 Found 3301 hits with Last Name = 'venkatesan' and Initial = 'h'
Found 3301 hits with Last Name = 'venkatesan' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50415081
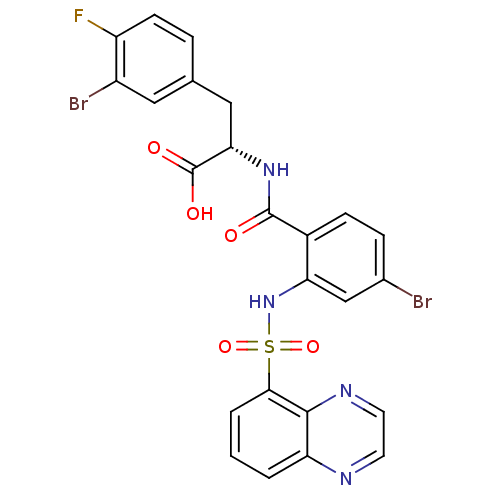
(CHEMBL571650)Show SMILES OC(=O)[C@H](Cc1ccc(F)c(Br)c1)NC(=O)c1ccc(Br)cc1NS(=O)(=O)c1cccc2nccnc12 |r| Show InChI InChI=1S/C24H17Br2FN4O5S/c25-14-5-6-15(23(32)30-20(24(33)34)11-13-4-7-17(27)16(26)10-13)19(12-14)31-37(35,36)21-3-1-2-18-22(21)29-9-8-28-18/h1-10,12,20,31H,11H2,(H,30,32)(H,33,34)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-2R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50415082

(CHEMBL583035)Show SMILES OC(=O)[C@H](Cc1ccc(F)c(Br)c1)NC(=O)c1ccc(I)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H15BrFIN4O5S2/c23-14-8-11(4-7-15(14)24)9-18(22(31)32)26-21(30)13-6-5-12(25)10-17(13)29-36(33,34)19-3-1-2-16-20(19)28-35-27-16/h1-8,10,18,29H,9H2,(H,26,30)(H,31,32)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-2R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50415080
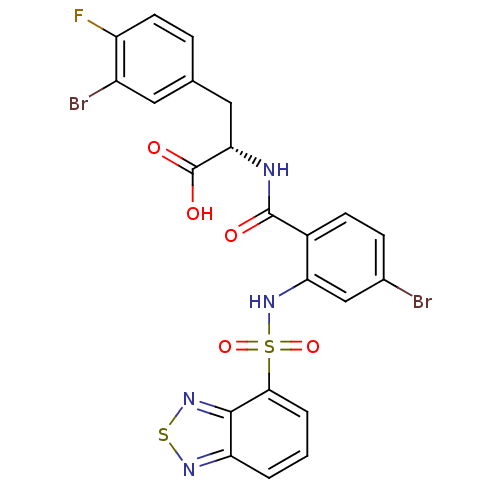
(CHEMBL585157)Show SMILES OC(=O)[C@H](Cc1ccc(F)c(Br)c1)NC(=O)c1ccc(Br)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H15Br2FN4O5S2/c23-12-5-6-13(21(30)26-18(22(31)32)9-11-4-7-15(25)14(24)8-11)17(10-12)29-36(33,34)19-3-1-2-16-20(19)28-35-27-16/h1-8,10,18,29H,9H2,(H,26,30)(H,31,32)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-2R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50415083

(CHEMBL566574)Show SMILES OC(=O)[C@H](Cc1ccc(F)c(Br)c1)NC(=O)c1cc(Cl)c(Cl)cc1NS(=O)(=O)c1cccc2nccnc12 |r| Show InChI InChI=1S/C24H16BrCl2FN4O5S/c25-14-8-12(4-5-17(14)28)9-20(24(34)35)31-23(33)13-10-15(26)16(27)11-19(13)32-38(36,37)21-3-1-2-18-22(21)30-7-6-29-18/h1-8,10-11,20,32H,9H2,(H,31,33)(H,34,35)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-2R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50415078
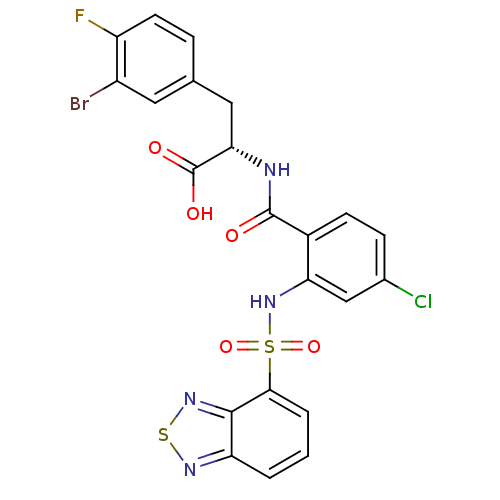
(CHEMBL565298)Show SMILES OC(=O)[C@H](Cc1ccc(F)c(Br)c1)NC(=O)c1ccc(Cl)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H15BrClFN4O5S2/c23-14-8-11(4-7-15(14)25)9-18(22(31)32)26-21(30)13-6-5-12(24)10-17(13)29-36(33,34)19-3-1-2-16-20(19)28-35-27-16/h1-8,10,18,29H,9H2,(H,26,30)(H,31,32)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-2R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50415084
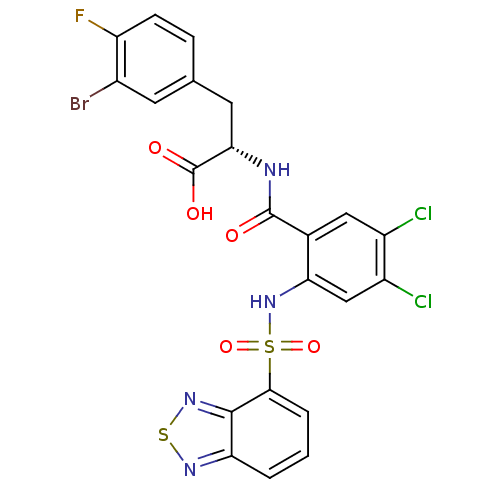
(CHEMBL565324)Show SMILES OC(=O)[C@H](Cc1ccc(F)c(Br)c1)NC(=O)c1cc(Cl)c(Cl)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H14BrCl2FN4O5S2/c23-12-6-10(4-5-15(12)26)7-18(22(32)33)27-21(31)11-8-13(24)14(25)9-17(11)30-37(34,35)19-3-1-2-16-20(19)29-36-28-16/h1-6,8-9,18,30H,7H2,(H,27,31)(H,32,33)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-2R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50415079
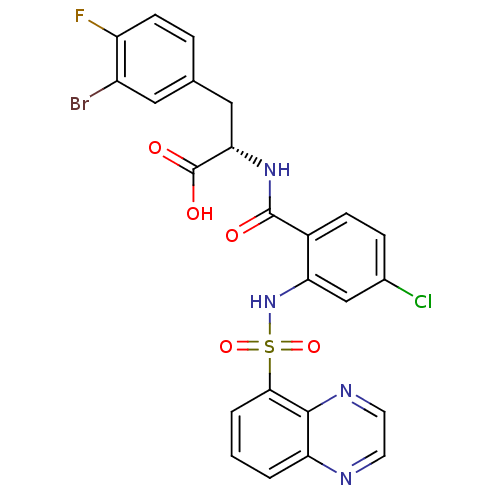
(CHEMBL569391)Show SMILES OC(=O)[C@H](Cc1ccc(F)c(Br)c1)NC(=O)c1ccc(Cl)cc1NS(=O)(=O)c1cccc2nccnc12 |r| Show InChI InChI=1S/C24H17BrClFN4O5S/c25-16-10-13(4-7-17(16)27)11-20(24(33)34)30-23(32)15-6-5-14(26)12-19(15)31-37(35,36)21-3-1-2-18-22(21)29-9-8-28-18/h1-10,12,20,31H,11H2,(H,30,32)(H,33,34)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-2R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50415077
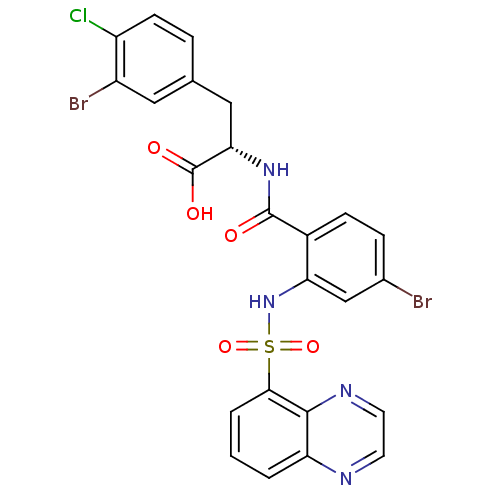
(CHEMBL584525)Show SMILES OC(=O)[C@H](Cc1ccc(Cl)c(Br)c1)NC(=O)c1ccc(Br)cc1NS(=O)(=O)c1cccc2nccnc12 |r| Show InChI InChI=1S/C24H17Br2ClN4O5S/c25-14-5-6-15(23(32)30-20(24(33)34)11-13-4-7-17(27)16(26)10-13)19(12-14)31-37(35,36)21-3-1-2-18-22(21)29-9-8-28-18/h1-10,12,20,31H,11H2,(H,30,32)(H,33,34)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-2R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50415085
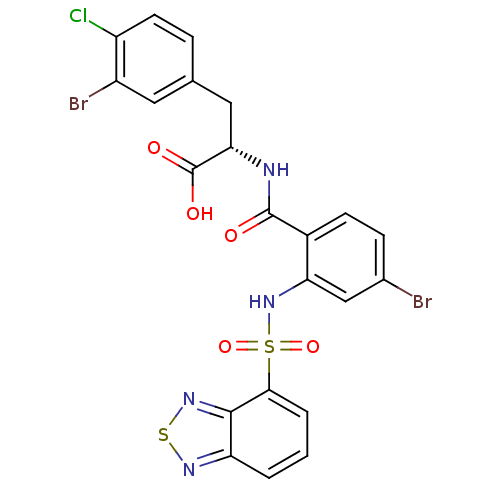
(CHEMBL570520)Show SMILES OC(=O)[C@H](Cc1ccc(Cl)c(Br)c1)NC(=O)c1ccc(Br)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H15Br2ClN4O5S2/c23-12-5-6-13(21(30)26-18(22(31)32)9-11-4-7-15(25)14(24)8-11)17(10-12)29-36(33,34)19-3-1-2-16-20(19)28-35-27-16/h1-8,10,18,29H,9H2,(H,26,30)(H,31,32)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-2R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50415066

