability to bind at alpha-1 adrenergic receptor by displacing [3H]WB-4101 | J Med Chem 32: 1147-56 (1989) BindingDB Entry DOI: 10.7270/Q2HD7W8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 161 total ) | Next | Last >> |