

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175251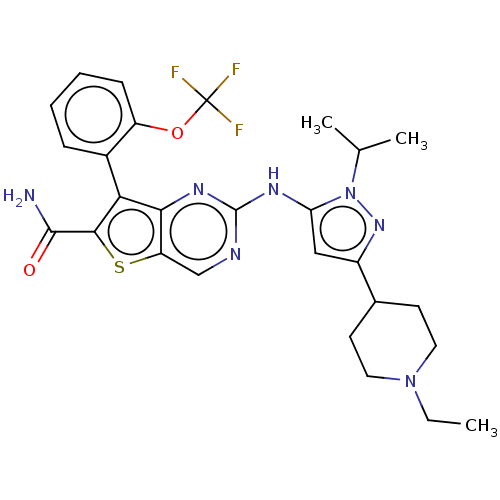 (US9115140, I-123) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175246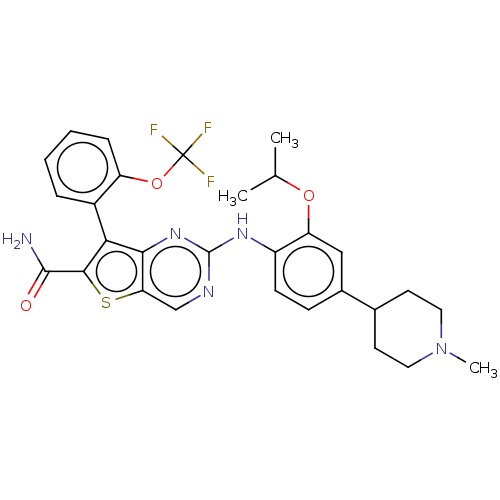 (US9115140, I-26) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM175253 (US9115140, I-131) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175250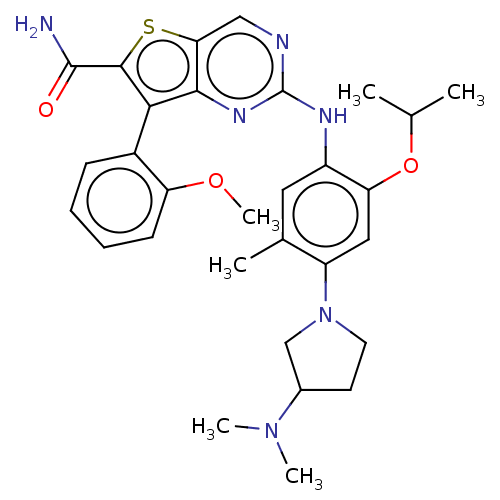 (US9115140, I-118) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM175250 (US9115140, I-118) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175253 (US9115140, I-131) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM175248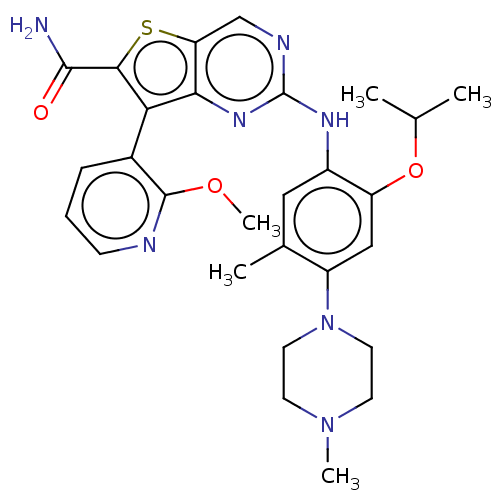 (US9115140, I-100) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175248 (US9115140, I-100) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50151638 (8''-chloro-5''-(5-hydroxy-1,2,4-oxadiazol-3-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human PDE7A1 expressed in baculovirus infected Sf9 cells | Bioorg Med Chem Lett 14: 4627-31 (2004) Article DOI: 10.1016/j.bmcl.2004.07.010 BindingDB Entry DOI: 10.7270/Q2TT4QDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50151562 (7-{5-[(Z)-Cyclohexylimino]-4-methyl-4,5-dihydro-[1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells | Bioorg Med Chem Lett 14: 4607-13 (2004) Article DOI: 10.1016/j.bmcl.2004.07.008 BindingDB Entry DOI: 10.7270/Q2377855 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50151635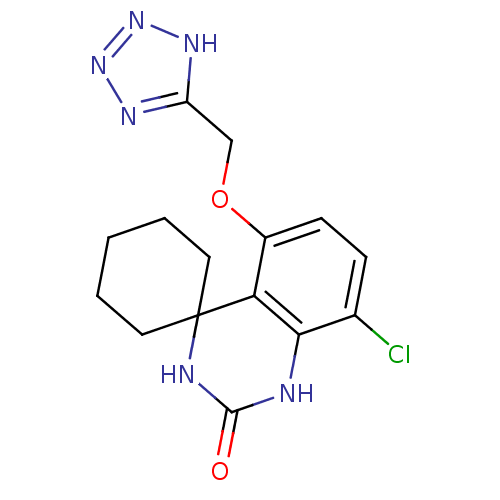 (8''-chloro-5''-(1H-1,2,3,4-tetraazol-5-ylmethoxy)s...