

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against human Prostaglandin G/H synthase 2 expressed in sf-9 cells infected with baculovirus | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50138441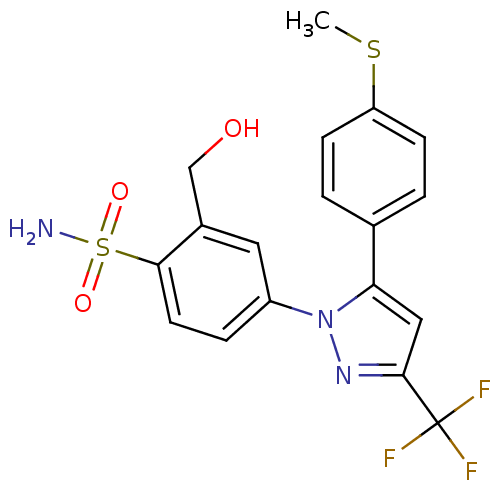 (2-(hydroxymethyl)-4-(5-(4-(methylthio)phenyl)-3-(t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 235 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against human Prostaglandin G/H synthase 2 expressed in sf-9 cells infected with baculovirus | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50138454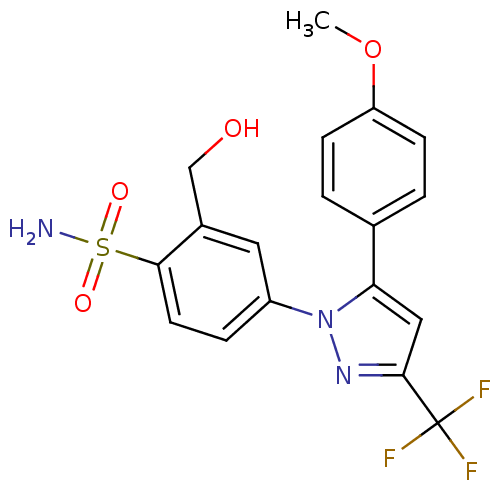 (2-Hydroxymethyl-4-[5-(4-methoxy-phenyl)-3-trifluor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 365 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against human Prostaglandin G/H synthase 2 expressed in sf-9 cells infected with baculovirus | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50452007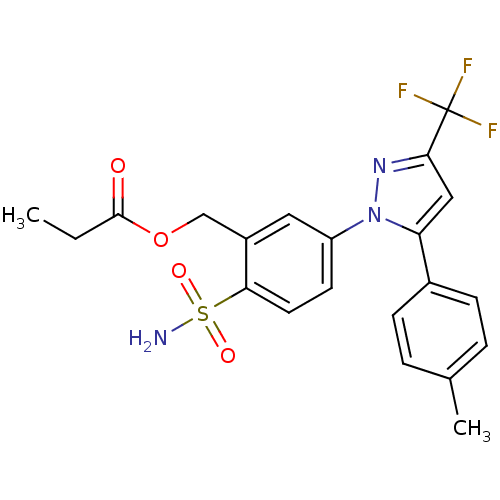 (CHEMBL2112872) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against human Prostaglandin G/H synthase 2 expressed in sf-9 cells infected with baculovirus | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50138444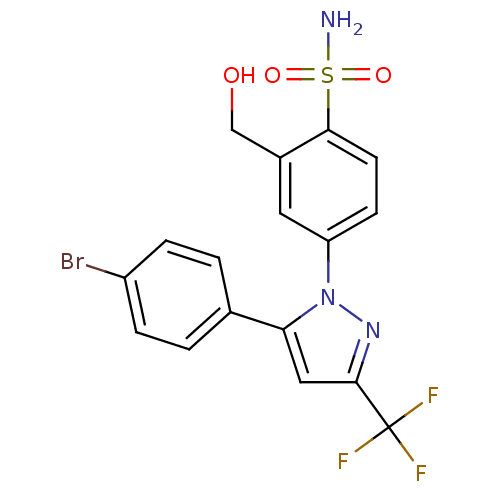 (4-(5-(4-bromophenyl)-3-(trifluoromethyl)-1H-pyrazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 592 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against human Prostaglandin G/H synthase 2 expressed in sf-9 cells infected with baculovirus | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50138447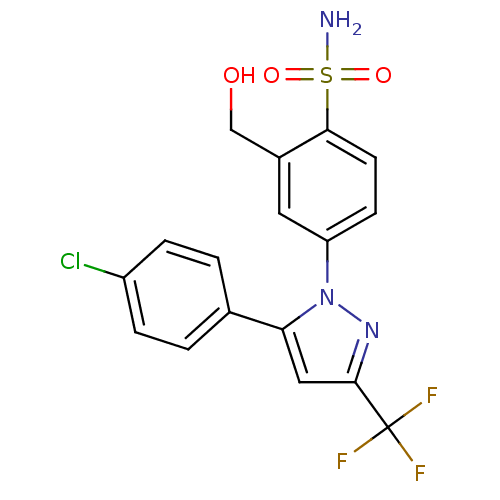 (4-[5-(4-Chloro-phenyl)-3-trifluoromethyl-pyrazol-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 712 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against human Prostaglandin G/H synthase 2 expressed in sf-9 cells infected with baculovirus | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50138458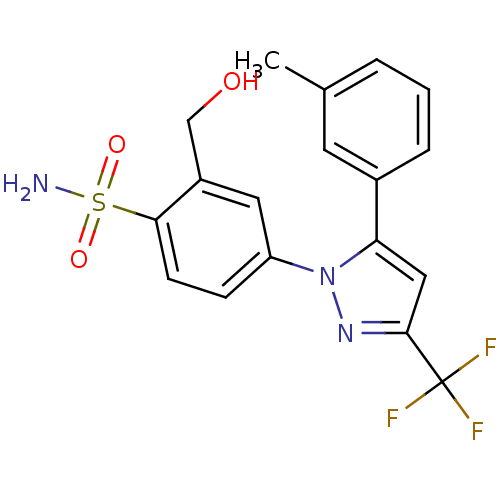 (2-Hydroxymethyl-4-(5-m-tolyl-3-trifluoromethyl-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 728 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against human Prostaglandin G/H synthase 2 expressed in sf-9 cells infected with baculovirus | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50138452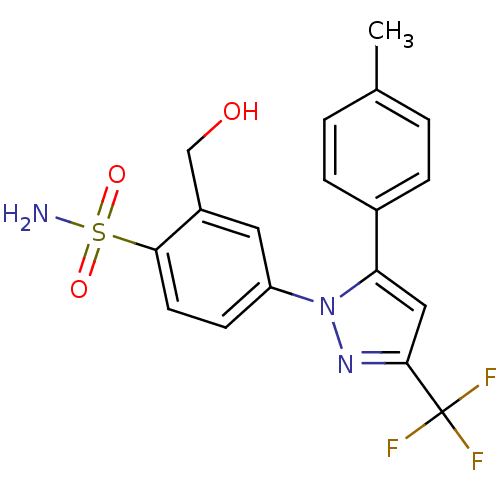 (2-Hydroxymethyl-4-(5-p-tolyl-3-trifluoromethyl-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against human Prostaglandin G/H synthase 2 expressed in sf-9 cells infected with baculovirus | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50138460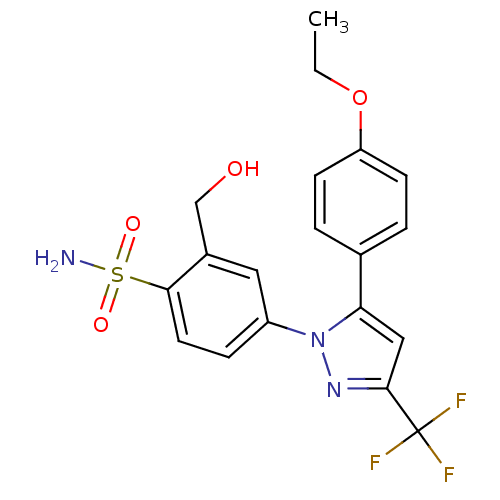 (4-[5-(4-Ethoxy-phenyl)-3-trifluoromethyl-pyrazol-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 901 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against human Prostaglandin G/H synthase 2 expressed in sf-9 cells infected with baculovirus | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50138445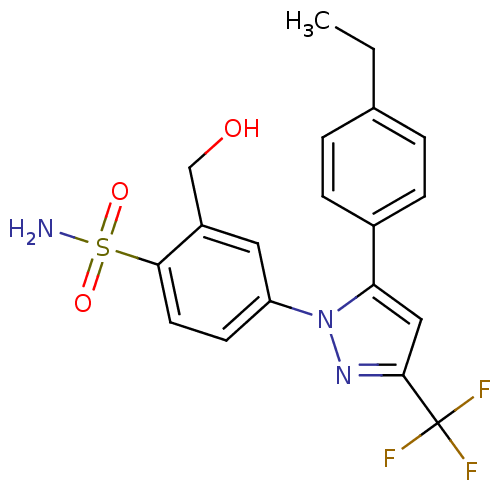 (4-[5-(4-Ethyl-phenyl)-3-trifluoromethyl-pyrazol-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against human Prostaglandin G/H synthase 2 expressed in sf-9 cells infected with baculovirus | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50138459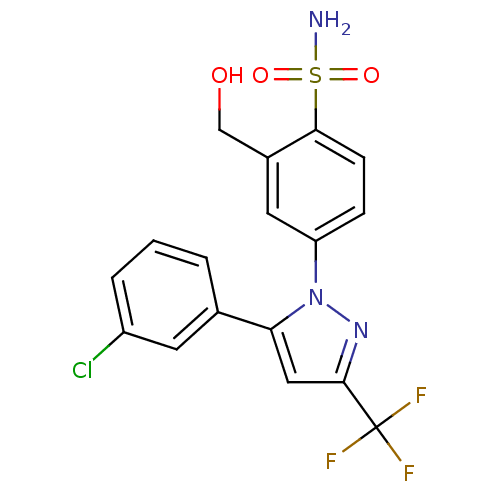 (4-[5-(3-Chloro-phenyl)-3-trifluoromethyl-pyrazol-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against human Prostaglandin G/H synthase 2 expressed in sf-9 cells infected with baculovirus | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50138442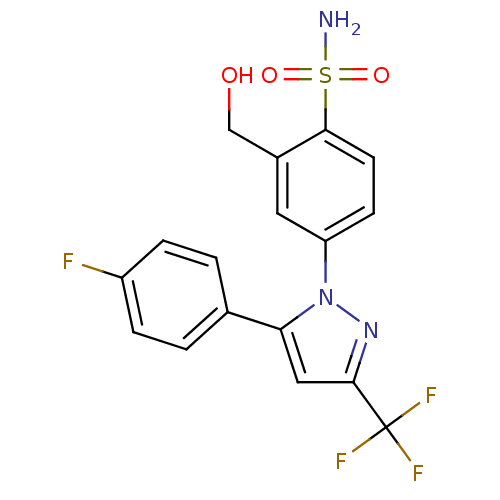 (4-[5-(4-Fluoro-phenyl)-3-trifluoromethyl-pyrazol-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against human Prostaglandin G/H synthase 2 expressed in sf-9 cells infected with baculovirus | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50138455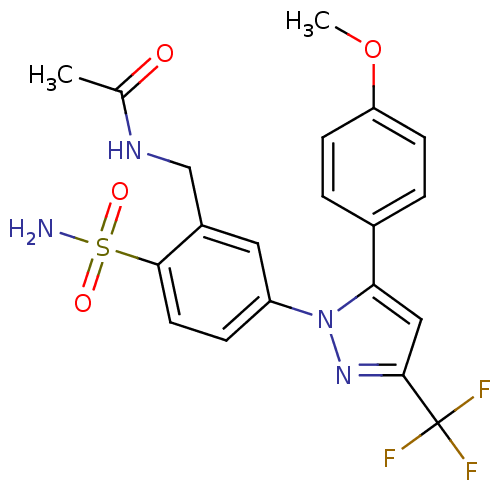 (CHEMBL344540 | N-{5-[5-(4-Methoxy-phenyl)-3-triflu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against human Prostaglandin G/H synthase 2 expressed in sf-9 cells infected with baculovirus | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50138446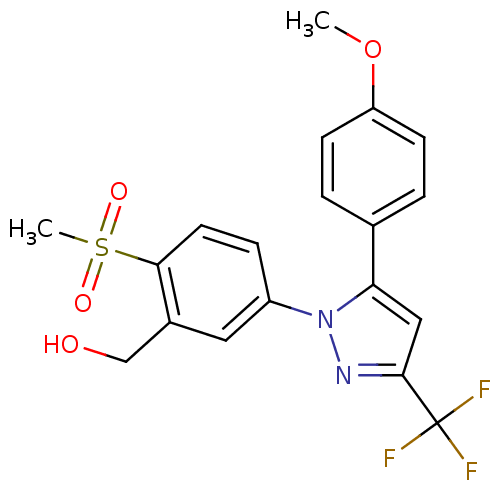 (CHEMBL358122 | {2-Methanesulfonyl-5-[5-(4-methoxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against human Prostaglandin G/H synthase 2 expressed in sf-9 cells infected with baculovirus | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50138461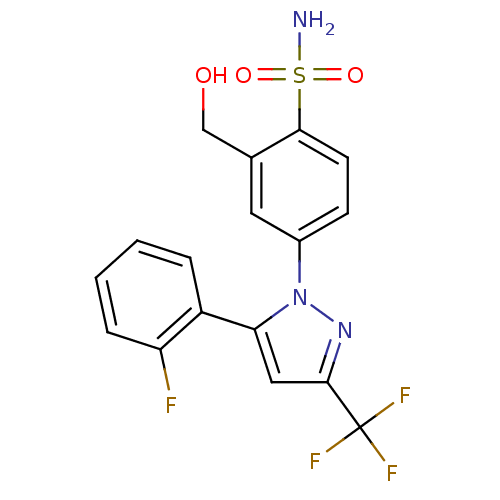 (4-[5-(2-Fluoro-phenyl)-3-trifluoromethyl-pyrazol-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against human Prostaglandin G/H synthase 2 expressed in sf-9 cells infected with baculovirus | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50138456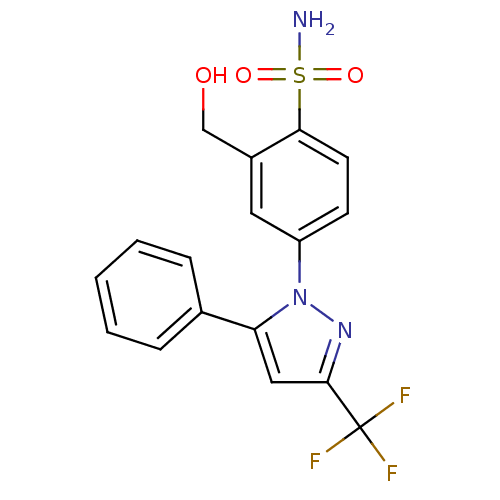 (2-Hydroxymethyl-4-(5-phenyl-3-trifluoromethyl-pyra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against human Prostaglandin G/H synthase 2 expressed in sf-9 cells infected with baculovirus | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50138448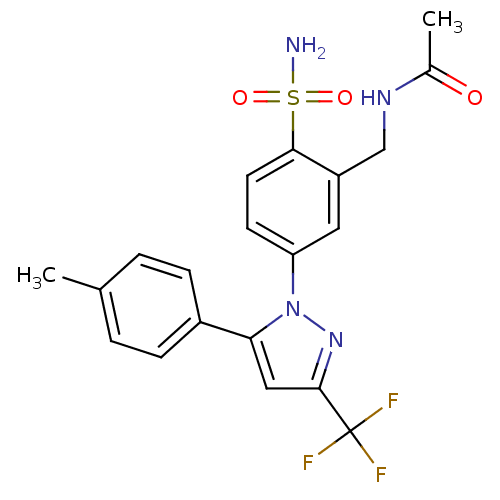 (CHEMBL149781 | N-[2-Sulfamoyl-5-(5-p-tolyl-3-trifl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 1 from ram seminal vesicles | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50138449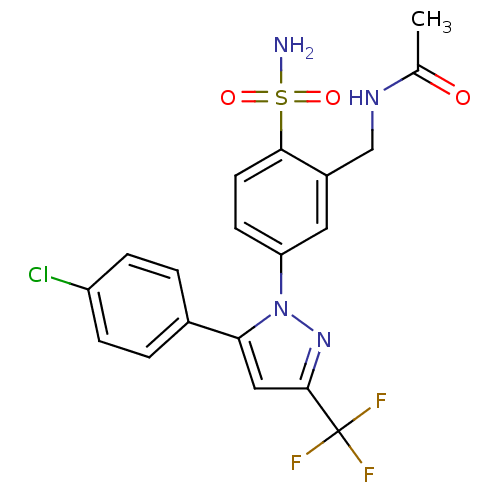 (CHEMBL146465 | N-{5-[5-(4-Chloro-phenyl)-3-trifluo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against human Prostaglandin G/H synthase 2 expressed in sf-9 cells infected with baculovirus | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50138443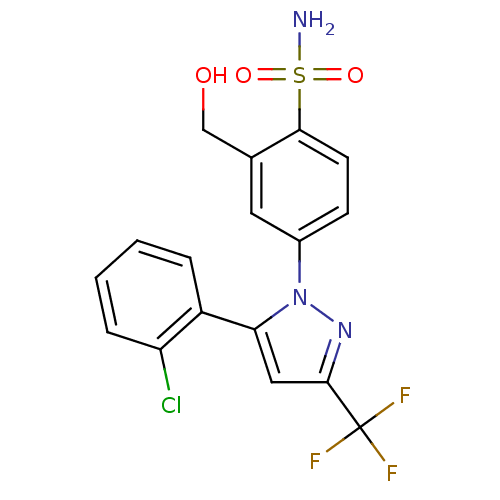 (4-[5-(2-Chloro-phenyl)-3-trifluoromethyl-pyrazol-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against human Prostaglandin G/H synthase 2 expressed in sf-9 cells infected with