(CHEMBL585914)Show SMILES OC(=O)[C@H](Cc1ccc(Cl)c(Cl)c1)NC(=O)c1ccc(Cl)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H15Cl3N4O5S2/c23-12-5-6-13(21(30)26-18(22(31)32)9-11-4-7-14(24)15(25)8-11)17(10-12)29-36(33,34)19-3-1-2-16-20(19)28-35-27-16/h1-8,10,18,29H,9H2,(H,26,30)(H,31,32)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-2R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50415076
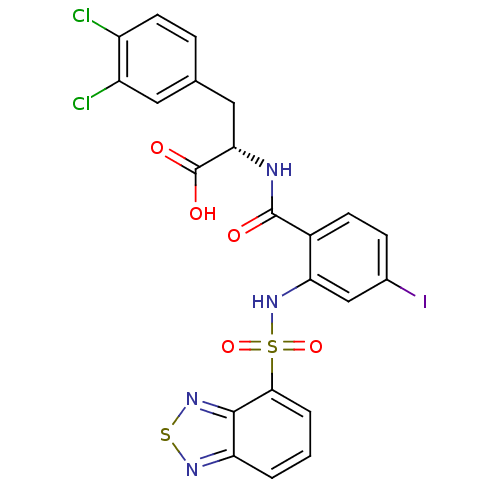
(CHEMBL583344)Show SMILES OC(=O)[C@H](Cc1ccc(Cl)c(Cl)c1)NC(=O)c1ccc(I)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H15Cl2IN4O5S2/c23-14-7-4-11(8-15(14)24)9-18(22(31)32)26-21(30)13-6-5-12(25)10-17(13)29-36(33,34)19-3-1-2-16-20(19)28-35-27-16/h1-8,10,18,29H,9H2,(H,26,30)(H,31,32)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-2R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415077

(CHEMBL584525)Show SMILES OC(=O)[C@H](Cc1ccc(Cl)c(Br)c1)NC(=O)c1ccc(Br)cc1NS(=O)(=O)c1cccc2nccnc12 |r| Show InChI InChI=1S/C24H17Br2ClN4O5S/c25-14-5-6-15(23(32)30-20(24(33)34)11-13-4-7-17(27)16(26)10-13)19(12-14)31-37(35,36)21-3-1-2-18-22(21)29-9-8-28-18/h1-10,12,20,31H,11H2,(H,30,32)(H,33,34)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415085

(CHEMBL570520)Show SMILES OC(=O)[C@H](Cc1ccc(Cl)c(Br)c1)NC(=O)c1ccc(Br)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H15Br2ClN4O5S2/c23-12-5-6-13(21(30)26-18(22(31)32)9-11-4-7-15(25)14(24)8-11)17(10-12)29-36(33,34)19-3-1-2-16-20(19)28-35-27-16/h1-8,10,18,29H,9H2,(H,26,30)(H,31,32)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415083

(CHEMBL566574)Show SMILES OC(=O)[C@H](Cc1ccc(F)c(Br)c1)NC(=O)c1cc(Cl)c(Cl)cc1NS(=O)(=O)c1cccc2nccnc12 |r| Show InChI InChI=1S/C24H16BrCl2FN4O5S/c25-14-8-12(4-5-17(14)28)9-20(24(34)35)31-23(33)13-10-15(26)16(27)11-19(13)32-38(36,37)21-3-1-2-18-22(21)30-7-6-29-18/h1-8,10-11,20,32H,9H2,(H,31,33)(H,34,35)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415082

(CHEMBL583035)Show SMILES OC(=O)[C@H](Cc1ccc(F)c(Br)c1)NC(=O)c1ccc(I)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H15BrFIN4O5S2/c23-14-8-11(4-7-15(14)24)9-18(22(31)32)26-21(30)13-6-5-12(25)10-17(13)29-36(33,34)19-3-1-2-16-20(19)28-35-27-16/h1-8,10,18,29H,9H2,(H,26,30)(H,31,32)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415084