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human PDE7A1 expressed in baculovirus infected Sf9 cells | Bioorg Med Chem Lett 14: 4627-31 (2004) Article DOI: 10.1016/j.bmcl.2004.07.010 BindingDB Entry DOI: 10.7270/Q2TT4QDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM175251 (US9115140, I-123) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175255 (US9115140, I-159) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175245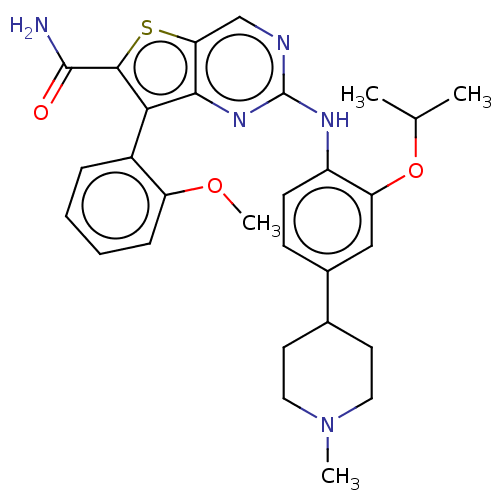 (US9115140, I-16) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175247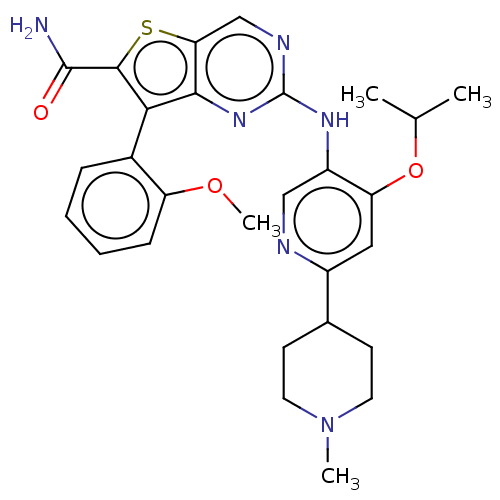 (US9115140, I-38) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175257 (US9115140, I-172) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM175255 (US9115140, I-159) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50151649 (8''-chloro-5''-hydroxyspiro[cyclohexane-1,4''-(1''...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human PDE7A1 expressed in baculovirus infected Sf9 cells | Bioorg Med Chem Lett 14: 4627-31 (2004) Article DOI: 10.1016/j.bmcl.2004.07.010 BindingDB Entry DOI: 10.7270/Q2TT4QDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50151644 (CHEMBL359890 | ethyl 5-[8''-chloro-2''-oxospiro[cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human PDE7A1 expressed in baculovirus infected Sf9 cells | Bioorg Med Chem Lett 14: 4627-31 (2004) Article DOI: 10.1016/j.bmcl.2004.07.010 BindingDB Entry DOI: 10.7270/Q2TT4QDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50151609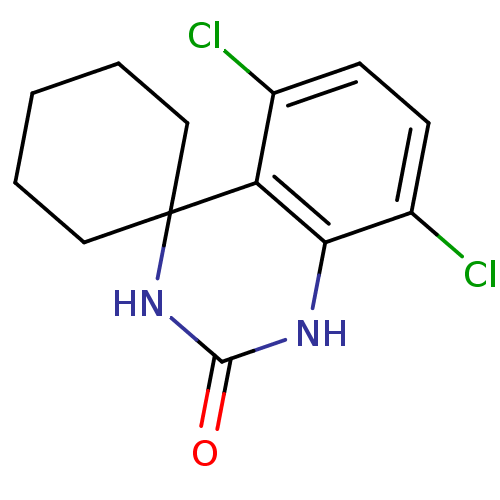 (5'',8''-dichlorospiro[cyclohexane-1,4''-(1'',2'',3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human