baculovirus | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50138448 (CHEMBL149781 | N-[2-Sulfamoyl-5-(5-p-tolyl-3-trifl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against human Prostaglandin G/H synthase 2 expressed in sf-9 cells infected with baculovirus | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50138451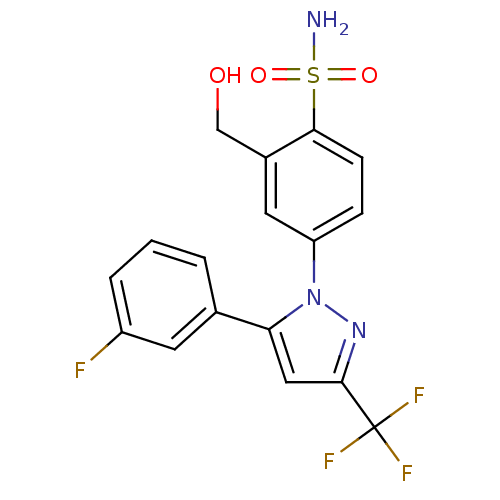 (4-[5-(3-Fluoro-phenyl)-3-trifluoromethyl-pyrazol-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against human Prostaglandin G/H synthase 2 expressed in sf-9 cells infected with baculovirus | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 1 from ram seminal vesicles | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50138453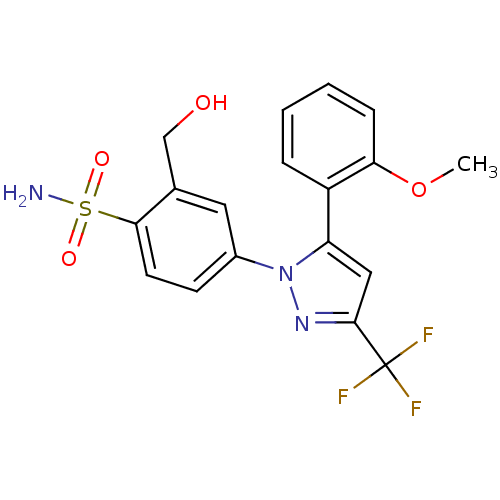 (2-Hydroxymethyl-4-[5-(2-methoxy-phenyl)-3-trifluor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against human Prostaglandin G/H synthase 2 expressed in sf-9 cells infected with baculovirus | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50452007 (CHEMBL2112872) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 1 from ram seminal vesicles | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50138457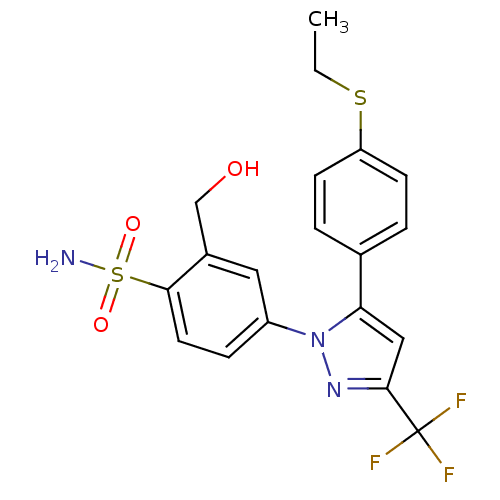 (4-[5-(4-Ethylsulfanyl-phenyl)-3-trifluoromethyl-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against human Prostaglandin G/H synthase 2 expressed in sf-9 cells infected with baculovirus | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50138441 (2-(hydroxymethyl)-4-(5-(4-(methylthio)phenyl)-3-(t...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 1 from ram seminal vesicles | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50138454 (2-Hydroxymethyl-4-[5-(4-methoxy-phenyl)-3-trifluor...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 1 from ram seminal vesicles | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50138455 (CHEMBL344540 | N-{5-[5-(4-Methoxy-phenyl)-3-triflu...