(CHEMBL565324)Show SMILES OC(=O)[C@H](Cc1ccc(F)c(Br)c1)NC(=O)c1cc(Cl)c(Cl)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H14BrCl2FN4O5S2/c23-12-6-10(4-5-15(12)26)7-18(22(32)33)27-21(31)11-8-13(24)14(25)9-17(11)30-37(34,35)19-3-1-2-16-20(19)29-36-28-16/h1-6,8-9,18,30H,7H2,(H,27,31)(H,32,33)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415076

(CHEMBL583344)Show SMILES OC(=O)[C@H](Cc1ccc(Cl)c(Cl)c1)NC(=O)c1ccc(I)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H15Cl2IN4O5S2/c23-14-7-4-11(8-15(14)24)9-18(22(31)32)26-21(30)13-6-5-12(25)10-17(13)29-36(33,34)19-3-1-2-16-20(19)28-35-27-16/h1-8,10,18,29H,9H2,(H,26,30)(H,31,32)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415075
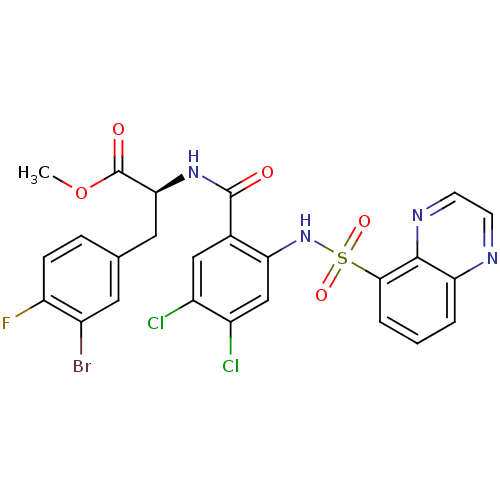
(CHEMBL566177)Show SMILES COC(=O)[C@H](Cc1ccc(F)c(Br)c1)NC(=O)c1cc(Cl)c(Cl)cc1NS(=O)(=O)c1cccc2nccnc12 |r| Show InChI InChI=1S/C25H18BrCl2FN4O5S/c1-38-25(35)21(10-13-5-6-18(29)15(26)9-13)32-24(34)14-11-16(27)17(28)12-20(14)33-39(36,37)22-4-2-3-19-23(22)31-8-7-30-19/h2-9,11-12,21,33H,10H2,1H3,(H,32,34)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415080

(CHEMBL585157)Show SMILES OC(=O)[C@H](Cc1ccc(F)c(Br)c1)NC(=O)c1ccc(Br)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H15Br2FN4O5S2/c23-12-5-6-13(21(30)26-18(22(31)32)9-11-4-7-15(25)14(24)8-11)17(10-12)29-36(33,34)19-3-1-2-16-20(19)28-35-27-16/h1-8,10,18,29H,9H2,(H,26,30)(H,31,32)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415078

(CHEMBL565298)Show SMILES OC(=O)[C@H](Cc1ccc(F)c(Br)c1)NC(=O)c1ccc(Cl)cc1NS(=O)(=O)c1cccc2nsnc12 |r| Show InChI InChI=1S/C22H15BrClFN4O5S2/c23-14-8-11(4-7-15(14)25)9-18(22(31)32)26-21(30)13-6-5-12(24)10-17(13)29-36(33,34)19-3-1-2-16-20(19)28-35-27-16/h1-8,10,18,29H,9H2,(H,26,30)(H,31,32)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50415075

(CHEMBL566177)Show SMILES COC(=O)[C@H](Cc1ccc(F)c(Br)c1)NC(=O)c1cc(Cl)c(Cl)cc1NS(=O)(=O)c1cccc2nccnc12 |r| Show InChI InChI=1S/C25H18BrCl2FN4O5S/c1-38-25(35)21(10-13-5-6-18(29)15(26)9-13)32-24(34)14-11-16(27)17(28)12-20(14)33-39(36,37)22-4-2-3-19-23(22)31-8-7-30-19/h2-9,11-12,21,33H,10H2,1H3,(H,32,34)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-2R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415081

(CHEMBL571650)Show SMILES OC(=O)[C@H](Cc1ccc(F)c(Br)c1)NC(=O)c1ccc(Br)cc1NS(=O)(=O)c1cccc2nccnc12 |r| Show InChI InChI=1S/C24H17Br2FN4O5S/c25-14-5-6-15(23(32)30-20(24(33)34)11-13-4-7-17(27)16(26)10-13)19(12-14)31-37(35,36)21-3-1-2-18-22(21)29-9-8-28-18/h1-10,12,20,31H,11H2,(H,30,32)(H,33,34)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50415079