PDE7A1 expressed in baculovirus infected Sf9 cells | Bioorg Med Chem Lett 14: 4627-31 (2004) Article DOI: 10.1016/j.bmcl.2004.07.010 BindingDB Entry DOI: 10.7270/Q2TT4QDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50151640 (8''-chloro-5''-methoxyspiro[cyclohexane-1,4''-(1''...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human PDE7A1 expressed in baculovirus infected Sf9 cells | Bioorg Med Chem Lett 14: 4627-31 (2004) Article DOI: 10.1016/j.bmcl.2004.07.010 BindingDB Entry DOI: 10.7270/Q2TT4QDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50151634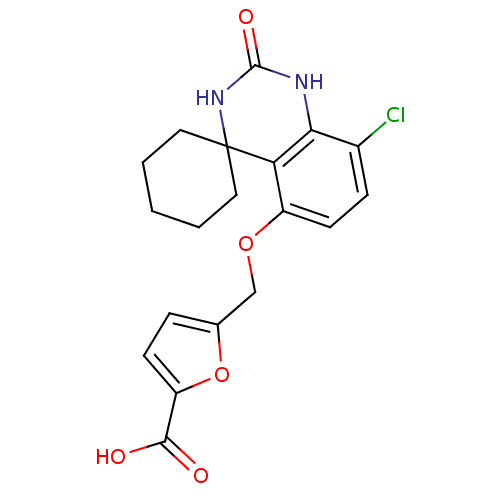 (5-[8''-chloro-2''-oxospiro[cyclohexane-1,4''-(1'',...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human PDE7A1 expressed in baculovirus infected Sf9 cells | Bioorg Med Chem Lett 14: 4627-31 (2004) Article DOI: 10.1016/j.bmcl.2004.07.010 BindingDB Entry DOI: 10.7270/Q2TT4QDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50151636 (3-[8''-chloro-2''-oxospiro[cyclohexane-1,4''-(1'',...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human PDE7A1 expressed in baculovirus infected Sf9 cells | Bioorg Med Chem Lett 14: 4627-31 (2004) Article DOI: 10.1016/j.bmcl.2004.07.010 BindingDB Entry DOI: 10.7270/Q2TT4QDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50151615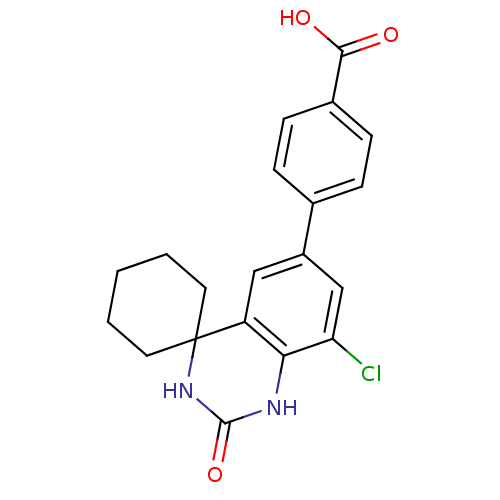 (4-[8''-chloro-2''-oxospiro[cyclohexane-1,4''-(1'',...