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 1 from ram seminal vesicles | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50138449 (CHEMBL146465 | N-{5-[5-(4-Chloro-phenyl)-3-trifluo...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 1 from ram seminal vesicles | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50138460 (4-[5-(4-Ethoxy-phenyl)-3-trifluoromethyl-pyrazol-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 1 from ram seminal vesicles | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50138444 (4-(5-(4-bromophenyl)-3-(trifluoromethyl)-1H-pyrazo...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 1 from ram seminal vesicles | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50138452 (2-Hydroxymethyl-4-(5-p-tolyl-3-trifluoromethyl-pyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.78E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 1 from ram seminal vesicles | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50138457 (4-[5-(4-Ethylsulfanyl-phenyl)-3-trifluoromethyl-py...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 1 from ram seminal vesicles | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50138446 (CHEMBL358122 | {2-Methanesulfonyl-5-[5-(4-methoxy-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 1 from ram seminal vesicles | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50138458 (2-Hydroxymethyl-4-(5-m-tolyl-3-trifluoromethyl-pyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.27E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 1 from ram seminal vesicles | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50138447 (4-[5-(4-Chloro-phenyl)-3-trifluoromethyl-pyrazol-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 1 from ram seminal vesicles | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50138459 (4-[5-(3-Chloro-phenyl)-3-trifluoromethyl-pyrazol-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.65E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 