

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50447108 (CHEMBL3112881) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013677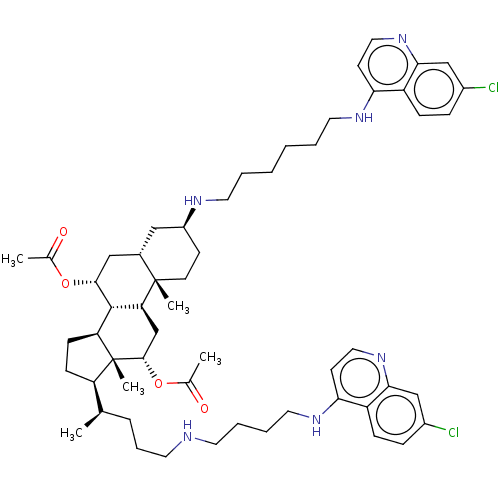 (CHEMBL3264512) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013673 (CHEMBL3264509) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 171 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013676 (CHEMBL3264511) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 285 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013678 (CHEMBL3264513) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013674 (CHEMBL3264510) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50384950 (CHEMBL2037386) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013672 (CHEMBL3264508) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 341 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013675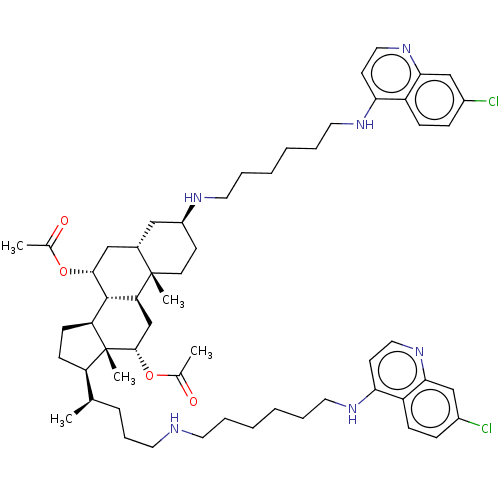 (CHEMBL3259867) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 389 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM23274 ((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013658 (CHEMBL3264499) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013659 (CHEMBL3264500) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013668 (CHEMBL3264171) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013667 (CHEMBL450398) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013645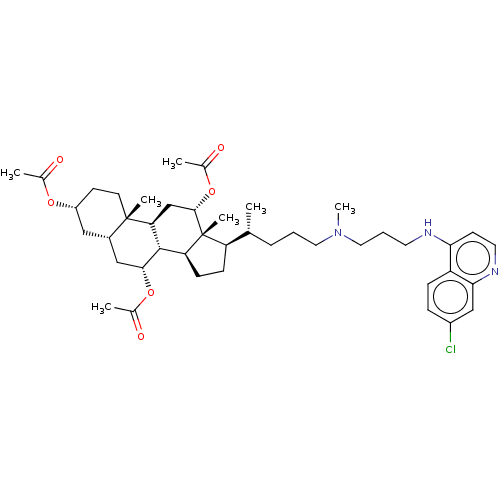 (CHEMBL3264183) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013644 (CHEMBL3264182) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50447108 (CHEMBL3112881) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013665 (CHEMBL3264507) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM23274 ((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013658 (CHEMBL3264499) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013656 (CHEMBL3264497) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013659 (CHEMBL3264500) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013661 (CHEMBL3264502) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013660 (CHEMBL3264501) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013663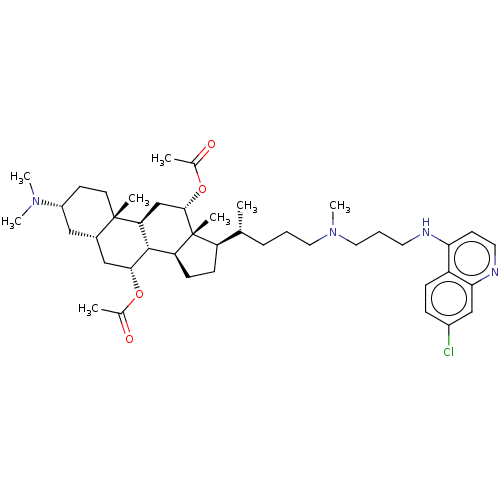 (CHEMBL3264504) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013657 (CHEMBL3264498) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013662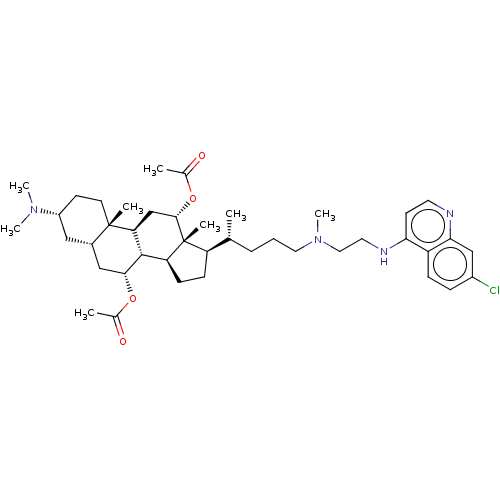 (CHEMBL3264503) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013668 (CHEMBL3264171) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013643 (CHEMBL3264179) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013646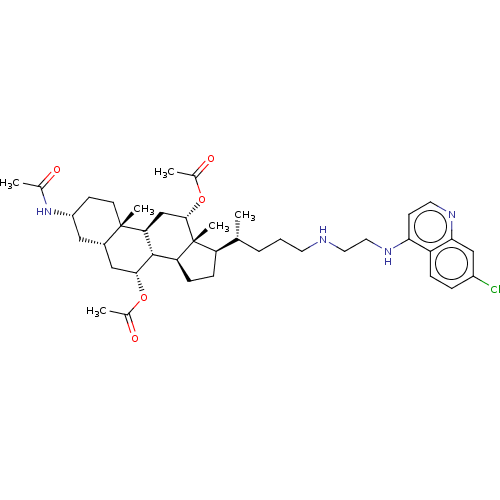 (CHEMBL3264184) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50100895 (2-(4-(4-carbamimidoylphenoxy)phenyl)-1H-indole-6-c...