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells | Bioorg Med Chem Lett 14: 4623-6 (2004) Article DOI: 10.1016/j.bmcl.2004.07.011 BindingDB Entry DOI: 10.7270/Q2ZG6RQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50151603 (1N-(3-dimethylaminopropyl)-3-[8''-chloro-2''-oxosp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells | Bioorg Med Chem Lett 14: 4623-6 (2004) Article DOI: 10.1016/j.bmcl.2004.07.011 BindingDB Entry DOI: 10.7270/Q2ZG6RQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50151605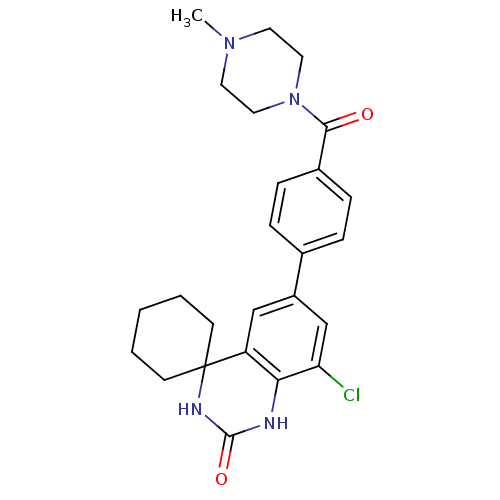 (4-[8''-chloro-2''-oxospiro[cyclohexane-1,4''-(1'',...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells | Bioorg Med Chem Lett 14: 4623-6 (2004) Article DOI: 10.1016/j.bmcl.2004.07.011 BindingDB Entry DOI: 10.7270/Q2ZG6RQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50151609 (5'',8''-dichlorospiro[cyclohexane-1,4''-(1'',2'',3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells | Bioorg Med Chem Lett 14: 4623-6 (2004) Article DOI: 10.1016/j.bmcl.2004.07.011 BindingDB Entry DOI: 10.7270/Q2ZG6RQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50151593 (8''-chloro-6''-phenylspiro[cyclohexane-1,4''-(1'',...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells | Bioorg Med Chem Lett 14: 4623-6 (2004) Article DOI: 10.1016/j.bmcl.2004.07.011 BindingDB Entry DOI: 10.7270/Q2ZG6RQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175249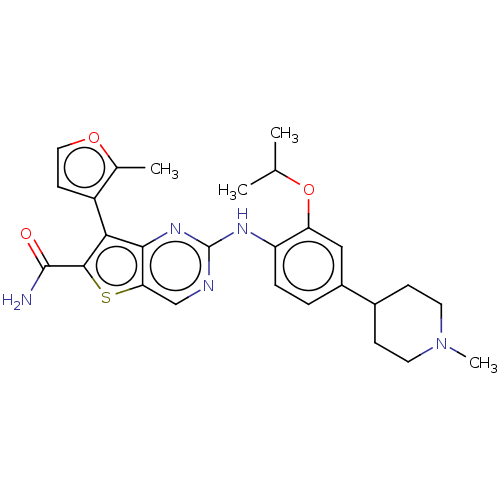 (US9115140, I-115) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM175257 (US9115140, I-172) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM60576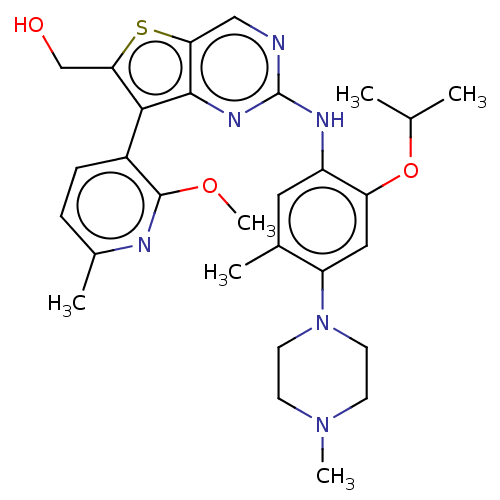 (US9115140, I-191) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175244 (US9115140, I-13) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175254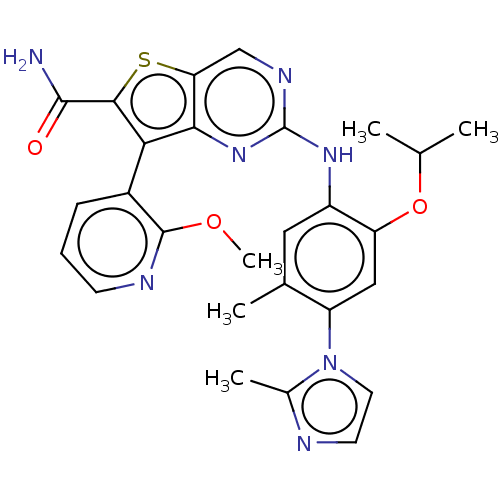 (US9115140, I-132) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM175249 (US9115140, I-115) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50151597 (3-[8''-chloro-2''-oxospiro[cyclohexane-1,4''-(1'',...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells | Bioorg Med Chem Lett 14: 4623-6 (2004) Article DOI: 10.1016/j.bmcl.2004.07.011 BindingDB Entry DOI: 10.7270/Q2ZG6RQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50151592 (1N-(3-dimethylaminopropyl)-4-[8''-chloro-2''-oxosp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells | Bioorg Med Chem Lett 14: 4623-6 (2004) Article DOI: 10.1016/j.bmcl.2004.07.011 BindingDB Entry DOI: 10.7270/Q2ZG6RQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50151602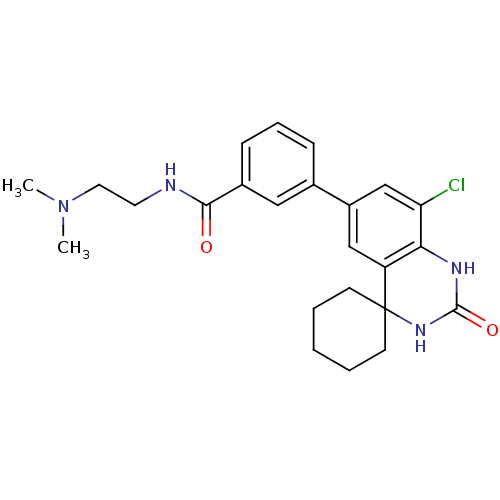 (1N-(2-dimethylaminoethyl)-3-[8''-chloro-2''-oxospi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells | Bioorg Med Chem Lett 14: 4623-6 (2004) Article DOI: 10.1016/j.bmcl.2004.07.011 BindingDB Entry DOI: 10.7270/Q2ZG6RQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50151594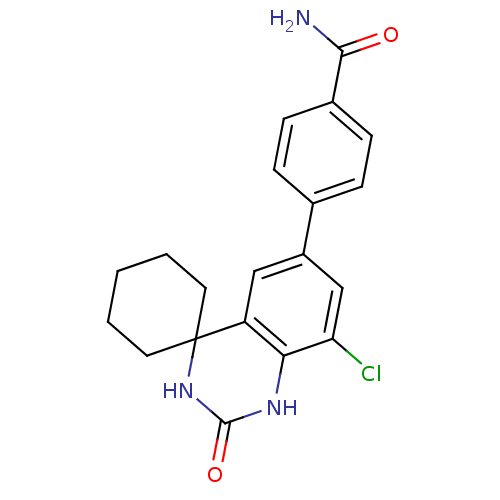 (4-[8''-chloro-2''-oxospiro[cyclohexane-1,4''-(1'',...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells | Bioorg Med Chem Lett 14: 4623-6 (2004) Article DOI: 10.1016/j.bmcl.2004.07.011 BindingDB Entry DOI: 10.7270/Q2ZG6RQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50151631 (2-[8''-chloro-2''-oxospiro[cyclohexane-1,4''-(1'',...