| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cholecystokinin receptor type A |
|---|
| Ligand | BDBM50196207 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_418718 (CHEMBL913512) |
|---|
| Ki | >10000±n/a nM |
|---|
| Citation |  Allison, BD; Phuong, VK; McAtee, LC; Rosen, M; Morton, M; Prendergast, C; Barrett, T; Lagaud, G; Freedman, J; Li, L; Wu, X; Venkatesan, H; Pippel, M; Woods, C; Rizzolio, MC; Hack, M; Hoey, K; Deng, X; King, C; Shankley, NP; Rabinowitz, MH Identification and optimization of anthranilic sulfonamides as novel, selective cholecystokinin-2 receptor antagonists. J Med Chem49:6371-90 (2006) [PubMed] Article Allison, BD; Phuong, VK; McAtee, LC; Rosen, M; Morton, M; Prendergast, C; Barrett, T; Lagaud, G; Freedman, J; Li, L; Wu, X; Venkatesan, H; Pippel, M; Woods, C; Rizzolio, MC; Hack, M; Hoey, K; Deng, X; King, C; Shankley, NP; Rabinowitz, MH Identification and optimization of anthranilic sulfonamides as novel, selective cholecystokinin-2 receptor antagonists. J Med Chem49:6371-90 (2006) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cholecystokinin receptor type A |
|---|
| Name: | Cholecystokinin receptor type A |
|---|
| Synonyms: | CCK-A receptor | CCK-AR | CCK1-R | CCKAR | CCKAR_HUMAN | CCKRA | Cholecystokinin receptor | Cholecystokinin receptor type A | Cholecystokinin-1 Receptor |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 47859.34 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Stable expression of human CCK-1 receptors in HEK 293 cells. |
|---|
| Residue: | 428 |
|---|
| Sequence: | MDVVDSLLVNGSNITPPCELGLENETLFCLDQPRPSKEWQPAVQILLYSLIFLLSVLGNT
LVITVLIRNKRMRTVTNIFLLSLAVSDLMLCLFCMPFNLIPNLLKDFIFGSAVCKTTTYF
MGTSVSVSTFNLVAISLERYGAICKPLQSRVWQTKSHALKVIAATWCLSFTIMTPYPIYS
NLVPFTKNNNQTANMCRFLLPNDVMQQSWHTFLLLILFLIPGIVMMVAYGLISLELYQGI
KFEASQKKSAKERKPSTTSSGKYEDSDGCYLQKTRPPRKLELRQLSTGSSSRANRIRSNS
SAANLMAKKRVIRMLIVIVVLFFLCWMPIFSANAWRAYDTASAERRLSGTPISFILLLSY
TSSCVNPIIYCFMNKRFRLGFMATFPCCPNPGPPGARGEVGEEEEGGTTGASLSRFSYSH
MSASVPPQ
|
|
|
|---|
| BDBM50196207 |
|---|
| n/a |
|---|
| Name | BDBM50196207 |
|---|
| Synonyms: | 1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4-chlorobenzoyl]hexahydro-1H-azepine | CHEMBL217720 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C19H19ClN4O3S2 |
|---|
| Mol. Mass. | 450.962 |
|---|
| SMILES | Clc1ccc(C(=O)N2CCCCCC2)c(NS(=O)(=O)c2cccc3nsnc23)c1 |
|---|
| Structure | 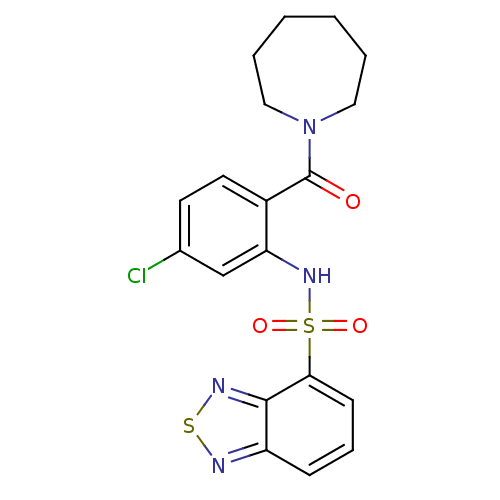
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Allison, BD; Phuong, VK; McAtee, LC; Rosen, M; Morton, M; Prendergast, C; Barrett, T; Lagaud, G; Freedman, J; Li, L; Wu, X; Venkatesan, H; Pippel, M; Woods, C; Rizzolio, MC; Hack, M; Hoey, K; Deng, X; King, C; Shankley, NP; Rabinowitz, MH Identification and optimization of anthranilic sulfonamides as novel, selective cholecystokinin-2 receptor antagonists. J Med Chem49:6371-90 (2006) [PubMed] Article
Allison, BD; Phuong, VK; McAtee, LC; Rosen, M; Morton, M; Prendergast, C; Barrett, T; Lagaud, G; Freedman, J; Li, L; Wu, X; Venkatesan, H; Pippel, M; Woods, C; Rizzolio, MC; Hack, M; Hoey, K; Deng, X; King, C; Shankley, NP; Rabinowitz, MH Identification and optimization of anthranilic sulfonamides as novel, selective cholecystokinin-2 receptor antagonists. J Med Chem49:6371-90 (2006) [PubMed] Article