(CHEMBL569391)Show SMILES OC(=O)[C@H](Cc1ccc(F)c(Br)c1)NC(=O)c1ccc(Cl)cc1NS(=O)(=O)c1cccc2nccnc12 |r| Show InChI InChI=1S/C24H17BrClFN4O5S/c25-16-10-13(4-7-17(16)27)11-20(24(33)34)30-23(32)15-6-5-14(26)12-19(15)31-37(35,36)21-3-1-2-18-22(21)29-9-8-28-18/h1-10,12,20,31H,11H2,(H,30,32)(H,33,34)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CCK-8S from human CCK-1R after 100 mins by liquid scintillation counting |
Bioorg Med Chem Lett 19: 6376-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.065
BindingDB Entry DOI: 10.7270/Q2X92CJK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50196208
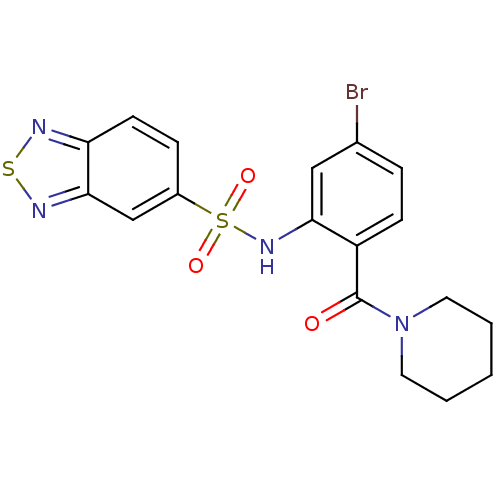
(1-[2-[(2,1,3-benzothiadiazol-5-ylsulfonyl)amino]-4...)Show SMILES Brc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2ccc3nsnc3c2)c1 Show InChI InChI=1S/C18H17BrN4O3S2/c19-12-4-6-14(18(24)23-8-2-1-3-9-23)16(10-12)22-28(25,26)13-5-7-15-17(11-13)21-27-20-15/h4-7,10-11,22H,1-3,8-9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8S from human CCK1R |
J Med Chem 49: 6371-90 (2006)
Article DOI: 10.1021/jm060590x
BindingDB Entry DOI: 10.7270/Q2MK6CHM |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50196209
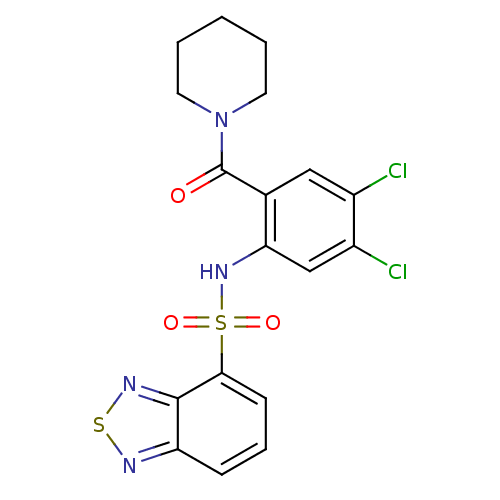
(1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...)Show SMILES Clc1cc(NS(=O)(=O)c2cccc3nsnc23)c(cc1Cl)C(=O)N1CCCCC1 Show InChI InChI=1S/C18H16Cl2N4O3S2/c19-12-9-11(18(25)24-7-2-1-3-8-24)15(10-13(12)20)23-29(26,27)16-6-4-5-14-17(16)22-28-21-14/h4-6,9-10,23H,1-3,7-8H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8S from human CCK1R |
J Med Chem 49: 6371-90 (2006)
Article DOI: 10.1021/jm060590x
BindingDB Entry DOI: 10.7270/Q2MK6CHM |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50196210
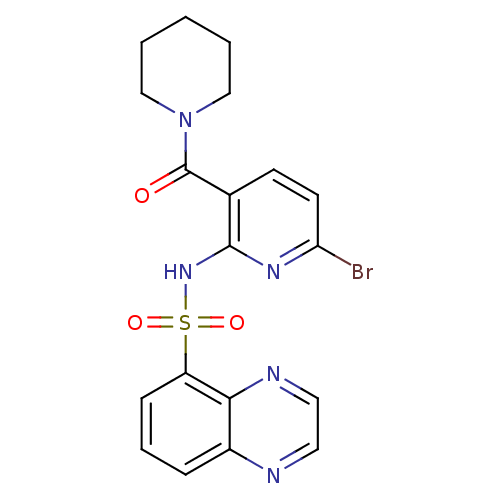
(1-[4-bromo-2-[(5-quinoxalinylsulfonyl)amino]benzoy...)Show SMILES Brc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3nccnc23)n1 Show InChI InChI=1S/C19H18BrN5O3S/c20-16-8-7-13(19(26)25-11-2-1-3-12-25)18(23-16)24-29(27,28)15-6-4-5-14-17(15)22-10-9-21-14/h4-10H,1-3,11-12H2,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8S from human CCK1R |
J Med Chem 49: 6371-90 (2006)
Article DOI: 10.1021/jm060590x
BindingDB Entry DOI: 10.7270/Q2MK6CHM |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50196200
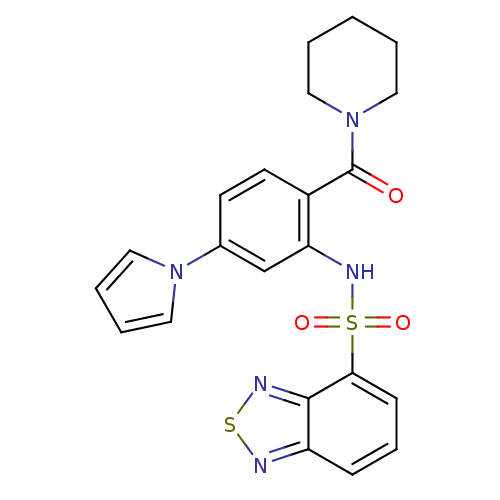
(1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...)Show SMILES O=C(N1CCCCC1)c1ccc(cc1NS(=O)(=O)c1cccc2nsnc12)-n1cccc1 Show InChI InChI=1S/C22H21N5O3S2/c28-22(27-13-2-1-3-14-27)17-10-9-16(26-11-4-5-12-26)15-19(17)25-32(29,30)20-8-6-7-18-21(20)24-31-23-18/h4-12,15,25H,1-3,13-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8S from human CCK1R |
J Med Chem 49: 6371-90 (2006)
Article DOI: 10.1021/jm060590x
BindingDB Entry DOI: 10.7270/Q2MK6CHM |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50196201