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human PDE7A1 expressed in baculovirus infected Sf9 cells | Bioorg Med Chem Lett 14: 4627-31 (2004) Article DOI: 10.1016/j.bmcl.2004.07.010 BindingDB Entry DOI: 10.7270/Q2TT4QDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50151572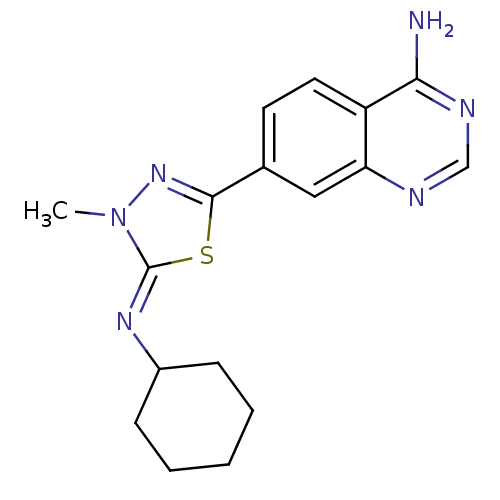 (7-{5-[(Z)-Cyclohexylimino]-4-methyl-4,5-dihydro-[1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells | Bioorg Med Chem Lett 14: 4607-13 (2004) Article DOI: 10.1016/j.bmcl.2004.07.008 BindingDB Entry DOI: 10.7270/Q2377855 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50151538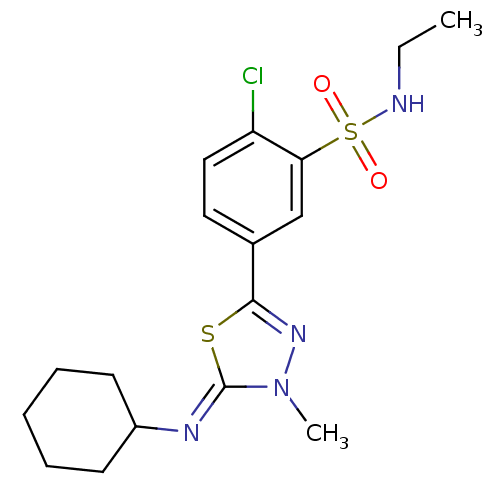 (2-Chloro-5-{5-[(Z)-cyclohexylimino]-4-methyl-4,5-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells | Bioorg Med Chem Lett 14: 4607-13 (2004) Article DOI: 10.1016/j.bmcl.2004.07.008 BindingDB Entry DOI: 10.7270/Q2377855 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50151598 (1N-(2-dimethylaminoethyl)-4-[8''-chloro-2''-oxospi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells | Bioorg Med Chem Lett 14: 4623-6 (2004) Article DOI: 10.1016/j.bmcl.2004.07.011 BindingDB Entry DOI: 10.7270/Q2ZG6RQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175258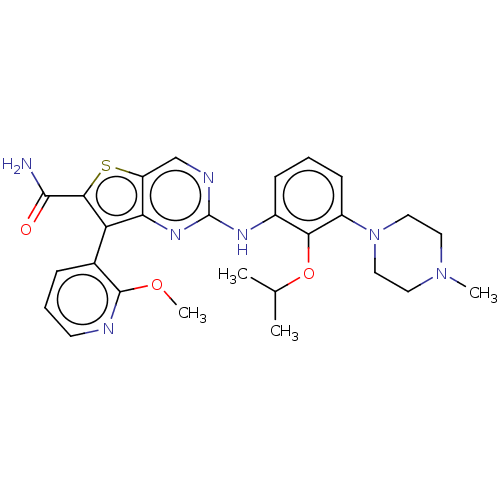 (US9115140, I-176) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175260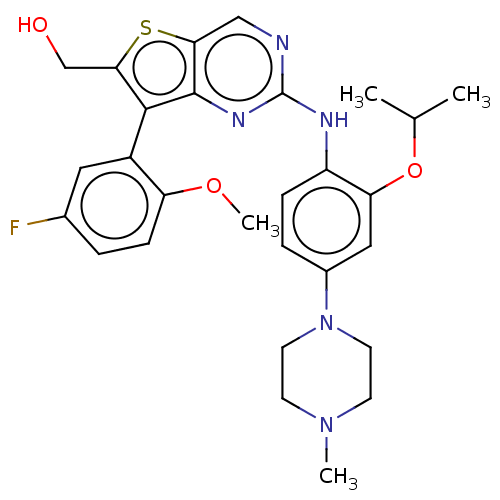 (US9115140, I-202) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM175243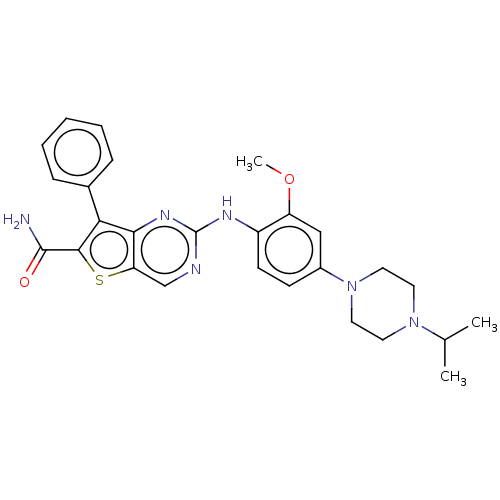 (US9115140, I-7) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM175260 (US9115140, I-202) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The reagents used