1 from ram seminal vesicles | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50138442 (4-[5-(4-Fluoro-phenyl)-3-trifluoromethyl-pyrazol-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 1 from ram seminal vesicles | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50138461 (4-[5-(2-Fluoro-phenyl)-3-trifluoromethyl-pyrazol-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 1 from ram seminal vesicles | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50138445 (4-[5-(4-Ethyl-phenyl)-3-trifluoromethyl-pyrazol-1-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 1 from ram seminal vesicles | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50138443 (4-[5-(2-Chloro-phenyl)-3-trifluoromethyl-pyrazol-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.12E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 1 from ram seminal vesicles | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50138451 (4-[5-(3-Fluoro-phenyl)-3-trifluoromethyl-pyrazol-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 1 from ram seminal vesicles | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50138456 (2-Hydroxymethyl-4-(5-phenyl-3-trifluoromethyl-pyra...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.09E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 1 from ram seminal vesicles | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50138453 (2-Hydroxymethyl-4-[5-(2-methoxy-phenyl)-3-trifluor...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.15E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Discovery Research-Dr. Reddy's Laboratories Ltd Curated by ChEMBL | Assay Description Inhibitory activity against prostaglandin G/H synthase 1 from ram seminal vesicles | Bioorg Med Chem Lett 14: 499-504 (2003) BindingDB Entry DOI: 10.7270/Q280522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||