(1-[2-[7-bromo(2,1,3-benzothiadiazol-4-ylsulfonyl)a...)Show SMILES Brc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2ccc(Br)c3nsnc23)c1 Show InChI InChI=1S/C18H16Br2N4O3S2/c19-11-4-5-12(18(25)24-8-2-1-3-9-24)14(10-11)23-29(26,27)15-7-6-13(20)16-17(15)22-28-21-16/h4-7,10,23H,1-3,8-9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8S from human CCK1R |
J Med Chem 49: 6371-90 (2006)
Article DOI: 10.1021/jm060590x
BindingDB Entry DOI: 10.7270/Q2MK6CHM |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50196184
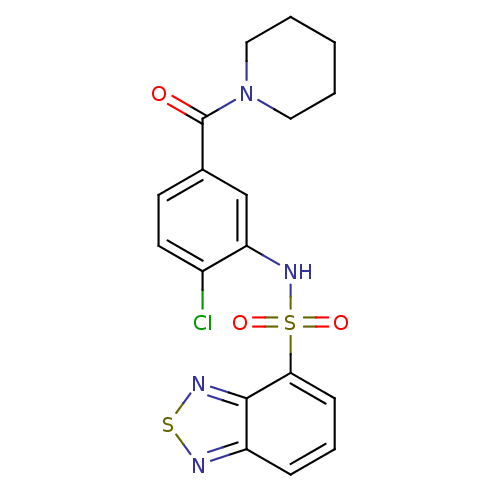
(1-[3-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...)Show SMILES Clc1ccc(cc1NS(=O)(=O)c1cccc2nsnc12)C(=O)N1CCCCC1 Show InChI InChI=1S/C18H17ClN4O3S2/c19-13-8-7-12(18(24)23-9-2-1-3-10-23)11-15(13)22-28(25,26)16-6-4-5-14-17(16)21-27-20-14/h4-8,11,22H,1-3,9-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8S from human CCK2R |
J Med Chem 49: 6371-90 (2006)
Article DOI: 10.1021/jm060590x
BindingDB Entry DOI: 10.7270/Q2MK6CHM |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50196202
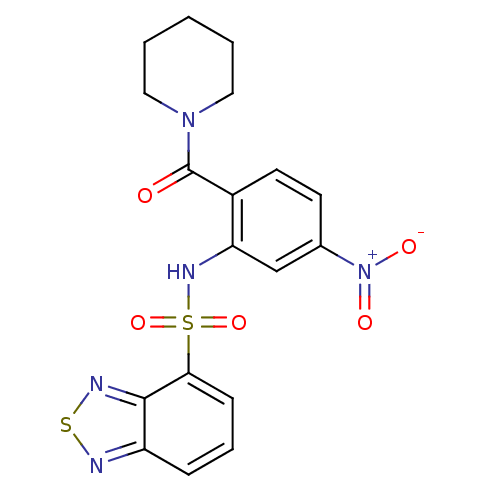
(1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...)Show SMILES [O-][N+](=O)c1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3nsnc23)c1 Show InChI InChI=1S/C18H17N5O5S2/c24-18(22-9-2-1-3-10-22)13-8-7-12(23(25)26)11-15(13)21-30(27,28)16-6-4-5-14-17(16)20-29-19-14/h4-8,11,21H,1-3,9-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8S from human CCK1R |
J Med Chem 49: 6371-90 (2006)
Article DOI: 10.1021/jm060590x
BindingDB Entry DOI: 10.7270/Q2MK6CHM |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50196185
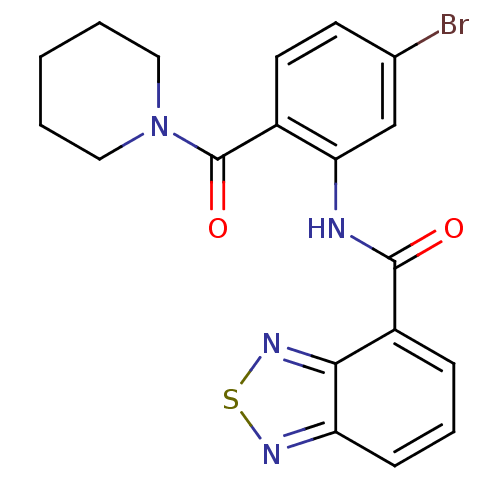
(1-[2-[(2,1,3-benzothiadiazol-4-ylcarbonyl)amino]-4...)Show SMILES Brc1ccc(C(=O)N2CCCCC2)c(NC(=O)c2cccc3nsnc23)c1 Show InChI InChI=1S/C19H17BrN4O2S/c20-12-7-8-13(19(26)24-9-2-1-3-10-24)16(11-12)21-18(25)14-5-4-6-15-17(14)23-27-22-15/h4-8,11H,1-3,9-10H2,(H,21,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8S from human CCK2R |
J Med Chem 49: 6371-90 (2006)
Article DOI: 10.1021/jm060590x
BindingDB Entry DOI: 10.7270/Q2MK6CHM |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50196216
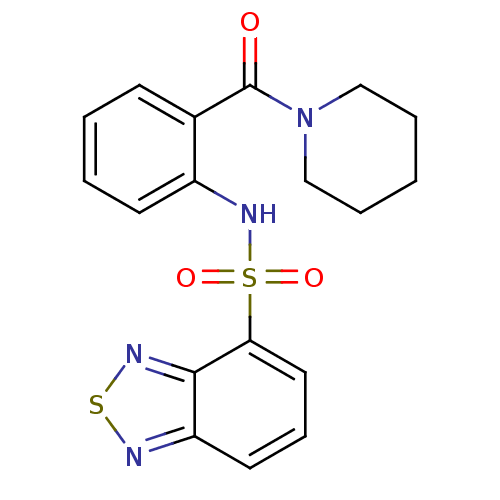
(1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-b...)Show SMILES O=C(N1CCCCC1)c1ccccc1NS(=O)(=O)c1cccc2nsnc12 Show InChI InChI=1S/C18H18N4O3S2/c23-18(22-11-4-1-5-12-22)13-7-2-3-8-14(13)21-27(24,25)16-10-6-9-15-17(16)20-26-19-15/h2-3,6-10,21H,1,4-5,11-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8S from human CCK2R |
J Med Chem 49: 6371-90 (2006)
Article DOI: 10.1021/jm060590x
BindingDB Entry DOI: 10.7270/Q2MK6CHM |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50196212
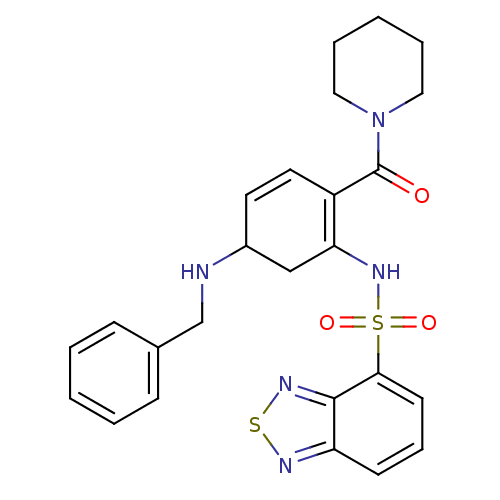