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013664 (CHEMBL3264505) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013651 (CHEMBL3264189) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013649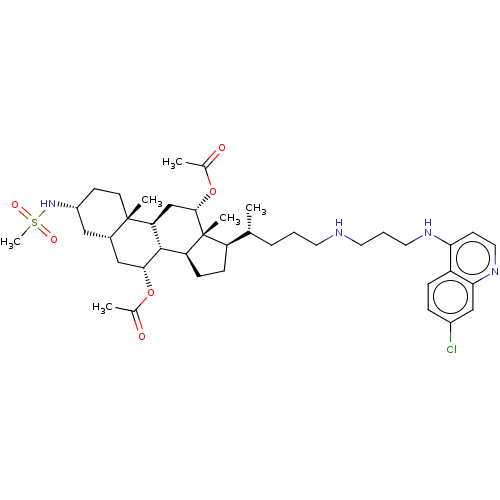 (CHEMBL3264187) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013667 (CHEMBL450398) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013645 (CHEMBL3264183) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013669 (CHEMBL3264174) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013650 (CHEMBL3264188) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013647 (CHEMBL3264185) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013648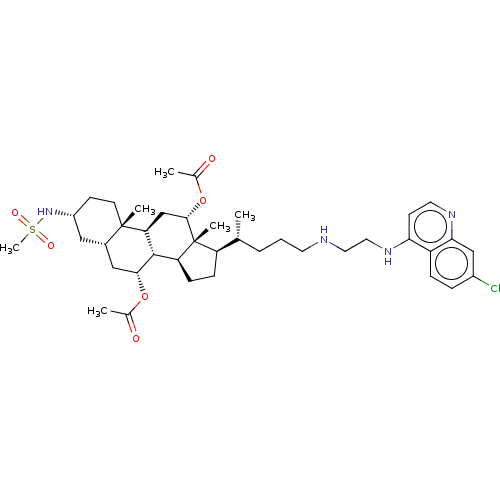 (CHEMBL3264186) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013670 (CHEMBL3264175) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013671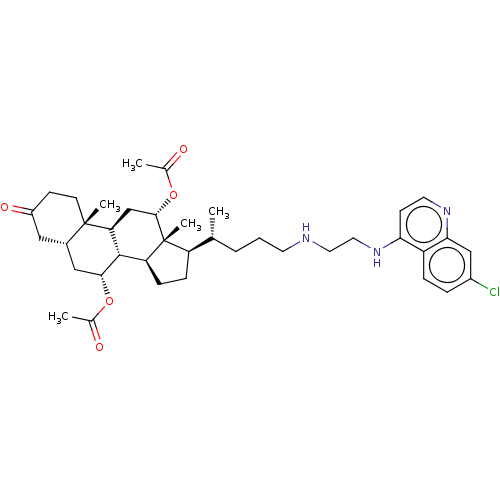 (CHEMBL3264178) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013655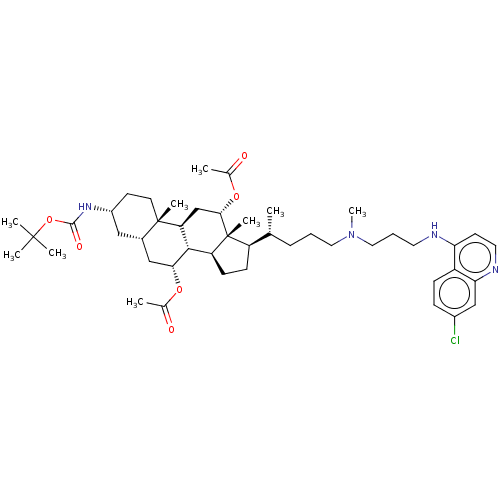 (CHEMBL3264496) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013654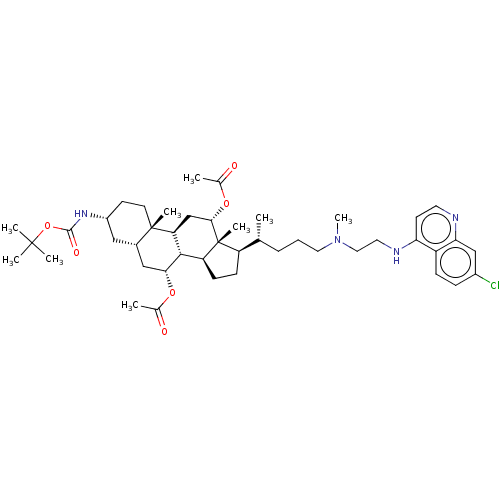 (CHEMBL3264192) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013653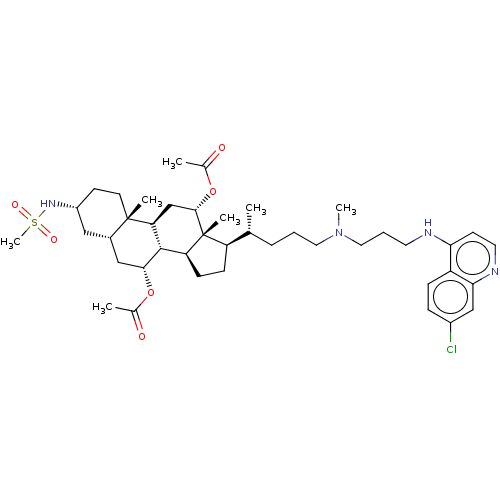 (CHEMBL3264191) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013666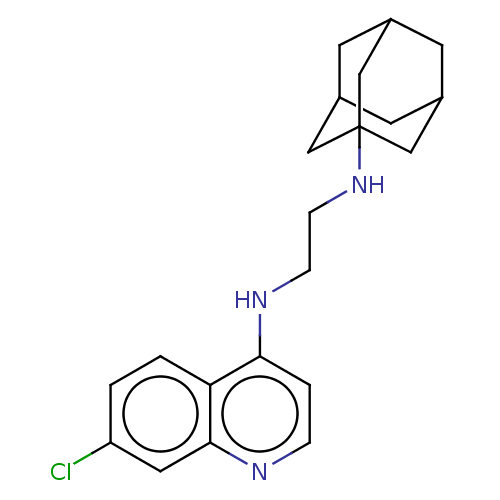 (CHEMBL3264170) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013652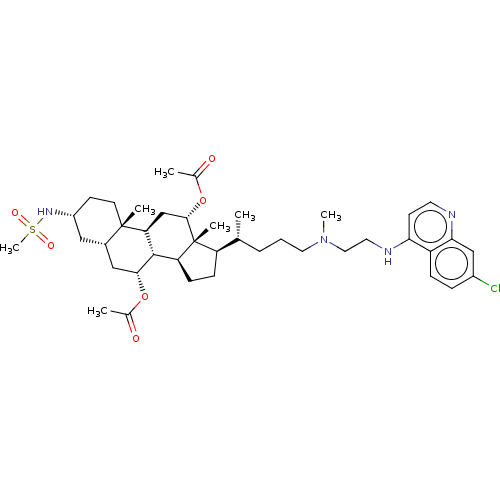 (CHEMBL3264190) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||