have the following composition: Enzyme buffer (EB): 50 mM HEPES (pH: 7.0) (Sigma H7523), 100 mM NaCl (Sigma S7653), NaN.sub.3 at 0.... | US Patent US9115140 (2015) BindingDB Entry DOI: 10.7270/Q2HX1BFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50151623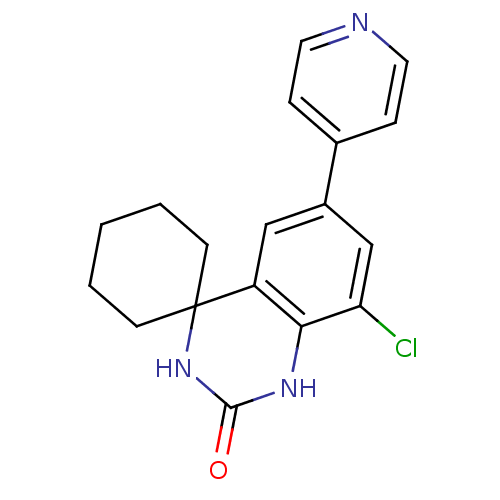 (8''-chloro-6''-(4-pyridyl)spiro[cyclohexane-1,4''-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells | Bioorg Med Chem Lett 14: 4623-6 (2004) Article DOI: 10.1016/j.bmcl.2004.07.011 BindingDB Entry DOI: 10.7270/Q2ZG6RQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50151645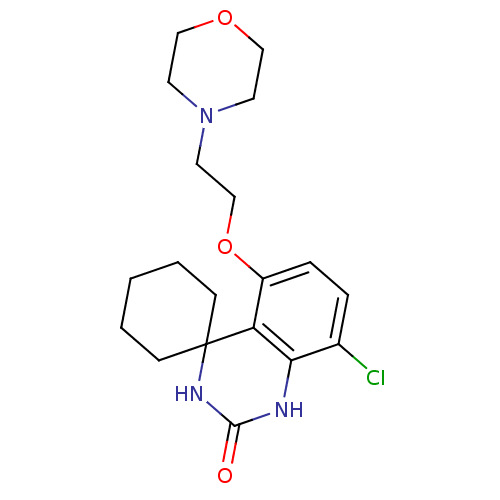 (8''-chloro-5''-[2-(1,4-oxazinan-4-yl)ethoxy]spiro[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human PDE7A1 expressed in baculovirus infected Sf9 cells | Bioorg Med Chem Lett 14: 4627-31 (2004) Article DOI: 10.1016/j.bmcl.2004.07.010 BindingDB Entry DOI: 10.7270/Q2TT4QDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50151599 (8''-chloro-6''-(2-pyridyl)spiro[cyclohexane-1,4''-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells | Bioorg Med Chem Lett 14: 4623-6 (2004) Article DOI: 10.1016/j.bmcl.2004.07.011 BindingDB Entry DOI: 10.7270/Q2ZG6RQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A (Homo sapiens (Human)) | BDBM50151527 (4-{5-[(Z)-Cyclohexylimino]-4-methyl-4,5-dihydro-[1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells | Bioorg Med Chem Lett 14: 4607-13 (2004) Article DOI: 10.1016/j.bmcl.2004.07.008 BindingDB Entry DOI: 10.7270/Q2377855 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 386 total ) | Next | Last >> |