(1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...)Show SMILES O=C(N1CCCCC1)C1=C(CC(NCc2ccccc2)C=C1)NS(=O)(=O)c1cccc2nsnc12 |c:22,t:9| Show InChI InChI=1S/C25H27N5O3S2/c31-25(30-14-5-2-6-15-30)20-13-12-19(26-17-18-8-3-1-4-9-18)16-22(20)29-35(32,33)23-11-7-10-21-24(23)28-34-27-21/h1,3-4,7-13,19,26,29H,2,5-6,14-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8S from human CCK1R |
J Med Chem 49: 6371-90 (2006)
Article DOI: 10.1021/jm060590x
BindingDB Entry DOI: 10.7270/Q2MK6CHM |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50196207
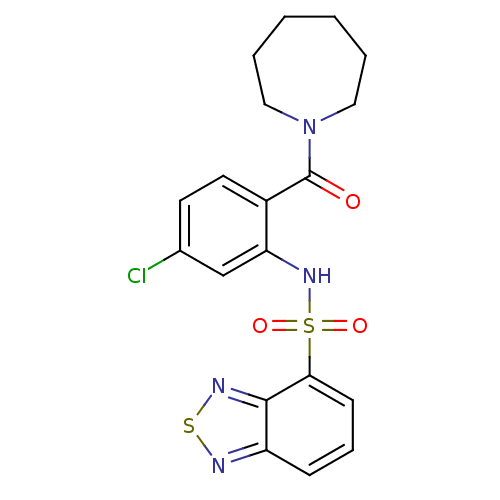
(1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...)Show SMILES Clc1ccc(C(=O)N2CCCCCC2)c(NS(=O)(=O)c2cccc3nsnc23)c1 Show InChI InChI=1S/C19H19ClN4O3S2/c20-13-8-9-14(19(25)24-10-3-1-2-4-11-24)16(12-13)23-29(26,27)17-7-5-6-15-18(17)22-28-21-15/h5-9,12,23H,1-4,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8S from human CCK1R |
J Med Chem 49: 6371-90 (2006)
Article DOI: 10.1021/jm060590x
BindingDB Entry DOI: 10.7270/Q2MK6CHM |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50196213
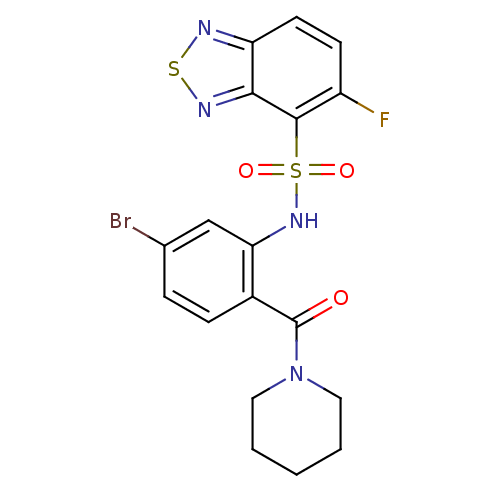
(1-[4-bromo-2-[5-fluoro(2,1,3-benzothiadiazol-4-yls...)Show SMILES Fc1ccc2nsnc2c1S(=O)(=O)Nc1cc(Br)ccc1C(=O)N1CCCCC1 Show InChI InChI=1S/C18H16BrFN4O3S2/c19-11-4-5-12(18(25)24-8-2-1-3-9-24)15(10-11)23-29(26,27)17-13(20)6-7-14-16(17)22-28-21-14/h4-7,10,23H,1-3,8-9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8S from human CCK1R |
J Med Chem 49: 6371-90 (2006)
Article DOI: 10.1021/jm060590x
BindingDB Entry DOI: 10.7270/Q2MK6CHM |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50196206
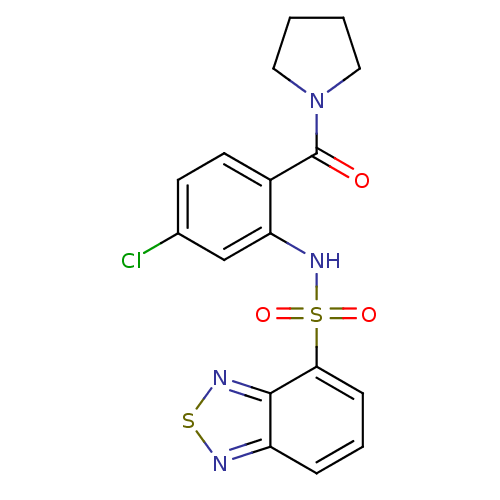
(1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...)Show SMILES Clc1ccc(C(=O)N2CCCC2)c(NS(=O)(=O)c2cccc3nsnc23)c1 Show InChI InChI=1S/C17H15ClN4O3S2/c18-11-6-7-12(17(23)22-8-1-2-9-22)14(10-11)21-27(24,25)15-5-3-4-13-16(15)20-26-19-13/h3-7,10,21H,1-2,8-9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8S from human CCK1R |
J Med Chem 49: 6371-90 (2006)
Article DOI: 10.1021/jm060590x
BindingDB Entry DOI: 10.7270/Q2MK6CHM |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50196219
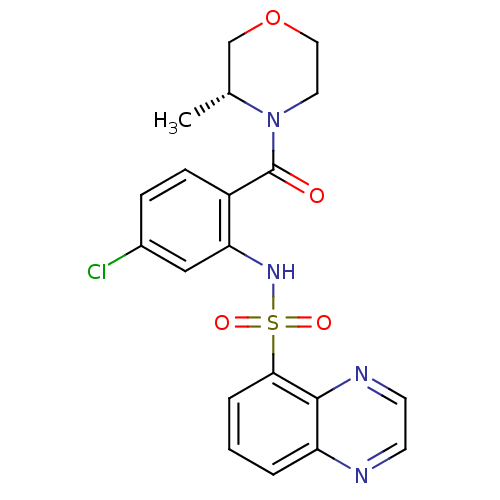
((R)-4-[4-chloro-2-[(5-quinoxalinylsulfonyl)amino]b...)Show SMILES C[C@@H]1COCCN1C(=O)c1ccc(Cl)cc1NS(=O)(=O)c1cccc2nccnc12 Show InChI InChI=1S/C20H19ClN4O4S/c1-13-12-29-10-9-25(13)20(26)15-6-5-14(21)11-17(15)24-30(27,28)18-4-2-3-16-19(18)23-8-7-22-16/h2-8,11,13,24H,9-10,12H2,1H3/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8S from human CCK1R |
J Med Chem 49: 6371-90 (2006)
Article DOI: 10.1021/jm060590x
BindingDB Entry DOI: 10.7270/Q2MK6CHM |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50196211
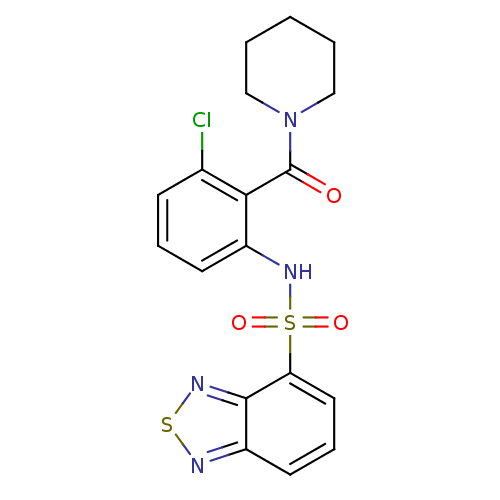
(1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-6...)Show SMILES Clc1cccc(NS(=O)(=O)c2cccc3nsnc23)c1C(=O)N1CCCCC1 Show InChI InChI=1S/C18H17ClN4O3S2/c19-12-6-4-7-13(16(12)18(24)23-10-2-1-3-11-23)22-28(25,26)15-9-5-8-14-17(15)21-27-20-14/h4-9,22H,1-3,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8S from human CCK1R |
J Med Chem 49: 6371-90 (2006)
Article DOI: 10.1021/jm060590x
BindingDB Entry DOI: 10.7270/Q2MK6CHM |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50196152
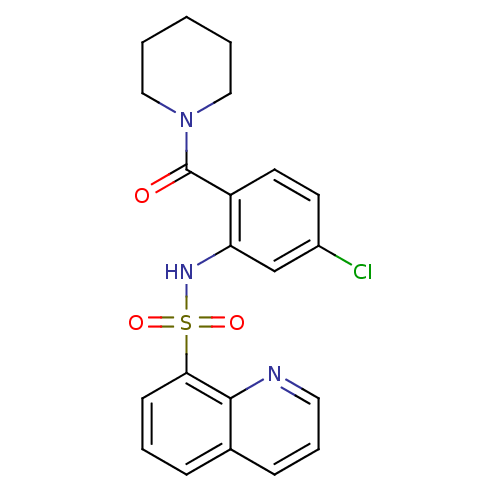
(1-[4-chloro-2-[(quinolin-8-ylsulfonyl)amino]-benzo...)Show SMILES Clc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3cccnc23)c1 Show InChI InChI=1S/C21H20ClN3O3S/c22-16-9-10-17(21(26)25-12-2-1-3-13-25)18(14-16)24-29(27,28)19-8-4-6-15-7-5-11-23-20(15)19/h4-11,14,24H,1-3,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8S from human CCK2R |
J Med Chem 49: 6371-90 (2006)
Article DOI: 10.1021/jm060590x
BindingDB Entry DOI: 10.7270/Q2MK6CHM |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50196161
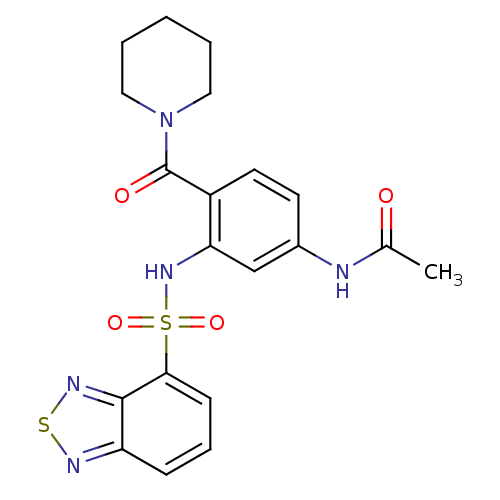
(1-[4-acetylamino-2-[(2,1,3-benzothiadiazol-4-ylsul...)Show SMILES CC(=O)Nc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3nsnc23)c1 Show InChI InChI=1S/C20H21N5O4S2/c1-13(26)21-14-8-9-15(20(27)25-10-3-2-4-11-25)17(12-14)24-31(28,29)18-7-5-6-16-19(18)23-30-22-16/h5-9,12,24H,2-4,10-11H2,1H3,(H,21,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8S from human CCK2R |
J Med Chem 49: 6371-90 (2006)
Article DOI: 10.1021/jm060590x
BindingDB Entry DOI: 10.7270/Q2MK6CHM |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50196215
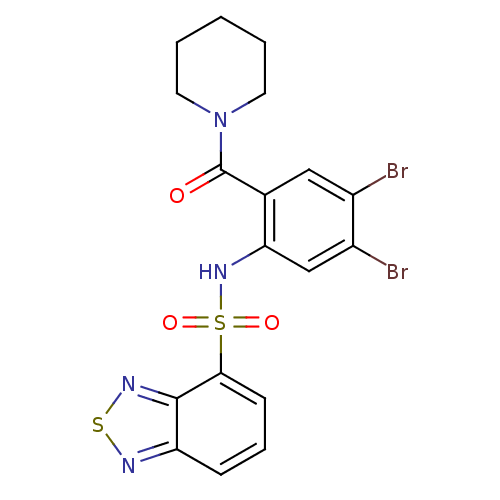
(1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...)Show SMILES Brc1cc(NS(=O)(=O)c2cccc3nsnc23)c(cc1Br)C(=O)N1CCCCC1 Show InChI InChI=1S/C18H16Br2N4O3S2/c19-12-9-11(18(25)24-7-2-1-3-8-24)15(10-13(12)20)23-29(26,27)16-6-4-5-14-17(16)22-28-21-14/h4-6,9-10,23H,1-3,7-8H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8S from human CCK1R |
J Med Chem 49: 6371-90 (2006)
Article DOI: 10.1021/jm060590x
BindingDB Entry DOI: 10.7270/Q2MK6CHM |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50196214
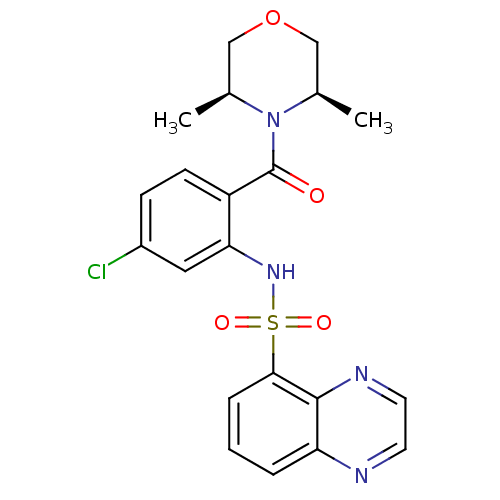
((3R,5S)-rel-4-[4-chloro-2-[(5-quinoxalinylsulfonyl...)Show SMILES C[C@H]1COC[C@@H](C)N1C(=O)c1ccc(Cl)cc1NS(=O)(=O)c1cccc2nccnc12 Show InChI InChI=1S/C21H21ClN4O4S/c1-13-11-30-12-14(2)26(13)21(27)16-7-6-15(22)10-18(16)25-31(28,29)19-5-3-4-17-20(19)24-9-8-23-17/h3-10,13-14,25H,11-12H2,1-2H3/t13-,14+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8S from human CCK1R |
J Med Chem 49: 6371-90 (2006)
Article DOI: 10.1021/jm060590x
BindingDB Entry DOI: 10.7270/Q2MK6CHM |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50196220
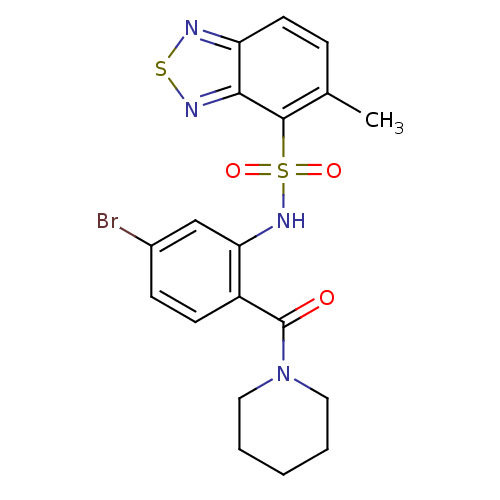
(1-[4-bromo-2-[5-methyl(2,1,3-benzothiadiazol-4-yls...)Show SMILES Cc1ccc2nsnc2c1S(=O)(=O)Nc1cc(Br)ccc1C(=O)N1CCCCC1 Show InChI InChI=1S/C19H19BrN4O3S2/c1-12-5-8-15-17(22-28-21-15)18(12)29(26,27)23-16-11-13(20)6-7-14(16)19(25)24-9-3-2-4-10-24/h5-8,11,23H,2-4,9-10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8S from human CCK1R |
J Med Chem 49: 6371-90 (2006)
Article DOI: 10.1021/jm060590x
BindingDB Entry DOI: 10.7270/Q2MK6CHM |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50196217

(1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...)Show SMILES CCc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3nsnc23)c1 Show InChI InChI=1S/C20H22N4O3S2/c1-2-14-9-10-15(20(25)24-11-4-3-5-12-24)17(13-14)23-29(26,27)18-8-6-7-16-19(18)22-28-21-16/h6-10,13,23H,2-5,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8S from human CCK1R |
J Med Chem 49: 6371-90 (2006)
Article DOI: 10.1021/jm060590x
BindingDB Entry DOI: 10.7270/Q2MK6CHM |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50196218
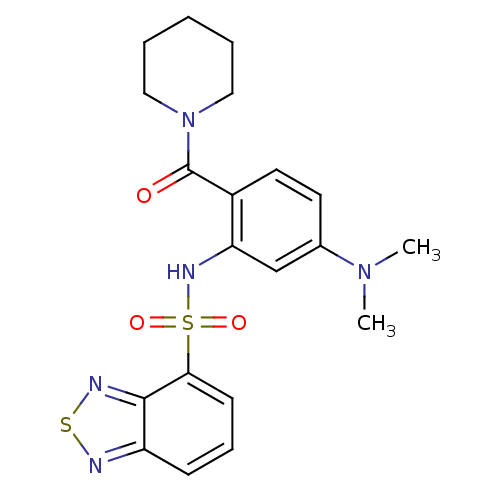
(1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...)Show SMILES CN(C)c1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3nsnc23)c1 Show InChI InChI=1S/C20H23N5O3S2/c1-24(2)14-9-10-15(20(26)25-11-4-3-5-12-25)17(13-14)23-30(27,28)18-8-6-7-16-19(18)22-29-21-16/h6-10,13,23H,3-5,11-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8S from human CCK1R |
J Med Chem 49: 6371-90 (2006)
Article DOI: 10.1021/jm060590x
BindingDB Entry DOI: 10.7270/Q2MK6CHM |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50196153
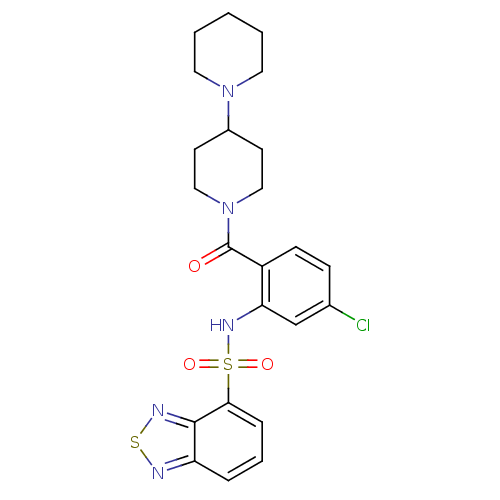
(1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...)Show SMILES Clc1ccc(C(=O)N2CCC(CC2)N2CCCCC2)c(NS(=O)(=O)c2cccc3nsnc23)c1 Show InChI InChI=1S/C23H26ClN5O3S2/c24-16-7-8-18(23(30)29-13-9-17(10-14-29)28-11-2-1-3-12-28)20(15-16)27-34(31,32)21-6-4-5-19-22(21)26-33-25-19/h4-8,15,17,27H,1-3,9-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8S from human CCK1R |
J Med Chem 49: 6371-90 (2006)
Article DOI: 10.1021/jm060590x
BindingDB Entry DOI: 10.7270/Q2MK6CHM |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50196172
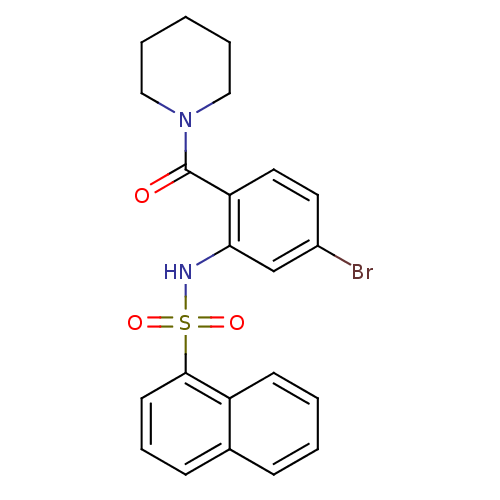
(1-[4-bromo-2-[[naphthalen-1-ylsulfonyl]amino]benzo...)Show SMILES Brc1ccc(C(=O)N2CCCCC2)c(NS(=O)(=O)c2cccc3ccccc23)c1 Show InChI InChI=1S/C22H21BrN2O3S/c23-17-11-12-19(22(26)25-13-4-1-5-14-25)20(15-17)24-29(27,28)21-10-6-8-16-7-2-3-9-18(16)21/h2-3,6-12,15,24H,1,4-5,13-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8S from human CCK2R |
J Med Chem 49: 6371-90 (2006)
Article DOI: 10.1021/jm060590x
BindingDB Entry DOI: 10.7270/Q2MK6CHM |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50196149
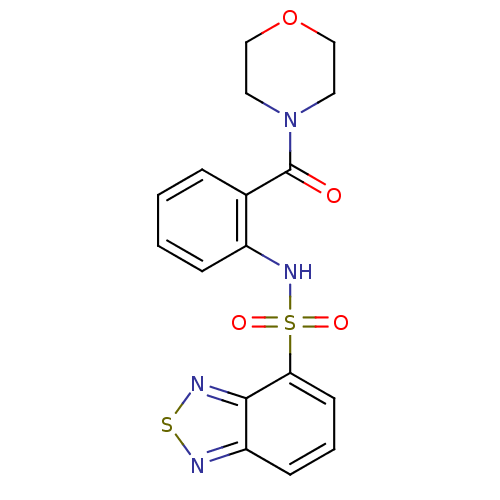
(4-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]be...)Show SMILES O=C(N1CCOCC1)c1ccccc1NS(=O)(=O)c1cccc2nsnc12 Show InChI InChI=1S/C17H16N4O4S2/c22-17(21-8-10-25-11-9-21)12-4-1-2-5-13(12)20-27(23,24)15-7-3-6-14-16(15)19-26-18-14/h1-7,20H,8-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8S from human CCK1R |
J Med Chem 49: 6371-90 (2006)
Article DOI: 10.1021/jm060590x
BindingDB Entry DOI: 10.7270/Q2MK6CHM |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50196150
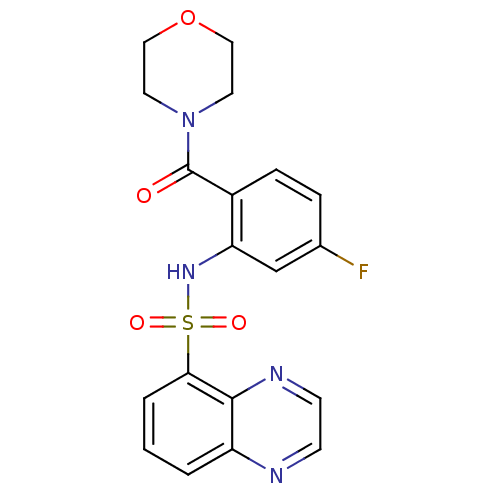
(4-[4-fluoro-2-[(5-quinoxalinylsulfonyl)amino]benzo...)Show SMILES Fc1ccc(C(=O)N2CCOCC2)c(NS(=O)(=O)c2cccc3nccnc23)c1 Show InChI InChI=1S/C19H17FN4O4S/c20-13-4-5-14(19(25)24-8-10-28-11-9-24)16(12-13)23-29(26,27)17-3-1-2-15-18(17)22-7-6-21-15/h1-7,12,23H,8-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8S from human CCK1R |
J Med Chem 49: 6371-90 (2006)
Article DOI: 10.1021/jm060590x
BindingDB Entry DOI: 10.7270/Q2MK6CHM |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50196151
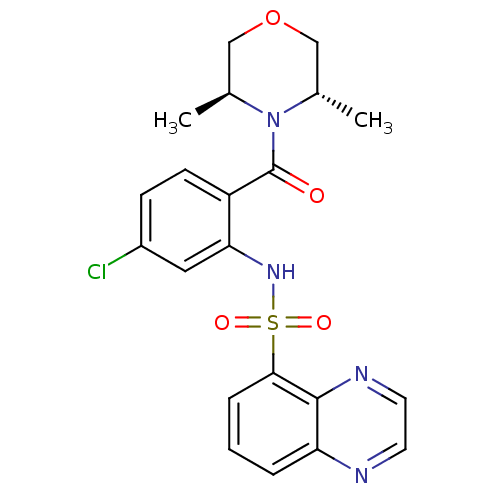
((3S,5S)-4-[4-chloro-2-[(5-quinoxalinylsulfonyl)ami...)Show SMILES C[C@H]1COC[C@H](C)N1C(=O)c1ccc(Cl)cc1NS(=O)(=O)c1cccc2nccnc12 Show InChI InChI=1S/C21H21ClN4O4S/c1-13-11-30-12-14(2)26(13)21(27)16-7-6-15(22)10-18(16)25-31(28,29)19-5-3-4-17-20(19)24-9-8-23-17/h3-10,13-14,25H,11-12H2,1-2H3/t13-,14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK-8S from human CCK1R |
J Med Chem 49: 6371-90 (2006)
Article DOI: 10.1021/jm060590x
BindingDB Entry DOI: 10.7270/Q2MK6CHM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data